Study of Electroless Nickel Coatings on EN-GJS-500-7 Spheroidal Graphite Cast Iron
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Experimental Setup for Electroless Ni-P Coating Deposition
2.3. Characterisation Techniques
3. Results and Discussion
3.1. Kinetic Study
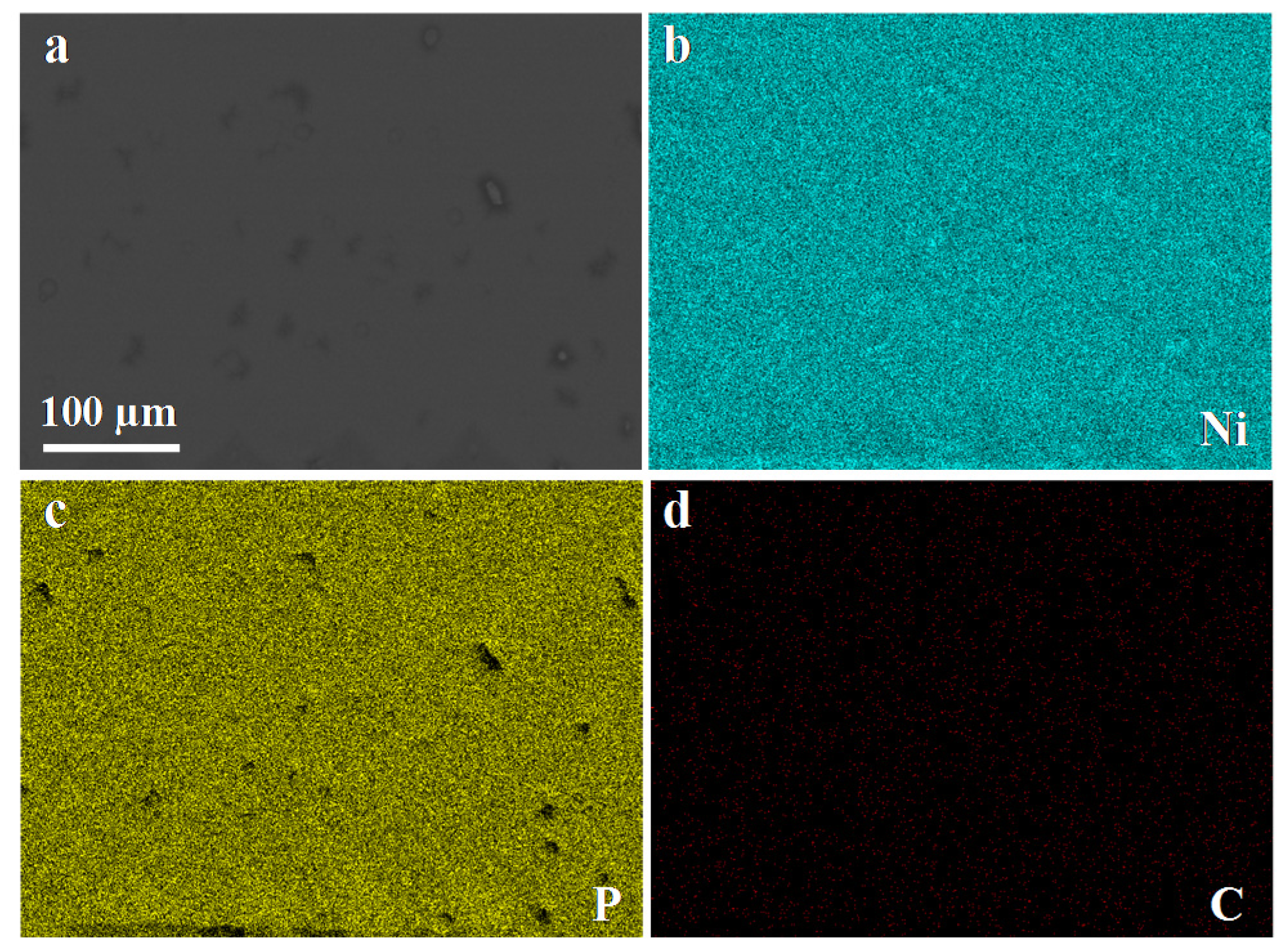
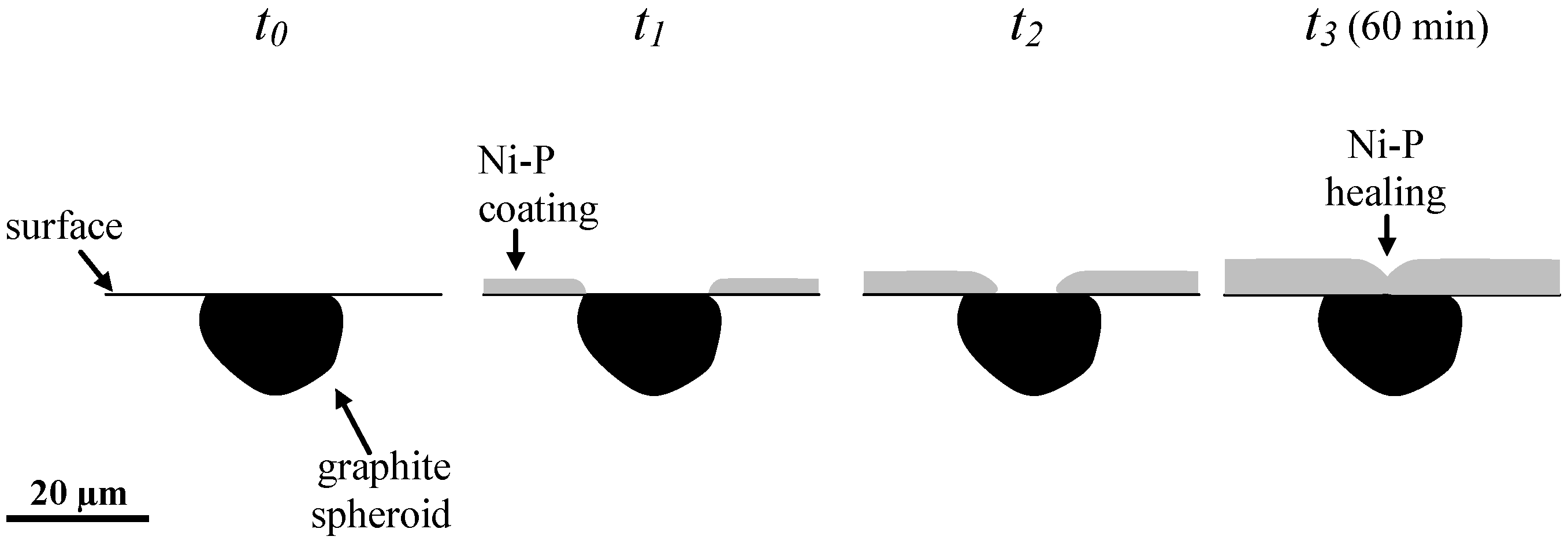
3.2. Morphology Study
- The diffusion of Ni2+ in the bath;
- The diffusion of in the bath;
- The adsorption of Ni2+ at the surface of graphite spheroid;
- The adsorption of at the surface of graphite spheroid;
- The charge transfer between and (as presented in Equation (5));
- The electro crystallization (crystal building) of Ni-P; and
- The desorption of at the surface of graphite spheroid.
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Murray, G. Handbook of Materials Selection for Engineering Applications; CRC Press: Boca Raton, FL, USA, 1997. [Google Scholar]
- Sugishita, J.; Fujiyoshi, S. The effect of cast iron graphite on friction and wear performance III: The lubricating effect of graphite under rolling-sliding contacts. Wear 1982, 77, 181–193. [Google Scholar] [CrossRef]
- Ghasemi, R.; Elmquist, L. Cast iron and the self-lubricating behaviour of graphite under abrasive wear conditions. In Proceedings of the 10th International Symposium on the Science and Processing of Cast Iron, Mar del Plata, Argentina, 13 November 2014. [Google Scholar]
- Gundlach, R.; Doane, D. Heat Treating of High-Alloy Irons; ASM Handbook: Materials Park, OH, USA, 1991; Volume 4, pp. 697–708. [Google Scholar]
- Hashmi, S. Comprehensive Materials Finishing; Elsevier: New York, NY, USA, 2016. [Google Scholar]
- Lai, G.Y. High-Temperature Corrosion and Materials Applications; ASM International: Materials Park, OH, USA, 2007. [Google Scholar]
- Rumble, J. CRC Handbook of Chemistry and Physics, 98th ed.; CRC Press LLC: Boca Raton, FL, USA, 2017. [Google Scholar]
- Ashassi-Sorkhabi, H.; Rafizadeh, S.H. Effect of coating time and heat treatment on structures and corrosion characteristics of electroless Ni–P alloy deposits. Surf. Coat. Technol. 2004, 176, 318–326. [Google Scholar] [CrossRef]
- Balaraju, J.N.; Narayanan, T.S.N.S.; Seshadri, S.K. Evaluation of the corrosion resistance of electroless NiP and NiP composite coatings by electrochemical impedance spectroscopy. J. Solid State Electr. 2001, 5, 334–338. [Google Scholar] [CrossRef] [Green Version]
- Ratzker, M.; Lashmore, D.S.; Pratt, K.W. Electrodeposition and corrosion performance of nickel–phosphorus amorphous alloys. Plat. Surf. Finish. 1986, 73, 74–82. [Google Scholar]
- Narayanan, T.S.N.S.; Baskaran, I.; Krishnaveni, K.; Parthiban, S. Deposition of electroless Ni–P graded coatings and evaluation of their corrosion resistance. Surf. Coat. Technol. 2006, 200, 3438–3445. [Google Scholar] [CrossRef]
- Morrough, H. Graphite formation in cast irons and in nickel-carbon and cobalt-carbon alloys. J. Iron Steel Inst. 1947, 155, 321–371. [Google Scholar]
- Brenner, A.; Riddell, G. Deposition of nickel and cobalt by chemical reaction. J. Res. Nat. Bur. Stand. 1947, 39, 385–395. [Google Scholar] [CrossRef]
- Balaraju, J.; Rajam, K. Electroless deposition and characterization of high phosphorus Ni-P-Si3N4 composite coatings. Int. J. Electrochem. Sci. 2007, 2, 747–761. [Google Scholar]
- Mallory, G.O.; Hajdu, J.B. Electroless Plating: Fundamentals and Applications; William Andrew: Norwich, NY, USA, 1990. [Google Scholar]
- Hu, B.; Sun, R.; Yu, G.; Liu, L.; Xie, Z.; He, X.; Zhang, X. Effect of bath pH and stabilizer on electroless nickel plating of magnesium alloys. Surf. Coat. Technol. 2013, 228, 84–91. [Google Scholar] [CrossRef]
- Xiao, Z.; Wang, W.; Ye, L.; Sha, Y.; Tu, S. Effect of Cd2+ as a stabilizer in the electroless nickel plating system. Surf. Coat. Technol. 2008, 202, 5008–5011. [Google Scholar] [CrossRef]
- Chen, B.-H.; Hong, L.; Ma, Y.; Ko, T.-M. Effects of surfactants in an electroless Nickel-Plating bath on the properties of Ni−P alloy deposits. Ind. Eng. Chem. Res. 2002, 41, 2668–2678. [Google Scholar] [CrossRef]
- Małecki, A.; Micek-Ilnicka, A. Electroless nickel plating from acid bath. Surf. Coat. Technol. 2000, 123, 72–77. [Google Scholar] [CrossRef]
- NACE 6A287 Electroless Nickel Coatings; NACE International: Houston, TX, USA, 1987.
- Hur, K.-H.; Jeong, J.-H.; Lee, D.N. Microstructures and crystallization of electroless Ni-P deposits. J. Mater. Sci. 1990, 25, 2573–2584. [Google Scholar] [CrossRef]
- Rajam, K.S.; Rajagopal, I.; Rajagopalan, S.R.; Viswanathan, B. DSC, X-ray and magnetic studies on electroless Ni-P films grown in alkaline ethanolamine baths. Mater. Chem. Phys. 1993, 33, 289–297. [Google Scholar] [CrossRef]
- Kumar, M.A.; Agarwala, R.C.; Agarwala, V. Synthesis and characterization of electroless Ni–P coated graphite particles. Bull. Mater. Sci. 2008, 31, 819–824. [Google Scholar] [CrossRef]
- Dong, Q.; Ma, T.; Yu, G.; Hu, B.; Guo, C.; Zhang, X. Investigation on the current efficiency of Ni/Graphite powders fabricated by electroplating. Russ. J. Electrochem. 2015, 51, 236–243. [Google Scholar] [CrossRef]
- Luo, L.; Lua, Z.; Tan, X.; Ding, X.; Huang, L.; Cheng, J.; Zhu, L.; Wu, Y. A specific chemical activation pretreatment for electroless nickel plating on SiC ceramic powders. Powder Technol. 2013, 249, 431–435. [Google Scholar] [CrossRef]
- Xu, X.; Cui, Z.D.; Zhu, S.L.; Liang, Y.Q.; Yang, X.J. Preparation of nickel-coated graphite by electroless plating under mechanical or ultrasonic agitation. Surf. Coat. Technol. 2014, 240, 425–431. [Google Scholar] [CrossRef]
- Song, S.Q.; Liu, Z.; Ortega, C.M.; Wu, X.S.; Sun, L. Electrochemical study of Ni deposition on carbon microfiber. Electrochim. Acta 2013, 94, 252–258. [Google Scholar] [CrossRef]
- Hammond, R.A.F. Nickel plating from sulphamate solutions. Pt. 1. Nickel sulphamate solution and its applications. Met. Finish. 1970, 16, 169–172. [Google Scholar]
- Wood, W.G. Metals Handbook. Vol. 5, Surface Cleaning, Finishing, and Coating, 9th ed.; ASM International: Materials Park, OH, USA, 1982; pp. 199–218. [Google Scholar]
- Apachitei, I.; Duszczyk, J. Hydrogen evolution, incorporation and removal in electroless nickel composite coatings on aluminum. J. Appl. Electrochem. 1999, 29, 835–841. [Google Scholar] [CrossRef]








| C | Si | Mn | Fe |
|---|---|---|---|
| 3.50–3.70 | 2.30–2.60 | max. 0.40 | bal. |
| Oxidation of Fe | Reduction of H+ | Reduction of Ni2+ |
|---|---|---|
| = −861 | = −589 | = −468 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forestier, I.; Berthomé, G.; Wouters, Y. Study of Electroless Nickel Coatings on EN-GJS-500-7 Spheroidal Graphite Cast Iron. Coatings 2018, 8, 239. https://doi.org/10.3390/coatings8070239
Forestier I, Berthomé G, Wouters Y. Study of Electroless Nickel Coatings on EN-GJS-500-7 Spheroidal Graphite Cast Iron. Coatings. 2018; 8(7):239. https://doi.org/10.3390/coatings8070239
Chicago/Turabian StyleForestier, Igor, Grégory Berthomé, and Yves Wouters. 2018. "Study of Electroless Nickel Coatings on EN-GJS-500-7 Spheroidal Graphite Cast Iron" Coatings 8, no. 7: 239. https://doi.org/10.3390/coatings8070239





