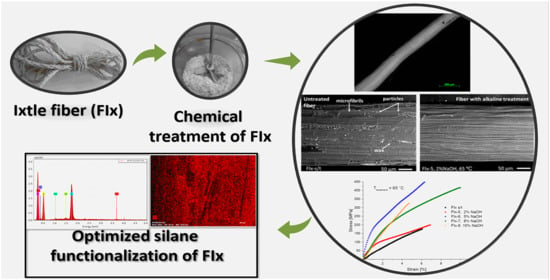
Optimization of the Alkali-Silane Treatment of Agave lechuguilla Fibers (Ixtle) for Potential Reinforcement in Polymeric Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization of Ixtle Fiber (FIx)
2.3. Pretreatment of FIx
2.4. Chemical Modification of FIx
2.4.1. Alkaline Treatment
2.4.2. Treatment with ACSi
3. Results and Discussion
3.1. Alkaline Treatment of FIx
3.1.1. Morphology Analysis
3.1.2. Thermogravimetric Analysis
3.1.3. Dynamic-Mechanical Analysis
3.1.4. X-ray Powder Diffraction Analysis (XRD)
3.2. Functionalization of FIx with Silane
3.2.1. FTIR Analysis of Non-Functionalized FIx
3.2.2. Analysis of FIx Functionalized with Silane Coupling Agents (ACSi)
Analysis of FIx Functionalized with PTMS
Analysis of FIx Functionalized with APTES
Analysis of FIx Functionalized with HDMS
3.2.3. Elemental Analysis and Chemical Mapping of FIx by SEM–EDS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soriano-Corral, F.; Calva-Nava, L.A.; Hernández-Gámez, J.F.; Hernández-Hernández, E.; González-Morones, P.; Ávila-Orta, C.A.; Soria-Arguello, G.; Fonseca-Florido, H.A.; Covarrubias-Gordillo, C.A.; Díaz de León-Gómez, R.E. Influence of Ethylene Plasma Treatment of Agave Fiber on the Cellular Morphology and Compressive Properties of Low-Density Polyethylene/Ethylene Vinyl Acetate Copolymer/Agave Fiber Composite Foams. Int. J. Polym. Sci. 2021, 2021, 9150310. [Google Scholar] [CrossRef]
- Muralidhar, B.A. Characterization of Sisal/Polypropylene Composites Treated with Plasma. Text. Leather Rev. 2020, 3, 202–212. [Google Scholar] [CrossRef]
- Frollini, E.; Bartolucci, N.; Sisti, L.; Celli, A. Poly(butylene succinate) reinforced with different lignocellulosic fibers. Ind. Crops Prod. 2013, 45, 160–169. [Google Scholar] [CrossRef]
- Valea, A.; Corcuera, M.A.; Eceiza, A.; Gonzalez, M.L. Modificación superficial de fibras de sisal para su utilización como refuerzo en materiales composites de matriz polipropileno. Mater. Compuestos 2019, 32, 69–75. [Google Scholar]
- Yan, Y.; Xiao, L.; Teng, Q.; Jiang, Y.; Deng, Q.; Li, X.; Huang, Y. Strong, Tough, and Adhesive Polyampholyte/Natural Fiber Composite Hydrogels. Polymers 2022, 14, 4984. [Google Scholar] [CrossRef] [PubMed]
- Annandarajah, C.; Li, P.; Michel, M.; Chen, Y.; Jamshidi, R.; Kiziltas, A.; Hoch, R.; Grewell, D.; Montazami, R. Study of Agave Fiber-Reinforced Biocomposite Films. Materials 2018, 12, 99. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Quiroz, D.; Berlanga-Reyes, C.; Pineda, A. Recolección, Extracción y Uso de la Fibra de Lechuguilla (Agave lechuguilla Torr.) en el Estado de Coahuila; INIFAP-CIRNE: Río Bravo, Mexico, 2005; pp. 1–13. [Google Scholar]
- Reyes-Agüero, J.; Aguirre-Rivera, J.; Peña-Valdivia, C. Biology and use of Agave lechuguilla Torrey. Bot. Sci. 2000, 67, 75–88. [Google Scholar] [CrossRef]
- Hernández, S.R.; Lugo, E.C.; Díaz, L.; Villanueva, S. Extraction and indirect quantification of saponins from the Agave lechuguilla Torrey. e-Gnosis 2005, 3, 1–9. [Google Scholar]
- Cruz, R.A.; Mendotza-Martínez, A.; Heinze, T. Synthesis and characterization of graft copolymers from natural fibers. Int. J. Polym. Mater. Polym. Biomater. 2002, 51, 661–674. [Google Scholar] [CrossRef]
- Carmona, J.E.; Morales-Martínez, T.K.; Mussatto, S.I.; Castillo-Quiroz, D.; Ríos-González, L.J. Propiedades químicas, estructurales y funcionales de la lechuguilla (Agave lechuguilla Torr.). Rev. Mex. Cienc. For. 2017, 8, 100–122. [Google Scholar] [CrossRef]
- Infante-Torres, O. El Aprovechamiento de Fibras Naturales del Altiplano Potosino como Materia Prima para el Desarrollo de Productos, a Través de un Modelo de Clasificación. Master’s Thesis, Universidad Autónoma de San Luis Potosí, San Luis Potosí, Mexico, 2011. [Google Scholar]
- Flores-Dávila, M.P. La Lechuguilla. Un recurso olvidado. Secretaria del Medio Ambiente. Bordeando Monte 2018, 51, 3–8. [Google Scholar]
- Madhu, P.; Sanjay, M.R.; Jawaid, M.; Siengchin, S.; Khan, A.; Pruncu, C.I. A new study on effect of various chemical treatments on Agave americana fiber for composite reinforcement: Physico-chemical, thermal, mechanical and morphological properties. Polym. Test. 2020, 85, 106437. [Google Scholar] [CrossRef]
- García Hernández, Z.; Miranda Teran, Z.N.; González-Morones, P.; Yañez-Macías, R.; Rosales, S.G.S.; Yolotzin-Romero, G.; Sifuentes-Nieves, I.; Hernández-Hernández, E. Performance of nylon 6 composites reinforced with modified agave fiber: Structural, morphological, and mechanical features. J. Appl. Polym. Sci. 2021, 138, 50857. [Google Scholar] [CrossRef]
- Moscoso-Sánchez, F.J.; Alvarad, A.; Martínez-Chávez, L.; Hernández-Montelongo, R.; Escamilla, V.V.F.; Escamilla, G.C. The effects of henequen cellulose treated with polyethylene glycol on properties of polylactic acid composites. BioResources 2019, 14, 2707–2726. [Google Scholar] [CrossRef]
- Hidalgo-Reyes, M.; Caballero-Caballero, M.; Hernández-Gómez, L.H.; Urriolagoitia-Calderón, G. Chemical and morphological characterization of Agave angustifolia bagasse fibers. Bot. Sci. 2015, 93, 807–817. [Google Scholar] [CrossRef]
- Caldera-Briseño, C.A.; Miramontes de León, D.; Hernández-Guerrero, A.; Trujillo-Barragán, M. Caracterización de materiales compuestos de matriz polimérica con fibra de ixtle. In Proceedings of the XVIII Congreso Internacional Anual de la SOMIM, Salamanca, Mexico, 19–21 September 2012; pp. 673–682. [Google Scholar]
- Torres-Arellano, M.; Renteria-Rodríguez, V.; Franco-Urquiza, E. Mechanical Properties of Natural-Fiber-Reinforced Biobased Epoxy Resins Manufactured by Resin Infusion Process. Polymers 2020, 12, 2841. [Google Scholar] [CrossRef] [PubMed]
- Franco-Urquiza, E.A.; Saleme-Osornio, R.S.; Ramírez-Aguilar, R. Mechanical Properties of Hybrid Carbonized Plant Fibers Reinforced Bio-Based Epoxy Laminates. Polymers 2021, 13, 3435. [Google Scholar] [CrossRef]
- Abhilash, S.S.; Singaravelu, D.L. Effect of Fiber Content on Mechanical, Morphological, and Vibration Damping Characteristics of Natural Fiber-reinforced Composite Fuel Tanks. J. Nat. Fibers 2022, 19, 14994–15007. [Google Scholar] [CrossRef]
- Narendiranath Babu, T.; Rajkumar, E.; George, G.; Jobai, J.; Rama Prabha, D. Tensile and flexural properties of Tampico fibres and E-glass fibre composites reinforced with epoxy resin. Proc. Inst. Mech. Eng. Part L J. Mater. Des. Appl. 2021, 235, 2783–2796. [Google Scholar] [CrossRef]
- Pérez-Pérez, A.; Bello-Silva, E.; Carro-Sánchez, S.; Romero-Mitre, R.D.; Cervantes-Hernández, B.A. Obtaining and Study of Cellulose Microcrystals from Agave Lechugilla. Int. Res. J. Eng. Technol. 2019, 6, 7238–7241. [Google Scholar]
- Jamilah, U.L.; Sujito, S. The improvement of ramie fiber properties as composite materials using alkalization treatment: NaOH concentration. Indones. J. Mater. Sci. 2021, 22, 62. [Google Scholar] [CrossRef]
- Sathish Kumar, R.; Muralidharan, N.; Sathyamurthy, R. Optimization of Alkali Treatment Process Parameters for Kenaf Fiber: Experiments Design. J. Nat. Fibers 2020, 19, 4276–4285. [Google Scholar] [CrossRef]
- Mochane, M.J.; Magagula, S.I.; Sefadi, J.S.; Mokhena, T.C. A Review on Green Composites Based on Natural Fiber-Reinforced Polybutylene Succinate (PBS). Polymers 2021, 13, 1200. [Google Scholar] [CrossRef]
- El Oudiani, A.; Ben Sghaier, R.; Chaabouni, Y.; Msahli, S.; Sakli, F. Physico-chemical and mechanical characterization of alkali-treated Agave americana L. fiber. J. Text. Inst. 2012, 103, 349–355. [Google Scholar] [CrossRef]
- Wang, X.; Chang, L.; Shi, X.; Wang, L. Effect of Hot-Alkali Treatment on the Structure Composition of Jute Fabrics and Mechanical Properties of Laminated Composites. Materials 2019, 12, 1386. [Google Scholar] [CrossRef] [PubMed]
- Quiceno-Jaramillo, N. Efecto del Proceso de Mercerización en el Comportamiento de la Fibra de Hoja de Piña (FHP) como Refuerzo en Una Matriz de Polipropileno. Master’s Thesis, Universidad Nacional de Colombia, Bogotá, Colombia, 2016. [Google Scholar]
- Xie, Y.; Hill, C.A.S.; Xiao, Z.; Militz, H.; Mai, C. Silane coupling agents used for natural fiber/polymer composites: A review. Compos. Part A Appl. Sci. Manuf. 2010, 41, 806–819. [Google Scholar] [CrossRef]
- Mondal, I.H.; Islam, K.; Ahmed, F. Effect of Silane Coupling Agents on Cotton Fibre Finishing. J. Nat. Fibers 2021, 19, 5451–5464. [Google Scholar] [CrossRef]
- Hidalgo-Salazar, M.A.; Luna-Vera, F.; Correa-Aguirre, J.P. Biocomposites from Colombian Sugarcane Bagasse with Polypropylene: Mechanical, Thermal and Viscoelastic Properties. In Characterizations of Some Composite Materials; IntechOpen: London, UK, 2019; p. 13. [Google Scholar]
- Correa-Aguirre, J.P.; Luna-Vera, F.; Caicedo, C.; Vera-Mondragón, B.; Hidalgo-Salazar, M.A. The Effects of Reprocessing and Fiber Treatments on the Properties of Polypropylene-Sugarcane Bagasse Biocomposites. Polymers 2020, 12, 1440. [Google Scholar] [CrossRef]
- Luna-Vera, F.; Melo Cortes, H.A.; Murcia, C.V.; Charry-Galvis, I.C. Modificación superficial de micro fibras de celulosa obtenidas a partir de bagazo de caña de azúcar usando silanización. Inf. Técnico 2014, 78, 106–114. [Google Scholar]
- Cappelletto, E.; Maggini, S.; Girardi, F.; Bochicchio, G.; Tessadri, B.; Di Maggio, R. Wood surface protection with different alkoxysilanes: A hydrophobic barrier. Cellulose 2012, 20, 3131–3141. [Google Scholar] [CrossRef]
- Cisneros-López, E.O.; Pérez-Fonseca, A.A.; Fuentes-Talavera, F.J.; Anzaldo, J.; González-Núñez, R.; Rodrigue, D.; Robledo-Ortíz, J.R. Rotomolded polyethylene-agave fiber composites: Effect of fiber surface treatment on the mechanical properties. Polym. Eng. Sci. 2016, 56, 856–865. [Google Scholar] [CrossRef]
- Sepe, R.; Bollino, F.; Boccarusso, L.; Caputo, F. Influence of chemical treatments on mechanical properties of hemp fiber reinforced composites. Compos. Part B Eng. 2018, 133, 210–217. [Google Scholar] [CrossRef]
- Muñoz-Vélez, M.F.; Idalgo-Salazar, M.; Mina-Hernandez, J. Fibras de fique una alternativa para el reforzamiento de plásticos. Influencia de la modificación superficial. Biotecnol. Sect. Agropecu. Agroindustrial 2014, 12, 60–70. [Google Scholar]
- Asumani, O.; Reid, R.; Paskaramoorthy, R. The effects of alkali–silane treatment on the tensile and flexural properties of short fibre non-woven kenaf reinforced polypropylene composites. Compos. Part A Appl. Sci. Manuf. 2012, 43, 1431–1440. [Google Scholar] [CrossRef]
- Orue, A.; Jauregi, A.; Unsuain, U.; Labidi, J.; Eceiza, A.; Arbelaiz, A. The effect of alkaline and silane treatments on mechanical properties and breakage of sisal fibers and poly(lactic acid)/sisal fiber composites. Compos. Part A Appl. Sci. Manuf. 2016, 84, 186–195. [Google Scholar] [CrossRef]
- Zhao, Y.; Qiu, J.; Feng, H.; Zhang, M. The interfacial modification of rice straw fiber reinforced poly(butylene succinate) composites: Effect of aminosilane with different alkoxy groups. J. Appl. Polym. Sci. 2012, 125, 3211–3220. [Google Scholar] [CrossRef]
- Chauhan, V.; Kärki, T.; Varis, J. Effect of Fiber Content and Silane Treatment on the Mechanical Properties of Recycled Acrylonitrile-Butadiene-Styrene Fiber Composites. Chemistry 2021, 3, 1258–1270. [Google Scholar] [CrossRef]
- Huda, M.S.; Drzal, L.T.; Mohanty, A.K.; Misra, M. Effect of fiber surface-treatments on the properties of laminated biocomposites from poly(lactic acid) (PLA) and kenaf fibers. Compos. Sci. Technol. 2008, 68, 424–432. [Google Scholar] [CrossRef]
- Huang, Y.; Qian, S.; Zhou, J.; Chen, W.; Liu, T.; Yang, S.; Long, S.; Li, X. Achieving Swollen yet Strengthened Hydrogels by Reorganizing Multiphase Network Structure. Adv. Funct. Mater. 2023, 33, 2213549. [Google Scholar] [CrossRef]
- Vieira, M.C.; Heinze, T.; Antonio-Cruz, R.; Mendoza-Martinez, A. Cellulose derivatives from cellulosic material isolated from Agave lechuguilla and fourcroydes. Cellulose 2002, 9, 203–212. [Google Scholar] [CrossRef]
- Hashim, M.; Amin, A.; Faizan-Marwah, O.; Othman, M.; Yunus, M.; Huat, N. The effect of alkali treatment under various conditions on physical properties of kenaf fiber. J. Phys. Conf. Ser. 2017, 914, 012030. [Google Scholar] [CrossRef]
- Sahu, P.; Gupta, M. A review on the properties of natural fibres and its bio-composites: Effect of alkali treatment. Proc. Inst. Mech. Eng. Part L J. Mater. Des. Appl. 2019, 234, 198–217. [Google Scholar] [CrossRef]
- Teli, M.; Jadhav, A. Effect of Mercerization on the Properties of Pandanus Odorifer Lignocellulosic Fibre. IOSR J. Polym. Text. Eng. 2017, 4, 7–15. [Google Scholar] [CrossRef]
- Jaramillo-Quiceno, N.; Vélez, J.M.; Cadena Ch, E.M.; Restrepo-Osorio, A.; Felipe Santa, J. Improvement of Mechanical Properties of Pineapple Leaf Fibers by Mercerization Process. Fibers Polym. 2018, 19, 2604–2611. [Google Scholar] [CrossRef]
- Peña-Alonso, R.; Rubio, F.; Rubio, J.; Oteo, J.L. Study of the hydrolysis and condensation of γ-Aminopropyltriethoxysilane by FT-IR spectroscopy. J. Mater. Sci. 2006, 42, 595–603. [Google Scholar] [CrossRef]
- Rubio, J.; Mazo, M.A.; Martín-Ilana, A.; Tamayo, A. FT-IR study of the hydrolysis and condensation of 3-(2-amino-ethylamino)propyl-trimethoxy silane. Boletín Soc. Española Cerámica Vidr. 2018, 57, 160–168. [Google Scholar] [CrossRef]
- Le Moigne, N.; Longerey, M.; Taulemesse, J.-M.; Bénézet, J.-C.; Bergeret, A. Study of the interface in natural fibres reinforced poly(lactic acid) biocomposites modified by optimized organosilane treatments. Ind. Crops Prod. 2014, 52, 481–494. [Google Scholar] [CrossRef]
- Lee, M.; Kim, Y.; Ryu, H.; Baeck, S.-H.; Shim, S.E. Effects of Silane Coupling Agent on the Mechanical and Thermal Properties of Silica/Polypropylene Composites. Polym. Korea 2017, 41, 599–609. [Google Scholar] [CrossRef]
- Liu, L.; Yu, J.; Cheng, L.; Qu, W. Mechanical properties of poly(butylene succinate) (PBS) biocomposites reinforced with surface modified jute fibre. Compos. Part A Appl. Sci. Manuf. 2009, 40, 669–674. [Google Scholar] [CrossRef]
- Perez-Pimienta, J.A.; Poggi-Varaldo, H.M.; Ponce-Noyola, T.; Ramos-Valdivia, A.C.; Chavez-Carvayar, J.A.; Stavila, V.; Simmons, B.A. Fractional pretreatment of raw and calcium oxalate-extracted agave bagasse using ionic liquid and alkaline hydrogen peroxide. Biomass Bioenergy 2016, 91, 48–55. [Google Scholar] [CrossRef]
- De Dios Naranjo, C.; Alamilla-Beltrán, L.; Gutiérrez-Lopez, G.G.; Terres-Rojas, E.; Solorza-Feria, J.; Romero-Vargas, S.; Yee-Madeira, H.T.; Flores-Morales, A.; Mora-Escobedo, R. Isolation and characterization of cellulose obtained form Agave salmiana fibers using two acid-alkali extractipon methods. Rev. Mex. Cienc. Agric. 2016, 7, 31–43. [Google Scholar]
- Asim, M.; Jawaid, M.; Abdan, K.; Nasir, M. Effect of Alkali treatments on physical and Mechanical strength of Pineapple leaf fibres. IOP Conf. Ser. Mater. Sci. Eng. 2018, 290, 012030. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Liang, W.; Wang, J.; Chen, Y. Effect of silane treatment on mechanical properties and thermal behavior of bamboo fibers reinforced polypropylene composites. J. Eng. Fibers Fabr. 2020, 15, 1558925020958195. [Google Scholar] [CrossRef]
- Anna Dilfi, K.F.; Balan, A.; Bin, H.; Xian, G.; Thomas, S. Effect of surface modification of jute fiber on the mechanical properties and durability of jute fiber-reinforced epoxy composites. Polym. Compos. 2018, 39, E2519–E2528. [Google Scholar] [CrossRef]
- Célino, A.; Fréour, S.; Jacquemin, F.; Casari, P. The hygroscopic behavior of plant fibers: A review. Front. Chem. 2014, 1, 43. [Google Scholar] [CrossRef] [PubMed]
- Gogoi, R.; Tyagi, A.K. Surface Modification of Jute Fabric by Treating with Silane Coupling Agent for Reducing Its Moisture Regain Characteristics. J. Nat. Fibers 2021, 18, 803–812. [Google Scholar] [CrossRef]
- García-Méndez, R.F.; Cortés-Martínez, C.I.; Almendárez-Camarillo, A. Thermochemical and Tensile Mechanical Properties of Fibers Mechanically Extracted from Leaves of Agave angustifolia Haw. J. Nat. Fibers 2022, 19, 3171–3185. [Google Scholar] [CrossRef]
- Medellín-Castillo, N.; Hernández-Ramírez, M.; Salazar-Rábago, J.; Labrada-Delgado, G.; Aragón-Piña, A. Bioadsorción de plomo (ii) presente en solución acuosa sobre residuos de fibras naturales procedentes de la industria ixtlera (Agave lechuguilla Torr. Y Yucca carnerosana (Trel.) McKelvey). Rev. Int. Contam. Ambient. 2017, 33, 269–280. [Google Scholar] [CrossRef]
- Muñoz, E.J.; Prieto-García, F.; Prieto-Méndez, J.; Acevedo-Sandoval, O.A.; Rodríguez-Laguna, R. Obtención de pulpa de celulosa a partir de residuos de Agave salmiana B. Otto ex Salm. Optimización. DYNA 2017, 84, 253–260. [Google Scholar] [CrossRef]
- Balam-Cocom, R.J.; Duarte-Aranda, S.; Canché-Escamilla, G. Obtención y caracterización de materiales compuestos de fibras de la “piña” de henequén y polipropileno. Rev. Mex. Ing. Quím. 2006, 5, 39–44. [Google Scholar]
- Márquez, A.; Cazaurang, N.; Gonzalez, I.; Colunga, P. Extraction of chemical cellulose from the fibers of Agave lechuguilla Torr. Econ. Bot. 1996, 50, 465–468. [Google Scholar] [CrossRef]
- Munawar, S.S.; Umemura, K.; Kawai, S. Characterization of the morphological, physical, and mechanical properties of seven nonwood plant fiber bundles. J. Wood Sci. 2007, 53, 108–113. [Google Scholar] [CrossRef]
- Ray, D.; Sarkar, B.K. Characterization of alkali-treated jute fibers for physical and mechanical properties. J. Appl. Polym. Sci. 2001, 80, 1013–1020. [Google Scholar] [CrossRef]
- Krishnaiah, P.; Ratnam, C.T.; Manickam, S. Enhancements in crystallinity, thermal stability, tensile modulus and strength of sisal fibres and their PP composites induced by the synergistic effects of alkali and high intensity ultrasound (HIU) treatments. Ultrason. Sonochem. 2017, 34, 729–742. [Google Scholar] [CrossRef]
- Borchani, K.E.; Carrot, C.; Jaziri, M. Untreated and alkali treated fibers from Alfa stem: Effect of alkali treatment on structural, morphological and thermal features. Cellulose 2015, 22, 1577–1589. [Google Scholar] [CrossRef]
- Sawpan, M.A.; Pickering, K.L.; Fernyhough, A. Effect of various chemical treatments on the fibre structure and tensile properties of industrial hemp fibres. Compos. Part A Appl. Sci. Manuf. 2011, 42, 888–895. [Google Scholar] [CrossRef]
- Vijay, R.; Singaravelu, D.L.; Vinod, A.; Sanjay, M.; Siengchin, S. Characterization of Alkali-Treated and Untreated Natural Fibers from the Stem of Parthenium Hysterophorus. J. Nat. Fibers 2019, 18, 80–90. [Google Scholar] [CrossRef]
- Khanjanzadeh, H.; Behrooz, R.; Bahramifar, N.; Gindl-Altmutter, W.; Bacher, M.; Edler, M.; Griesser, T. Surface chemical functionalization of cellulose nanocrystals by 3-aminopropyltriethoxysilane. Int. J. Biol. Macromol. 2018, 106, 1288–1296. [Google Scholar] [CrossRef]
- Abdelmouleh, M.; Boufi, S.; Belgacem, M.; Duarte, A.; Ben Salah, A.; Gandini, A. Modification of cellulosic fibres with functionalised silanes: Development of surface properties. Int. J. Adhes. Adhes. 2004, 24, 43–54. [Google Scholar] [CrossRef]
- Valadez-Gonzalez, A.; Cervantes-Uc, J.; Olayo, R.; Herrera-Franco, P. Chemical modification of henequén fibers with an organosilane coupling agent. Compos. Part B Eng. 1999, 30, 321–331. [Google Scholar] [CrossRef]
- Saha, P.K.; Mia, R.; Zhou, Y.; Ahmed, T. Functionalization of hydrophobic nonwoven cotton fabric for oil and water repellency. SN Appl. Sci. 2021, 3, 586. [Google Scholar] [CrossRef]
- Zang, D.; Zhang, M.; Liu, F.; Wang, C. Superhydrophobic/superoleophilic corn straw fibers as effective oil sorbents for the recovery of spilled oil. J. Chem. Technol. Biotechnol. 2016, 91, 2449–2456. [Google Scholar] [CrossRef]
- Majoul, N.; Aouida, S.; Bessaïs, B. Progress of porous silicon APTES-functionalization by FTIR investigations. Appl. Surf. Sci. 2015, 331, 388–391. [Google Scholar] [CrossRef]
- Rao, X.; Hassan, A.A.; Guyon, C.; Zhang, M.; Ognier, S.; Tatoulian, M. Plasma Polymer Layers with Primary Amino Groups for Immobilization of Nano- and Microparticles. Plasma Chem. Plasma Process. 2020, 40, 589–606. [Google Scholar] [CrossRef]
- Sundar, S.; Mariappan, R.; Piraman, S. Synthesis and characterization of amine modified magnetite nanoparticles as carriers of curcumin-anticancer drug. Powder Technol. 2014, 266, 321–328. [Google Scholar] [CrossRef]













| Sample | Temp (°C) | NaOH (%) | T5% a (°C) | T10% a (°C) | T50% a (°C) | Tmax b (°C) |
|---|---|---|---|---|---|---|
| FIx s/t (w/o treatment) | 25 | 0 | 72.2 | 273.3 | 358.4 | 364.5 |
| FIx-1 | 25 | 2 | 122.6 | 269.1 | 342.7 | 343.5 |
| FIx-2 | 25 | 5 | 99.16 | 261.4 | 361.5 | 365.5 |
| FIx-3 | 25 | 8 | 108.6 | 269.6 | 357.5 | 361.5 |
| FIx-4 | 25 | 10 | 76.3 | 255.4 | 354.8 | 359.6 |
| FIx-5 | 65 | 2 | 73.4 | 259.3 | 356.6 | 359.7 |
| FIx-6 | 65 | 5 | 76.2 | 266.3 | 360.9 | 366.1 |
| FIx-7 | 65 | 8 | 233.5 | 291.9 | 360.4 | 365.7 |
| FIx-8 | 65 | 10 | 188.5 | 281.4 | 357.4 | 363.3 |
| Sample | Temp. (°C) | NaOH (%) | Tensile Strength a (MPa) | Young’s Modulus a (GPa) | Strain at Breakage a (%) | Wb b (MJ m−3) |
|---|---|---|---|---|---|---|
| FIx s/t (w/o treatment) | - | 0 | 242.33 ± 9.40 | 7.38 ± 2.56 | 4.90 ± 0.57 | 386.4 ± 57.9 |
| FIx-1 | 25 | 2 | 355.53 ± 28.57 | 7.77 ± 0.22 | 5.19 ± 0.21 | 1143.5 ± 184.8 |
| FIx-2 | 25 | 5 | 194.43 ± 11.67 | 7.40 ± 0.79 | 5.13 ± 0.14 | 468.4 ± 170.3 |
| FIx-3 | 25 | 8 | 289.15 ± 30.19 | 8.86 ± 1.32 | 8.78 ± 1.66 | 1532.4 ± 92.2 |
| FIx-4 | 25 | 10 | 293.83 ± 54.59 | 9.64 ± 1.05 | 11.82 ± 3.87 | 1923.4 ± 332.4 |
| FIx-5 | 65 | 2 | 347.73 ± 51.97 | 10.00 ± 1.42 | 6.81 ± 0.13 | 881.8 ± 160.7 |
| FIx-6 | 65 | 5 | 524.25 ± 18.60 | 14.41 ± 5.06 | 7.27 ± 1.42 | 2107.1 ± 557.9 |
| FIx-7 | 65 | 8 | 365.50 ± 12.59 | 10.21 ± 0.49 | 8.48 ± 0.67 | 1879.6 ± 455.7 |
| FIx-8 | 65 | 10 | 329.97 ± 24.68 | 11.88 ± 1.68 | 5.56 ± 0.78 | 1614.6 ± 132.4 |
| T = 25 °C | T = 65 °C | |||
|---|---|---|---|---|
| NaOH (%) | Sample | CI a (%) | Sample | CI a (%) |
| 0 | FIx s/t | 67.4 | - | - |
| 2 | FIx-1 | 63.1 | FIx-5 | 62.2 |
| 5 | FIx-2 | 73.4 | FIx-6 | 76.9 |
| 8 | FIx-3 | 66.9 | FIx-7 | 74.0 |
| 10 | FIx-4 | 80.5 | FIx-8 | 78.2 |
| Sample | Methanol/Water Ratio | ACSi [%] a | Si [%] b (Average) |
|---|---|---|---|
| FIx s/t | 0 | 0 | 0.02 |
| FIx-PTMS | 8/2 | 8 | 0.84 |
| FIx-PTMS | 6/4 | 5 | 0.23 |
| FIx-HDMS | 8/2 | 5 | 0.62 |
| FIx-HDMS | 6/4 | 8 | 0.33 |
| FIx-APTES | 8/2 | 8 | 2.12 |
| FIx-APTES | 6/4 | 8 | 8.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jardon-Maximino, N.; Dehonor Gómez, M.; Villa Moreno, R.; Baeza-Alvarado, M.D.; Lugo Uribe, L.E. Optimization of the Alkali-Silane Treatment of Agave lechuguilla Fibers (Ixtle) for Potential Reinforcement in Polymeric Composites. Fibers 2023, 11, 86. https://doi.org/10.3390/fib11100086
Jardon-Maximino N, Dehonor Gómez M, Villa Moreno R, Baeza-Alvarado MD, Lugo Uribe LE. Optimization of the Alkali-Silane Treatment of Agave lechuguilla Fibers (Ixtle) for Potential Reinforcement in Polymeric Composites. Fibers. 2023; 11(10):86. https://doi.org/10.3390/fib11100086
Chicago/Turabian StyleJardon-Maximino, Noemi, Mariamne Dehonor Gómez, Rolando Villa Moreno, M. D. Baeza-Alvarado, and Luis Edmundo Lugo Uribe. 2023. "Optimization of the Alkali-Silane Treatment of Agave lechuguilla Fibers (Ixtle) for Potential Reinforcement in Polymeric Composites" Fibers 11, no. 10: 86. https://doi.org/10.3390/fib11100086