The Role of the Protein Corona in Fiber Structure-Activity Relationships
Abstract
:1. Introduction
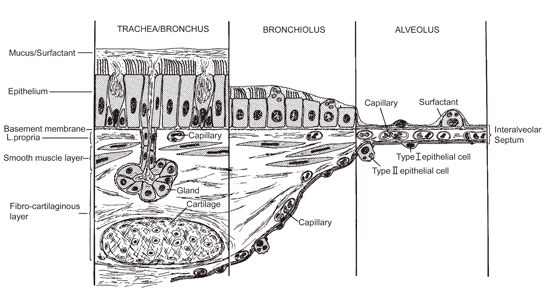
Human Exposure to (Nano)fibers

2. Methods to Determine the Protein Coating of (Nano)materials
3. Interactions of Proteins and Fibers
3.1. Asbestos-Protein Interactions
| Year of publication | Type of study | Type of adsorbed protein(s) | Major outcome | Reference |
|---|---|---|---|---|
| 1974 | Protein adsorption | Human serum albumin | The surface charge of the asbestos fibers had a strong influence on the adsorption of proteins. | [31] |
| 1977 | In vitro, in vivo | Human serum albumin | The capacity of asbestos fibers to adsorb proteins is dependent from the magnesium content in the fibers. | [33] |
| 1980 | Protein adsorption | Bovine serum albumin, Ferritin | Magnesium depletion of the asbestos fibers leads to a decrease of albumin adsorption, while the specific adsorption offerritin increased. | [34] |
| 1986 | Protein adsorption | Fetal serum proteins | Strong electrostatic interactions between the charges of the fibers and the proteins were responsible for the protein-fiber adsorption. | [35] |
| 1987 | Protein adsorption | Different types of proteins | The protein-fiber affinity was correlated with the specific area of the fiber and the protein charge density. | [36] |
| 1990 | In vitro | Serum proteins | The cytotoxic effects of asbestos fibers was serum-dose dependent. | [37] |
| Immunoglobulin G, Bovine serum albumin, Cytochrome c | Certain proteins were selectively adsorbed onto the asbestos fibers. | [38] | ||
| 1995 | In vitro | Vitronectin, Fibronectin | Vitronectin specifically enhanced the internalization of asbestos fibers via αvβ5 integrin receptors. | [39] |
| 2000 | In vitro | Vitronectin | The adsorption of vitronectin onto the asbestos fibers increased the fiber uptake and the cytotoxic effects of asbestos. | [40] |
| Vitronectin adsorption to chrysotile asbestos fibers increased fiber phagocytosis and toxicity for mesothelial cells. | [41] |
3.2. CNT-Protein Interactions
3.2.1. Mechanism of Interaction
3.2.2. Influence of Solvents, Surfactants, Surface-Functionalization, and Pre-Coating on CNT-Protein Interactions
3.2.3. Alternative Theory to CNT Protein Interactions
- -
- Electrochemical and chemical nature of the CNT and proteins are essential for strong CNT-protein interaction
- -
- Protein-CNT binding is based on non-covalent π-π stacking hydrophobic interactions
- -
- The diameter, size and surface curvature of the CNT is essential for a significant protein-CNT binding
- -
- Protein-binding is dependent on the three-dimensional arrangement of the carbon atoms of the CNTs
| Year of publication | Type of CNT | Type of adsorbed protein(s) | Major outcome | Reference |
|---|---|---|---|---|
| 2001 | SWCNT | Ferritin, streptavidin | Proteins with primary and secondary amines adsorbed onto f-SWCNT via π-π stacking interactions. | [82] |
| Proteins rich in surface amines (antibody for C60) | SWCNT with a curved hydrophobic π-electron-rich surface bound on the hydrophobic binding sites of proteins. | [54] | ||
| 2002 | SWCNT | Metalloproteins, Enzymes | Enzymes immobilized on SWCNTs retain their catalytic activity. | [68] |
| Streptavidin (various proteins) | Pre-coating of SWCNTs with triton X-100 prior PEG coating prevented the adsorption of small proteins onto SWCNT nearly completely. | [78] | ||
| 2003 | SWCNT | Amphiphilic α-helical peptide | The apolar residues of amphiphilic proteins bound to the surface of SWCNT and the polar residues of the proteins were located against the solvent face. | [55] |
| MWCNT | Phage and other types of peptides | Peptides that were rich in histidine and tryptophan bound at special locations of the MWCNTs by hydrophobic interactions. | [62] | |
| 2004 | SWCNT | α-Chymotrypsin, soybean peroxidase | The enzymes changed their secondary structures upon adsorption onto the SWCNTs, which caused a decrease or nearly complete loss of their activity. | [66] |
| Ferritin | A covalent coating of SWCNT with PEG was alleviating or even completely eliminating the natural protein affinity of the SWCNTs. | [77] | ||
| Amphiphilic α-helical peptide | The binding of polar residues of amphiphilic proteins onto the surface of SWCNTs increased the dispersion of the SWCNTs in water. | [56] | ||
| CNT | Streptavidin | Protein adsorption onto CNTs occurred through interactions between the amine groups of the protein and the hydrophobic surface of the CNTs. | [83] | |
| 2005 | SWCNT | Amphiphilic α-helical peptide | Amphiphilic peptides bound non-covalently with their apolar residues onto the SWCNTs, which resulted in a better solubilisation of the SWCNTs. | [51] |
| Amphiphilic peptide helix (nano-1) | The aromatic residues of the peptides interacted with the SWCNT surface, which was leading to a better dispersion of the SWCNTs. | [52] | ||
| 2006 | SWCNT | Model proteins | Protein coated SWCNTs were incorporated by the cells via energy dependent endocytosis through clathrin-coated pits. | [84] |
| Different types of proteins | Proteins adsorbed onto SWCNTs via π-π stacking as well as amine interactions, whereas the hydrophilic protein moieties were located towards the water face. | [57] | ||
| Polyline, polytryptophan | A strong adhesive force was registrated between the protonated amine-groups of the protein (polylysine) and the carboxyl-groups of the oxidized CNTs. | [58] | ||
| Lysozyme | π-π stacking and hydrophobic interactions as well as protonated amine interactions between proteins and SWCNT were responsible for the dispersion of the SWCNTs. | [49] | ||
| Fibrinogen, apolipoproteins (AI, AIV, CIII) | Protein binding onto SWCNT was highly selective. | [45] | ||
| Peptides from phage libraries | Hydrophobic as well as π-π interactions between proteins and SWCNTs were important for a selective protein binding onto SWCNTs. | [46] | ||
| 2007 | SWCNT | Foetal bovine plasma, human serum/plasma protein | The uptake of SWCNT occurred by pathways associated with the adsorbed proteins. The proteins modulated in addition the toxicity of the SWCNTs. | [43] |
| Different types of proteins | The primary, secondary and tertiary structures of proteins and the pH of the dispersion medium were important to obtain a high yield of de-bundeled CNTs | [61]. | ||
| Different types of peptides | Disulfide bonds adsorbed onto the SWCNTs and by that they solubilize the SWCNTs without altering their electronic structure. | [60] | ||
| DWCNT | Surfactant proteins A and D | Supernatant protein A and D adsorbed selectively onto DWCNTs out of different pulmonary surfactant protein samples. | [59] | |
| 2008 | SWCNT, MWCNT | Ribonuclease A | CNTs functionalized with carboxylic groups interacted with the enzyme and caused a reduction of its activity by changing its conformation. | [64] |
| MWCNT, f-MWCNT | Bovine serum albumin (BSA) and different types of proteins | Electrostatic and stereo-chemical properties of the MWCNTs and the proteins as well as the curvature of the MWCNTs were affecting the protein binding affinity onto the MWCNTs. | [81] | |
| Human plasma and serum proteins | Functionalization of the MWCNTs affected the patterns of adsorbed proteins onto the MWCNT, which resulted in a better biocompatibility of the MWCNTs. | [76] | ||
| CNT | A-sub-domain of human serum albumin | The adsorption of proteins onto CNTs caused a conformation change of the secondary protein structure, which resulted in a decrease of the protein activity. | [67] | |
| 2009 | MWCNT, f-MWCNT | α-Chymotrypsin | Enzymes bound onto MWCNTs through π-π stacking and hydrophobic interactions, which resulted in a competitive inhibition of the enzyme activity. | [65] |
| 2010 | SWCNT | Model surfactant | Surfactants with a larger hydrophilic head group was leading to a significant better dispersion stability of SWCNTs. | [75] |
| Model protein | Hydrophobic interactions between the hydrophobic core of the proteins and the SWCNTs formed stable complexes, which caused a blockage of the active sides of the proteins. | [63] | ||
| MWCNT, f-MWCNT | Pulmonary surfactant (Curosurf®) | The pre-coating of MWCNTs with a lung surfactant influenced the protein binding onto the MWCNTs and resulted in characteristic binding patterns. | [70] | |
| 2011 | SWCNT, MWCNT | Serum proteins | The adsorption capacity of CNTs for proteins was dependent on the type, arrangement model, size and surface modification of the CNTs. | [42] |
| SWCNT | Human serum proteins | Competitive binding of blood proteins onto the SWCNT surface can alter the cellular interaction pathways, resulting in a reduced cytotoxicity. | [44] | |
| 2011 | SWCNT | Bovine serum albumin (BSA) | Bovine serum albumin dispersed SWCNTs readily entered into the cells and did not acute deleterious cellular effects. | [73] |
| SWCNT, DWCNT | Serum proteins | The adsorption of enzymes of the immune system to the hydrophobic SWCNT surface didn’t caused an activation of the enzymes. | [85] | |
| MWCNT | Blood proteins | A surface modification of the MWCNT affected their patterns of adsorbed proteins, which resulted in a modification of the biocompatibility of the MWCNTs. | [48] | |
| 2012 | SWCNT | Bovine serum albumin (BSA) | Bovine serum albumin coated SWCNTs were taken up by the cells within seconds. However, the cells were able to expel the incorporated BSA-SWCNT complexes over hours and days. | [72] |
| Different types of proteins | The stability of a SWCNT-protein complex had a substantial influence on the cellular uptake and the uptake of a certain protein was dependent from the cell type. | [86] | ||
| MWCNT | Pulmonary surfactant (Curosurf) | The pre-coating of MWCNTs with a lung surfactant affected the uptake of the MWCNTs without significantly altering the cytotoxicity of the MWCNTs. | [71] | |
| 2013 | MWCNT | Human cellular proteins (HeLa cells lysate) | Electrostatic, stereochemical properties, diameter and curvature of the MWCNTs were significantly affecting the adsorption of proteins onto the MWCNTs. | [79] |
| SWCNT | Plasma proteins | The surface PEG conformation of SWCNT-PEG complexes affected the pattern of adsorbed plasma proteins onto the SWCNTs and influenced the biodistribution of the SWCNT-PEG complexes. | [53] | |
| SWCNT, f-SWCNT, MWCNT, f-MWCNT | Foetal Bovine serum (FBS) | Functionalized CNTs were able to bind a number of unique proteins, which implied that electrostatic interactions and specific covalent bonding were involved. | [80] | |
| CNT | Different types of proteins | π-π stacking and hydrophobic interactions were responsible for the adsorption of proteins onto CNTs. The protein adsorption leaded to a reduction of the cytotoxicity and to a loss of the enzymatic activity of the proteins. | [47] |
4. Discussion
4.1. Comparison of Protein Coronas
4.2. Methodology and Challenges
4.3. Influence of Shape on Nanomaterial-Protein Interaction
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Casals, E.; Puntes, V.F. Inorganic nanoparticle biomolecular corona: Formation, evolution and biological impact. Nanomedicine (Lond) 2012, 7, 1917–1930. [Google Scholar] [CrossRef]
- Monopoli, M.P.; Aberg, C.; Salvati, A.; Dawson, K.A. Biomolecular coronas provide the biological identity of nanosized materials. Nat. Nanotechnol. 2012, 7, 779–786. [Google Scholar] [CrossRef]
- Aggarwal, P.; Hall, J.B.; McLeland, C.B.; Dobrovolskaia, M.A.; McNeil, S.E. Nanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacy. Adv. Drug Deliv. Rev. 2009, 61, 428–437. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Donaldson, K.; Murphy, F.A.; Duffin, R.; Poland, C.A. Asbestos, carbon nanotubes and the pleural mesothelium: A review of the hypothesis regarding the role of long fibre retention in the parietal pleura, inflammation and mesothelioma. Part. Fibre Toxicol. 2010, 7, 1–17. [Google Scholar] [CrossRef]
- Dogan, A.U.; Dogan, M.; Hoskins, J.A. Erionite series minerals: Mineralogical and carcinogenic properties. Environ. Geochem. Health 2008, 30, 367–381. [Google Scholar] [CrossRef]
- Carbone, M.; Baris, Y.I.; Bertino, P.; Brass, B.; Comertpay, S.; Dogan, A.U.; Gaudino, G.; Jube, S.; Kanodia, S.; Partridge, C.R.; et al. Erionite exposure in North Dakota and Turkish villages with mesothelioma. Proc. Natl. Acad. Sci. USA 2011, 108, 13618–13623. [Google Scholar] [CrossRef]
- Faller, A.; Schünke, M. Der Körper des Menschen; Thieme: Stuttgart, Germany, 1999. (in German) [Google Scholar]
- Gil, J.; Bachofen, H.; Gehr, P.; Weibel, E.R. Alveolar volume-surface area relation in air- and saline-filled lungs fixed by vascular perfusion. J. Appl. Physiol. 1979, 47, 990–1001. [Google Scholar]
- Gehr, P.; Bachofen, M.; Weibel, E.R. The normal human lung: Ultrastructure and morphometric estimation of diffusion capacity. Respir. Physiol. 1978, 32, 121–140. [Google Scholar]
- Ochs, M.; Weibel, E.R. Functional design of the human lung for gas exchange. In Fishman’s Pulmonary Diseases and Disorders; Fishman, A.P., Elias, J., Fishman, J., Grippi, M., Senior, R., Eds.; McGraw-Hill Professional: New York, NY, USA, 2008; pp. 23–70. [Google Scholar]
- Lohmann-Matthes, M.L.; Steinmüller, C.; Franke-Ullmann, G. Pulmonary macrophages. Eur. Respir. J. 1994, 7, 1678–1689. [Google Scholar]
- Holt, P.G.; Stumbles, P.A. Characterization of dendritic cell populations in the respiratory tract. J. Aerosol. Med. 2000, 13, 361–367. [Google Scholar] [CrossRef]
- Donaldson, K.; Seaton, A. A short history of the toxicology of inhaled particles. Part. Fibre Toxicol. 2012, 9, 13. [Google Scholar] [CrossRef]
- Brown, D.M.; Kinloch, I.A.; Bangert, U.; Windleb, A.H.; Walterd, D.M.; Walkerd, G.S.; Scotchfordd, C.A.; Donaldsone, K.; Stonea, V. An in vitro study of the potential of carbon nanotubes and nanofibres to induce inflammatory mediators and frustrated phagocytosis. Carbon 2007, 45, 1743–1756. [Google Scholar] [CrossRef]
- Oberdorster, G. Toxicokinetics and effects of fibrous and nonfibrous particles. Inhal. Toxicol. 2002, 14, 29–56. [Google Scholar] [CrossRef]
- Liu, W.; Rose, J.; Plantevin, S.; Auffan, M.; Bottero, J.Y.; Vidaud, C. Protein corona formation for nanomaterials and proteins of a similar size: Hard or soft corona? Nanoscale 2013, 5, 1658–1668. [Google Scholar] [CrossRef]
- Wick, P.; Clift, M.J.; Rösslein, M.; Rothen-Rutishauser, B. A brief summary of carbon nanotubes science and technology: A health and safety perspective. ChemSusChem 2011, 4, 905–911. [Google Scholar] [CrossRef]
- Poland, C.A.; Duffin, R.; Kinloch, I.; Maynard, A.; Wallace, W.A.; Seaton, A.; Stone, V.; Brown, S.; Macnee, W.; Donaldson, K. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat. Nanotechnol. 2008, 3, 423–428. [Google Scholar] [CrossRef]
- Donaldson, K.; Tran, C.L. An introduction to the short-term toxicology of respirable industrial fibres. Mutat. Res. 2004, 553, 5–9. [Google Scholar] [CrossRef]
- Miller, B.G.; Jones, A.D.; Searl, A.; Buchanan, D.; Cullen, R.T.; Soutar, C.A.; Davis, J.M.; Donaldson, K. Influence of characteristics of inhaled fibres on development of tumours in the rat lung. Ann. Occup. Hyg. 1999, 43, 167–179. [Google Scholar] [CrossRef]
- Stanton, M.F.; Wrench, C. Mechanisms of mesothelioma induction with asbestos and fibrous glass. J. Natl. Cancer Inst. 1972, 48, 797–821. [Google Scholar]
- Kamstrup, O.; Ellehauge, A.; Chevalier, J.; Davis, J.M.; McConnell, E.E.; Thévenaz, P. Chronic inhalation studies of two types of stone wool fibers in rats. Inhal. Toxicol. 2001, 13, 603–621. [Google Scholar] [CrossRef]
- UmweltWissen—Produkte & Abfall: Künstliche Mineralfasern. 2013. Available online: http://www.lfu.bayern.de/umweltwissen/doc/uw_32_kuenstliche_mineralfasern.pdf (accessed on 19 June 2014).
- Jaurand, M.C.; Renier, A.; Daubriac, J. Mesothelioma: Do asbestos and carbon nanotubes pose the same health risk? Part. Fibre Toxicol. 2009, 6, 16. [Google Scholar] [CrossRef] [Green Version]
- Lynch, I.; Dawson, K.A. Protein-nanoparticle interactions. Nano Today 2008, 3, 40–47. [Google Scholar] [CrossRef]
- Sempf, K.; Arrey, T.; Gelperina, S.; Schorge, T.; Meyer, B.; Karas, M.; Kreuter, J. Adsorption of plasma proteins on uncoated PLGA nanoparticles. Eur. J. Pharm. Biopharm. 2013, 85, 53–60. [Google Scholar] [CrossRef]
- Small, I.; Peeters, N.; Legeai, F.; Lurin, C. Predotar: A tool for rapidly screening proteomes for N-terminal targeting sequences. Proteomics 2004, 4, 1581–1590. [Google Scholar] [CrossRef]
- Cedervall, T.; Lynch, I.; Foy, M.; Berggård, T.; Donnelly, S.C.; Cagney, G.; Linse, S.; Dawson, K.A. Detailed identification of plasma proteins adsorbed on copolymer nanoparticles. Angew. Chem. 2007, 119, 5856–5858. [Google Scholar] [CrossRef]
- Cedervall, T.; Lynch, I.; Lindman, S.; Berggård, T.; Thulin, E.; Nilsson, H.; Dawson, K.A.; Linse, S. Understanding the nanoparticle-protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles. Proc. Natl. Acad. Sci. USA 2007, 104, 2050–2055. [Google Scholar] [CrossRef]
- Morgan, A. Absorption of human serum albumin by asbestiform minerals and its application to the measurement of surface areas of dispersed samples of chrysotile. Environ. Res. 1974, 7, 330–341. [Google Scholar] [CrossRef]
- Light, W.G.; Wei, E.T. Surface charge and asbestos toxicity. Nature 1977, 265, 537–539. [Google Scholar] [CrossRef]
- Morgan, A.; Davies, P.; Wagner, J.C.; Berry, G.; Holmes, A. The biological effects of magnesium-leached chrysotile asbestos. Br. J. Exp. Pathol. 1977, 58, 465–473. [Google Scholar]
- Valerio, F.; Veggi, M.; Santi, L. Adsorption isotherms of albumin and ferritin on rhodesian chrysotile. Environ. Res. 1980, 21, 186–189. [Google Scholar]
- Valerio, F.; Balducci, D.; Scarabelli, L. Selective adsorption of serum proteins by chrysotile and crocidolite. Environ. Res. 1986, 41, 432–439. [Google Scholar] [CrossRef]
- Valerio, F.; Balducci, D.; Lazzarotto, A. Adsorption of proteins by chrysotile and crocidolite: role of molecular weight and charge density. Environ. Res. 1987, 44, 312–320. [Google Scholar] [CrossRef]
- Kamp, D.W.; Dunne, M.; Anderson, J.A.; Weitzman, S.A.; Dunn, M.M. Serum promotes asbestos-induced injury to human pulmonary epithelial cells. J. Lab. Clin. Med. 1990, 116, 289–297. [Google Scholar]
- Scheule, R.K.; Holian, A. Modification of asbestos bioactivity for the alveolar macrophage by selective protein adsorption. Am. J. Respir. Cell Mol. Biol. 1990, 2, 441–448. [Google Scholar] [CrossRef]
- Boylan, A.M.; Sanan, D.A.; Sheppard, D.; Broaddus, V.C. Vitronectin enhances internalization of crocidolite asbestos by rabbit pleural mesothelial cells via the integrin alpha v beta 5. J. Clin. Invest. 1995, 96, 1987–2001. [Google Scholar] [CrossRef]
- Liu, W.; Ernst, J.D.; Broaddus, V.C. Phagocytosis of crocidolite asbestos induces oxidative stress, DNA damage, and apoptosis in mesothelial cells. Am. J. Respir. Cell Mol. Biol. 2000, 23, 371–378. [Google Scholar] [CrossRef]
- Wu, J.; Liu, W.; Koenig, K.; Idell, S.; Broaddus, V.C. Vitronectin adsorption to chrysotile asbestos increases fiber phagocytosis and toxicity for mesothelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000, 279, L916–L923. [Google Scholar]
- Du, J.; Ge, C.; Liu, Y.; Bai, R.; Li, D.; Yang, Y.; Liao, L.; Chen, C. The interaction of serum proteins with carbon nanotubes depend on the physicochemical properties of nanotubes. J. Nanosci. Nanotechnol. 2011, 11, 10102–10110. [Google Scholar] [CrossRef]
- Dutta, D.; Sundaram, S.K.; Teeguarden, J.G.; Riley, B.J.; Fifield, L.S.; Jacobs, J.M.; Addleman, S.R.; Kaysen, G.A.; Moudgil, B.M.; Weber, T.J. Adsorbed proteins influence the biological activity and molecular targeting of nanomaterials. Toxicol. Sci. 2007, 100, 303–315. [Google Scholar] [CrossRef]
- Ge, C.; Du, J.; Zhao, L.; Wang, L.; Liu, Y.; Li, D.; Yang, Y.; Zhou, R.; Zhao, Y.; Chai, Z.; et al. Binding of blood proteins to carbon nanotubes reduces cytotoxicity. PNAS 2011, 108, 16968–16973. [Google Scholar]
- Salvador-Morales, C.; Flahaut, E.; Sim, E.; Sloan, J.; Green, M.L.; Sim, R.B. Complement activation and protein adsorption by carbon nanotubes. Mol. Immunol. 2006, 43, 193–201. [Google Scholar] [CrossRef] [Green Version]
- Su, Z.; Leung, T.; Honek, J.F. Conformational selectivity of peptides for single-walled carbon nanotubes. J. Phys. Chem. B 2006, 110, 23623–23627. [Google Scholar] [CrossRef]
- Zuo, G.; Kang, S.G.; Xiu, P.; Zhao, Y.; Zhou, R. Interactions between proteins and carbon-based nanoparticles: Exploring the origin of nanotoxicity at the molecular level. Small 2013, 9, 1546–1556. [Google Scholar] [CrossRef]
- Yan, L.; Zhao, F.; Li, S.; Hu, Z.; Zhao, Y. Low-toxic and safe nanomaterials by surface-chemical design, carbon nanotubes, fullerenes, metallofullerenes, and graphenes. Nanoscale 2011, 3, 362–382. [Google Scholar] [CrossRef]
- Nepal, D.; Geckeler, K.E. pH-sensitive dispersion and debundling of single-walled carbon nanotubes: Lysozyme as a tool. Small 2006, 2, 406–412. [Google Scholar]
- Ortiz-Acevedo, A.; Xie, H.; Zorbas, V.; Sampson, W.M.; Dalton, A.B.; Baughman, R.H.; Draper, R.K.; Musselman, I.H.; Dieckmann, G.R. Diameter-selective solubilization of single-walled carbon nanotubes by reversible cyclic peptides. J. Am. Chem. Soc. 2005, 127, 9512–9517. [Google Scholar] [CrossRef]
- Zorbas, V.; Smith, A.L.; Xie, H.; Ortiz-Acevedo, A.; Dalton, A.B.; Dieckmann, G.R.; Draper, R.K.; Baughman, R.H.; Musselman, I.H. Importance of aromatic content for peptide/single-walled carbon nanotube interactions. J. Am. Chem. Soc. 2005, 127, 12323–12328. [Google Scholar] [CrossRef]
- Wick, P.; Manser, P.; Limbach, L.K.; Dettlaff-Weglikowska, U.; Krumeich, F.; Roth, S.; Stark, W.J.; Bruinink, A. The degree and kind of agglomeration affect carbon nanotube cytotoxicity. Toxicol. Lett. 2007, 168, 121–131. [Google Scholar] [CrossRef]
- Sacchetti, C.; Motamedchaboki, K.; Magrini, A.; Palmieri, G.; Mattei, M.; Bernardini, S.; Rosato, N.; Bottini, N.; Bottini, M. Surface polyethylene glycol conformation influences the protein corona of polyethylene glycol-modified single-walled carbon nanotubes: Potential implications on biological performance. ACS Nano 2013, 7, 1974–1989. [Google Scholar] [CrossRef]
- Erlanger, B.F.; Chen, B.-X.; Zhu, M.; Brus, L. Binding of an anti-fullerene IgG monoclonal antibody to single wall carbon nanotubes. Nano Lett. 2001, 1, 465–467. [Google Scholar] [CrossRef]
- Dieckmann, G.R.; Dalton, A.B.; Johnson, P.A.; Razal, J.; Chen, J.; Giordano, G.M.; Muñoz, E.; Musselman, I.H.; Baughman, R.H.; Draper, R.K. Controlled assembly of carbon nanotubes by designed amphiphilic Peptide helices. J. Am. Chem. Soc. 2003, 125, 1770–1777. [Google Scholar] [CrossRef]
- Zorbas, V.; Ortiz-Acevedo, A.; Dalton, A.B.; Yoshida, M.M.; Dieckmann, G.R.; Draper, R.K.; Baughman, R.H.; Jose-Yacaman, M.; Musselman, I.H. Preparation and characterization of individual peptide-wrapped single-walled carbon nanotubes. J. Am. Chem. Soc. 2004, 126, 7222–7227. [Google Scholar] [CrossRef]
- Karajanagi, S.S.; Yang, H.; Asuri, P.; Sellitto, E.; Dordick, J.S.; Kane, R.S. Protein-assisted solubilization of single-walled carbon nanotubes. Langmuir 2006, 22, 1392–1395. [Google Scholar] [CrossRef]
- Li, X.; Chen, W.; Zhan, Q.; Dai, L.; Sowards, L.; Pender, M.; Naik, R.R. Direct measurements of interactions between polypeptides and carbon nanotubes. J. Phys. Chem. B 2006, 110, 12621–12625. [Google Scholar]
- Salvador-Morales, C.; Townsend, P.; Flahaut, E.; Vénien-Bryan, C.; Vlandas, A.; Green, M.L.H.; Sim, R.B. Binding of pulmonary surfactant proteins to carbon nanotubes; potential for damage to lung immune defense mechanisms. Carbon 2007, 45, 607–617. [Google Scholar] [CrossRef] [Green Version]
- Witus, L.S.; Rocha, J.-D.R.; Yuwono, V.M.; Paramonov, S.E.; Weismana, R.B.; Hartgerink, J.D. Peptides that non-covalently functionalize single-walled carbon nanotubes to give controlled solubility characteristics. J. Mater. Chem. 2007, 17, 1909–1915. [Google Scholar] [CrossRef]
- Nepal, D.; Geckeler, K.E. Proteins and carbon nanotubes: Close encounter in water. Small 2007, 3, 1259–1265. [Google Scholar] [CrossRef]
- Wang, S.; Humphreys, E.S.; Chung, S.Y.; Delduco, D.F.; Lustig, S.R.; Wang, H.; Parker, K.N.; Rizzo, N.W.; Subramoney, S.; Chiang, Y.M. Peptides with selective affinity for carbon nanotubes. Nat. Mater. 2003, 2, 196–200. [Google Scholar] [CrossRef]
- Zuo, G.; Huang, Q.; Wei, G.; Zhou, R.; Fang, H. Plugging into proteins: Poisoning protein function by a hydrophobic nanoparticle. ACS Nano 2010, 4, 7508–7514. [Google Scholar] [CrossRef]
- Yi, C.; Fong, C.C.; Zhang, Q.; Lee, S.T.; Yang, M. The structure and function of ribonuclease A upon interacting with carbon nanotubes. Nanotechnology 2008, 19, 095102. [Google Scholar] [CrossRef]
- Zhang, B.; Xing, Y.; Li, Z.; Zhou, H.; Mu, Q.; Yan, B. Functionalized carbon nanotubes specifically bind to alpha-chymotrypsin’s catalytic site and regulate its enzymatic function. Nano Lett. 2009, 9, 2280–2284. [Google Scholar] [CrossRef]
- Karajanagi, S.S.; Vertegel, A.A.; Kane, R.S.; Dordick, J.S. Structure and function of enzymes adsorbed onto single-walled carbon nanotubes. Langmuir 2004, 20, 11594–11599. [Google Scholar] [CrossRef]
- Shen, J.W.; Wu, T.; Wang, Q.; Kang, Y. Induced stepwise conformational change of human serum albumin on carbon nanotube surfaces. Biomaterials 2008, 29, 3847–3855. [Google Scholar] [CrossRef]
- Azamian, B.R.; Davis, J.J.; Coleman, K.S.; Bagshaw, C.B.; Green, M.L. Bioelectrochemical single-walled carbon nanotubes. J. Am. Chem. Soc. 2002, 124, 12664–12665. [Google Scholar] [CrossRef]
- Bussy, C.; Kostarelos, K. Carbon nanotubes in medicine and biology—Safety and toxicology. Adv. Drug Deliv. Rev. 2013, 65, 2061–2062. [Google Scholar] [CrossRef]
- Gasser, M.; Rothen-Rutishauser, B.; Krug, H.F.; Gehr, P.; Nelle, M.; Yan, B.; Wick, P. The adsorption of biomolecules to multi-walled carbon nanotubes is influenced by both pulmonary surfactant lipids and surface chemistry. J. Nanobiotechnology 2010, 8, 31. [Google Scholar] [CrossRef]
- Gasser, M.; Wick, P.; Clift, M.J.; Blank, F.; Diener, L.; Yan, B.; Gehr, P.; Krug, H.F.; Rothen-Rutishauser, B. Pulmonary surfactant coating of multi-walled carbon nanotubes (MWCNTs) influences their oxidative and pro-inflammatory potential in vitro. Part. Fibre Toxicol. 2012, 9, 17. [Google Scholar] [CrossRef] [Green Version]
- Holt, B.D.; Dahl, K.N.; Islam, M.F. Cells take up and recover from protein-stabilized single-wall carbon nanotubes with two distinct rates. ACS Nano 2012, 6, 3481–3490. [Google Scholar] [CrossRef]
- Holt, B.D.; Dahl, K.N.; Islam, M.F. Quantification of uptake and localization of bovine serum albumin-stabilized single-wall carbon nanotubes in different human cell types. Small 2011, 7, 2348–2355. [Google Scholar] [CrossRef]
- Kostarelos, K.; Lacerda, L.; Pastorin, G.; Wu, W.; Wieckowski, S.; Luangsivilay, J.; Godefroy, S.; Pantarotto, D.; Briand, J.P.; Muller, S. Cellular uptake of functionalized carbon nanotubes is independent of functional group and cell type. Nat. Nanotechnol. 2007, 2, 108–113. [Google Scholar] [CrossRef]
- Angelikopoulos, P.; Schou, K.; Bock, H. Surfactant-induced forces between carbon nanotubes. Langmuir 2010, 26, 18874–18883. [Google Scholar] [CrossRef]
- Salvador-Morales, C.; Basiuk, E.V.; Basiuk, V.A.; Green, M.L.; Sim, R.B. Effects of covalent functionalization on the biocompatibility characteristics of multi-walled carbon nanotubes. J. Nanosci. Nanotechnol. 2008, 8, 2347–2356. [Google Scholar] [CrossRef]
- Lin, Y.; Allard, L.F.; Sun, Y.P. Protein-affinity of single-walled carbon nanotubes in water. J. Phys. Chem. B 2004, 108, 3760–3764. [Google Scholar] [CrossRef]
- Shim, M.; Kam, N.W. S.; Chen, R.J.; Li, Y.; Dai, H. Functionalization of carbon nanotubes for biocompatibility and biomolecular recognition. Nano Lett. 2002, 2, 285–288. [Google Scholar] [CrossRef]
- Cai, X.; Ramalingam, R.; Wong, H.S.; Cheng, J.; Ajuh, P.; Cheng, S.H.; Lam, Y.W. Characterization of carbon nanotube protein corona by using quantitative proteomics. Nanomedicine 2013, 9, 583–593. [Google Scholar]
- Shannahan, J.H.; Brown, J.M.; Chen, R.; Ke, P.C.; Lai, X.; Mitra, S.; Witzmann, F.A. Comparison of nanotube-protein corona composition in cell culture media. Small 2013, 9, 2171–2181. [Google Scholar] [CrossRef]
- Mu, Q.; Liu, W.; Xing, Y.; Zhou, H.; Li, Z.; Zhang, Y.; Ji, L.; Wang, F.; Si, Z.; Zhang, B.; Yan, B. Protein binding by functionalized multiwalled carbon nanotubes is governed by the surface chemistry of both parties and the nanotube diameter. J. Phys. Chem. C 2008, 112, 3300–3307. [Google Scholar]
- Chen, R.J.; Zhang, Y.; Wang, D.; Dai, H. Noncovalent sidewall functionalization of single-walled carbon nanotubes for protein immobilization. J. Am. Chem. Soc. 2001, 123, 3838–3839. [Google Scholar] [CrossRef]
- Bradley, K.; Briman, M.; Star, A.; Grüner, G. Charge transfer from adsorbed proteins. Nano Lett. 2004, 4, 253–256. [Google Scholar] [CrossRef]
- Kam, N.W.; Liu, Z.; Dai, H. Carbon nanotubes as intracellular transporters for proteins and DNA: An investigation of the uptake mechanism and pathway. Angew. Chem. Int. Ed. Engl. 2006, 45, 577–581. [Google Scholar] [CrossRef]
- Ling, W.L.; Biro, A.; Bally, I.; Tacnet, P.; Deniaud, A.; Doris, E.; Frachet, P.; Schoehn, G.; Pebay-Peyroula, E.; Arlaud, G.J. Proteins of the innate immune system crystallize on carbon nanotubes but are not activated. ACS Nano 2011, 5, 730–737. [Google Scholar] [CrossRef]
- Holt, B.D.; McCorry, M.C.; Boyer, P.D.; Dahl, K.N.; Islam, M.F. Not all protein-mediated single-wall carbon nanotube dispersions are equally bioactive. Nanoscale 2012, 4, 7425–7434. [Google Scholar] [CrossRef]
- Tenzer, S.; Docter, D.; Rosfa, S.; Wlodarski, A.; Kuharev, J.; Rekik, A.; Knauer, S.K.; Bantz, C.; Nawroth, T.; Bier, C.; et al. Nanoparticle size is a critical physicochemical determinant of the human blood plasma corona: A comprehensive quantitative proteomic analysis. ACS Nano 2011, 5, 7155–7167. [Google Scholar] [CrossRef]
- Tenzer, S.; Docter, D.; Kuharev, J.; Musyanovych, A.; Fetz, V.; Hecht, R.; Schlenk, F.; Fischer, D.; Kiouptsi, K.; Reinhardt, C.; et al. Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology. Nat. Nanotechnol. 2013, 8, 772–781. [Google Scholar] [CrossRef]
- Dobrovolskaia, M.A.; Patri, A.K.; Zheng, J.; Clogston, J.D.; Ayub, N.; Aggarwal, P.; Neun, B.W.; Hall, J.B.; McNeil, S.E. Interaction of colloidal gold nanoparticles with human blood: Effects on particle size and analysis of plasma protein binding profiles. Nanomedicine 2009, 5, 106–117. [Google Scholar] [CrossRef]
- Schäffler, M.; Semmler-Behnke, M.; Sarioglu, H.; Takenaka, S.; Wenk, A.; Schleh, C.; Hauck, S.M.; Johnston, B.D.; Kreyling, W.G. Serum protein identification and quantification of the corona of 5, 15 and 80 nm gold nanoparticles. Nanotechnology 2013, 24, 265103. [Google Scholar] [CrossRef]
- Maiorano, G.; Sabella, S.; Sorce, B.; Brunetti, V.; Malvindi, M.A.; Cingolani, R.; Pompa, P.P. Effects of cell culture media on the dynamic formation of protein-nanoparticle complexes and influence on the cellular response. ACS Nano 2010, 4, 7481–7491. [Google Scholar] [CrossRef]
- Lundqvist, M.; Stigler, J.; Elia, G.; Lynch, I.; Cedervall, T.; Dawson, K.A. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proc. Natl. Acad. Sci. USA 2008, 105, 14265–14270. [Google Scholar]
- Lind, K.; Kresse, M.; Müller, R.H. Comparison of protein adsorption patterns onto differently charged hydrophilic superparamagnetic iron oxide particles obtained in vitro and ex vivo. Electrophoresis 2001, 22, 3514–3521. [Google Scholar] [CrossRef]
- Jansch, M.; Stumpf, P.; Graf, C.; Rühl, E.; Müller, R.H. Adsorption kinetics of plasma proteins on ultrasmall superparamagnetic iron oxide (USPIO) nanoparticles. Int. J. Pharm. 2012, 428, 125–133. [Google Scholar] [CrossRef]
- Gagner, J.E.; Lopez, M.D.; Dordick, J.S.; Siegel, R.W. Effect of gold nanoparticle morphology on adsorbed protein structure and function. Biomaterials 2011, 32, 7241–7252. [Google Scholar] [CrossRef]
- Gagner, J.E.; Qian, X.; Lopez, M.M.; Dordick, J.S.; Siegel, R.W. Effect of gold nanoparticle structure on the conformation and function of adsorbed proteins. Biomaterials 2012, 33, 8503–8516. [Google Scholar] [CrossRef]
- Vertegel, A.A.; Siegel, R.W.; Dordick, J.S. Silica nanoparticle size influences the structure and enzymatic activity of adsorbed lysozyme. Langmuir 2004, 20, 6800–6807. [Google Scholar] [CrossRef]
- Deng, J.; Sun, M.; Zhu, J.; Gao, C. Molecular interactions of different size AuNP-COOH nanoparticles with human fibrinogen. Nanoscale 2013, 5, 8130–8137. [Google Scholar] [CrossRef]
- Asuri, P.; Karajanagi, S.S.; Yang, H.; Yim, T.J.; Kane, R.S.; Dordick, J.S. Increasing protein stability through control of the nanoscale environment. Langmuir 2006, 22, 5833–5836. [Google Scholar] [CrossRef]
- Deng, Z.J.; Mortimer, G.; Schiller, T.; Musumeci, A.; Martin, D.; Minchin, R.F. Differential plasma protein binding to metal oxide nanoparticles. Nanotechnology 2009, 20, 455101. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kucki, M.; Kaiser, J.-P.; Clift, M.J.D.; Rothen-Rutishauser, B.; Petri-Fink, A.; Wick, P. The Role of the Protein Corona in Fiber Structure-Activity Relationships. Fibers 2014, 2, 187-210. https://doi.org/10.3390/fib2030187
Kucki M, Kaiser J-P, Clift MJD, Rothen-Rutishauser B, Petri-Fink A, Wick P. The Role of the Protein Corona in Fiber Structure-Activity Relationships. Fibers. 2014; 2(3):187-210. https://doi.org/10.3390/fib2030187
Chicago/Turabian StyleKucki, Melanie, Jean-Pierre Kaiser, Martin J. D. Clift, Barbara Rothen-Rutishauser, Alke Petri-Fink, and Peter Wick. 2014. "The Role of the Protein Corona in Fiber Structure-Activity Relationships" Fibers 2, no. 3: 187-210. https://doi.org/10.3390/fib2030187
APA StyleKucki, M., Kaiser, J.-P., Clift, M. J. D., Rothen-Rutishauser, B., Petri-Fink, A., & Wick, P. (2014). The Role of the Protein Corona in Fiber Structure-Activity Relationships. Fibers, 2(3), 187-210. https://doi.org/10.3390/fib2030187