Mammalian Skeletal Muscle Fibres Promote Non-Muscle Stem Cells and Non-Stem Cells to Adopt Myogenic Characteristics
Abstract
:1. Introduction
2. Material and Methods
2.1. Ethical Approval
2.2. Animal Maintenance
2.3. Cell Lines and Cultures
2.4. Single Myofibre Isolation and Culture
2.5. Cytoskeletal Inhibitor Preparation
2.6. Time-Lapse Microscopy
2.7. Myotube Formation
2.8. Immunocytochemistry
2.9. Fluorescence Microscopy
2.10. Scanning Electron Microscopy
2.11. Image and Movie Analysis
2.12. Statistical Analysis
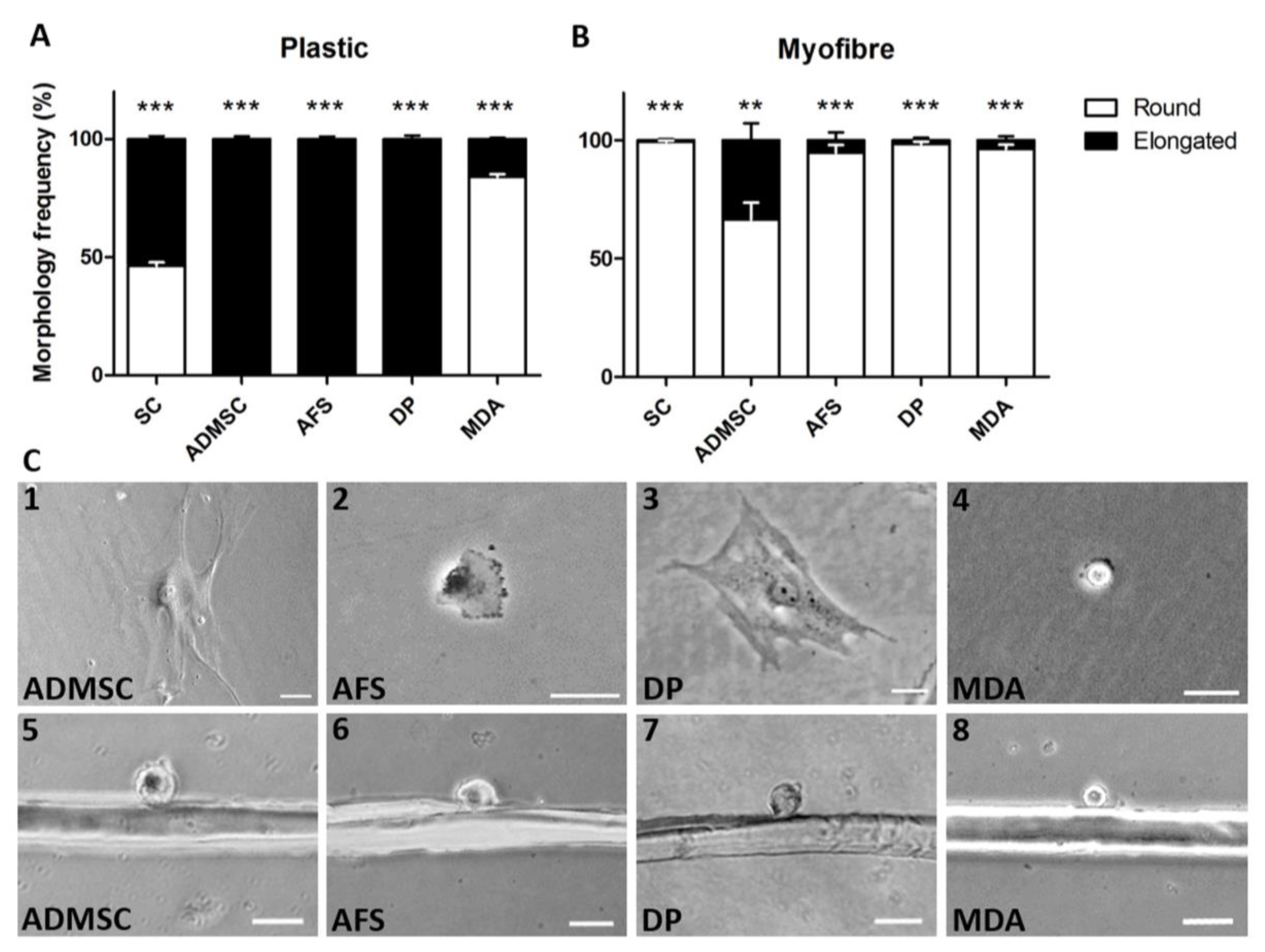
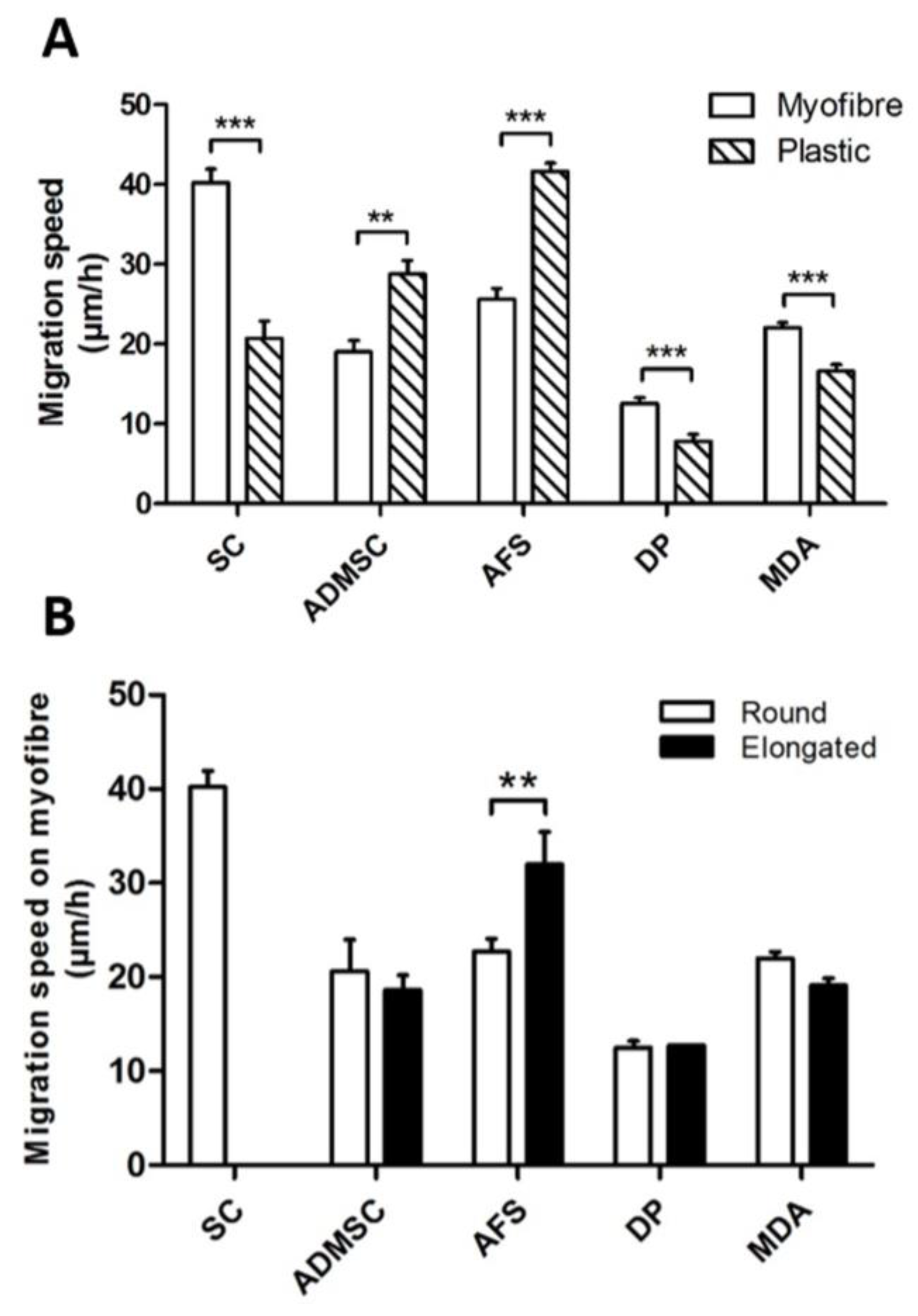
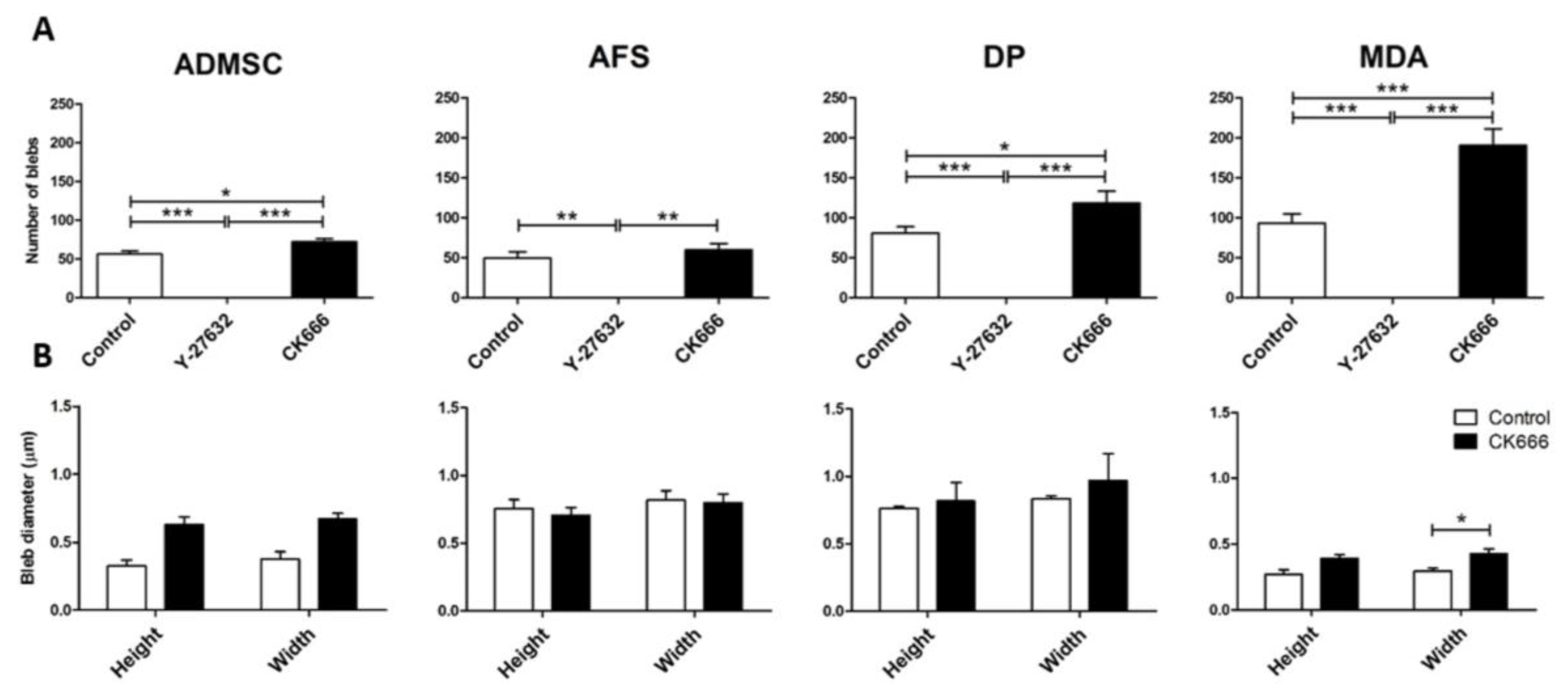
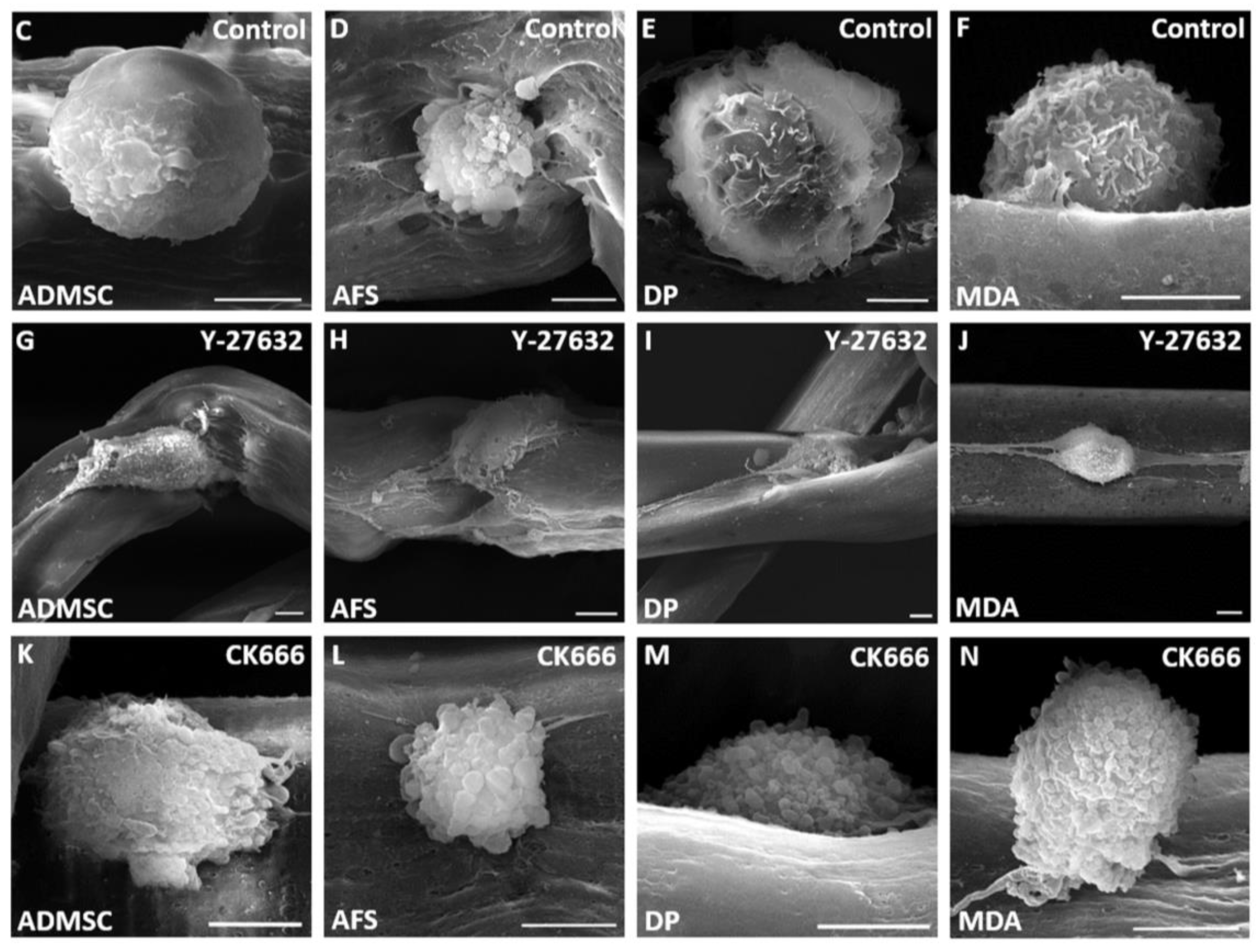
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Collins, C.A.; Olsen, I.; Zammit, P.S.; Heslop, L.; Petrie, A.; Partridge, T.A.; Morgan, J.E. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell 2005, 122, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Watt, D.J.; Karasinski, J.; Moss, J.; England, M.A. Migration of muscle cells. Nature 1994, 368, 406–407. [Google Scholar] [CrossRef] [PubMed]
- Watt, D.J.; Morgan, J.E.; Clifford, M.A.; Partridge, T.A. The movement of muscle precursor cells between adjacent regenerating muscles in the mouse. Anat. Embryol. (Berl.) 1987, 175, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Sacco, A.; Mourkioti, F.; Tran, R.; Choi, J.; Llewellyn, M.; Kraft, P.; Shkreli, M.; Delp, S.; Pomerantz, J.H.; Artandi, S.E.; et al. Short telomeres and stem cell exhaustion model duchenne muscular dystrophy in mdx/mTR mice. Cell 2010, 143, 1059–1071. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Urso, M.L.; Murray, K.; Fu, F.; Li, Y. Relaxin regulates MMP expression and promotes satellite cell mobilization during muscle healing in both young and aged mice. Am. J. Pathol. 2010, 177, 2399–2410. [Google Scholar] [CrossRef] [PubMed]
- Peault, B.; Rudnicki, M.; Torrente, Y.; Cossu, G.; Tremblay, J.P.; Partridge, T.; Gussoni, E.; Kunkel, L.M.; Huard, J. Stem and progenitor cells in skeletal muscle development, maintenance, and therapy. Mol. Ther. 2007, 15, 867–877. [Google Scholar] [CrossRef] [PubMed]
- Lafreniere, J.F.; Mills, P.; Tremblay, J.P.; El Fahime, E. Growth factors improve the in vivo migration of human skeletal myoblasts by modulating their endogenous proteolytic activity. Transplantation 2004, 77, 1741–1747. [Google Scholar] [CrossRef] [PubMed]
- Satoh, A.; Huard, J.; Labrecque, C.; Tremblay, J.P. Use of fluorescent latex microspheres (flms) to follow the fate of transplanted myoblasts. J. Histochem. Cytochem. 1993, 41, 1579–1582. [Google Scholar] [CrossRef] [PubMed]
- Collins-Hooper, H.; Woolley, T.E.; Dyson, L.; Patel, A.; Potter, P.; Baker, R.E.; Gaffney, E.A.; Maini, P.K.; Dash, P.R.; Patel, K. Age-related changes in speed and mechanism of adult skeletal muscle stem cell migration. Stem Cells 2012, 30, 1182–1195. [Google Scholar] [CrossRef] [PubMed]
- Otto, A.; Collins-Hooper, H.; Patel, A.; Dash, P.R.; Patel, K. Adult skeletal muscle stem cell migration is mediated by a blebbing/amoeboid mechanism. Rejuvenation Res. 2011, 14, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Charras, G.; Paluch, E. Blebs lead the way: How to migrate without lamellipodia. Nat. Rev. Mol. Cell Biol. 2008, 9, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Tozluoglu, M.; Tournier, A.L.; Jenkins, R.P.; Hooper, S.; Bates, P.A.; Sahai, E. Matrix geometry determines optimal cancer cell migration strategy and modulates response to interventions. Nat. Cell Biol. 2013, 15, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, P.M.; Havenstrite, K.L.; Magnusson, K.E.; Sacco, A.; Leonardi, N.A.; Kraft, P.; Nguyen, N.K.; Thrun, S.; Lutolf, M.P.; Blau, H.M. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science 2010, 329, 1078–1081. [Google Scholar] [CrossRef] [PubMed]
- Bajek, A.; Gurtowska, N.; Olkowska, J.; Kazmierski, L.; Maj, M.; Drewa, T. Adipose-derived stem cells as a tool in cell-based therapies. Arch. Immunol. Ther. Exp. (Warsz.) 2016, 64, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Conde-Green, A.; Marano, A.A.; Lee, E.S.; Reisler, T.; Price, L.A.; Milner, S.M.; Granick, M.S. Fat grafting and adipose-derived regenerative cells in burn wound healing and scarring: A systematic review of the literature. Plast. Reconstr. Surg. 2016, 137, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Mead, B.; Logan, A.; Berry, M.; Leadbeater, W.; Scheven, B.A. Dental pulp stem cells: A novel cell therapy for retinal and central nervous system repair. Stem Cells 2016, 35, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Collart-Dutilleul, P.Y.; Chaubron, F.; De Vos, J.; Cuisinier, F.J. Allogenic banking of dental pulp stem cells for innovative therapeutics. World J. Stem Cells 2015, 7, 1010–1021. [Google Scholar] [PubMed]
- Tajiri, N.; Acosta, S.; Portillo-Gonzales, G.S.; Aguirre, D.; Reyes, S.; Lozano, D.; Pabon, M.; Dela Pena, I.; Ji, X.; Yasuhara, T.; et al. Therapeutic outcomes of transplantation of amniotic fluid-derived stem cells in experimental ischemic stroke. Front. Cell. Neurosci. 2014, 8, 227. [Google Scholar] [CrossRef] [PubMed]
- Waddington, R.J.; Youde, S.J.; Lee, C.P.; Sloan, A.J. Isolation of distinct progenitor stem cell populations from dental pulp. Cells Tissues Organs 2009, 189, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Otto, A.; Schmidt, C.; Luke, G.; Allen, S.; Valasek, P.; Muntoni, F.; Lawrence-Watt, D.; Patel, K. Canonical Wnt signalling induces satellite-cell proliferation during adult skeletal muscle regeneration. J. Cell Sci. 2008, 121, 2939–2950. [Google Scholar] [CrossRef] [PubMed]
- Cornelison, D.D.; Wold, B.J. Single-cell analysis of regulatory gene expression in quiescent and activated mouse skeletal muscle satellite cells. Dev. Biol. 1997, 191, 270–283. [Google Scholar] [CrossRef] [PubMed]
- Zammit, P.S.; Golding, J.P.; Nagata, Y.; Hudon, V.; Partridge, T.A.; Beauchamp, J.R. Muscle satellite cells adopt divergent fates: A mechanism for self-renewal? J. Cell Biol. 2004, 166, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Asokan, S.B.; Berginski, M.E.; Haynes, E.M.; Sharpless, N.E.; Griffith, J.D.; Gomez, S.M.; Bear, J.E. Arp2/3 is critical for lamellipodia and response to extracellular matrix cues but is dispensable for chemotaxis. Cell 2012, 148, 973–987. [Google Scholar] [CrossRef] [PubMed]
- Krawetz, R.J.; Taiani, J.; Greene, A.; Kelly, G.M.; Rancourt, D.E. Inhibition of rho kinase regulates specification of early differentiation events in P19 embryonal carcinoma stem cells. PLoS ONE 2011, 6, e26484. [Google Scholar] [CrossRef] [PubMed]
- Hetrick, B.; Han, M.S.; Helgeson, L.A.; Nolen, B.J. Small molecules CK-666 and CK-869 inhibit actin-related protein 2/3 complex by blocking an activating conformational change. Chem. Biol. 2013, 20, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Otto, A.; Macharia, R.; Matsakas, A.; Valasek, P.; Mankoo, B.S.; Patel, K. A hypoplastic model of skeletal muscle development displaying reduced foetal myoblast cell numbers, increased oxidative myofibres and improved specific tension capacity. Dev. Biol. 2010, 343, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the european working group on sarcopenia in older people. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Nagano, M.; Hoshino, D.; Koshikawa, N.; Akizawa, T.; Seiki, M. Turnover of focal adhesions and cancer cell migration. Int. J. Cell Biol. 2012, 2012, 310616. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Moreno, V.; Gadea, G.; Ahn, J.; Paterson, H.; Marra, P.; Pinner, S.; Sahai, E.; Marshall, C.J. Rac activation and inactivation control plasticity of tumor cell movement. Cell 2008, 135, 510–523. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zheng, Y.; Zhang, W.; Wang, Z.; Xiao, J.; Yuan, Y. Progression and variation of fatty infiltration of the thigh muscles in duchenne muscular dystrophy, a muscle magnetic resonance imaging study. Neuromuscul. Disord. 2015, 25, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Cordani, N.; Pisa, V.; Pozzi, L.; Sciorati, C.; Clementi, E. Nitric oxide controls fat deposition in dystrophic skeletal muscle by regulating fibro-adipogenic precursor differentiation. Stem Cells 2014, 32, 874–885. [Google Scholar] [CrossRef] [PubMed]
- Amthor, H.; Christ, B.; Weil, M.; Patel, K. The importance of timing differentiation during limb muscle development. Curr. Biol. 1998, 8, 642–652. [Google Scholar] [CrossRef]
- Condic, M.L.; Rao, M. Alternative sources of pluripotent stem cells: Ethical and scientific issues revisited. Stem Cells Dev. 2010, 19, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Porzionato, A.; Sfriso, M.M.; Pontini, A.; Macchi, V.; Petrelli, L.; Pavan, P.G.; Natali, A.N.; Bassetto, F.; Vindigni, V.; De Caro, R. Decellularized human skeletal muscle as biologic scaffold for reconstructive surgery. Int. J. Mol. Sci. 2015, 16, 14808–14831. [Google Scholar] [CrossRef] [PubMed]
- Manchineella, S.; Thrivikraman, G.; Khanum, K.K.; Ramamurthy, P.C.; Basu, B.; Govindaraju, T. Pigmented silk nanofibrous composite for skeletal muscle tissue engineering. Adv. Healthc. Mater. 2016, 5, 1222–1232. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Kwon, C.H.; Lee, C.; An, J.; Phuong, T.T.; Park, S.H.; Lima, M.D.; Baughman, R.H.; Kang, T.M.; Kim, S.J. Bio-inspired hybrid carbon nanotube muscles. Sci. Rep. 2016, 6, 26687. [Google Scholar] [CrossRef] [PubMed]
- Omairi, S.; Matsakas, A.; Degens, H.; Kretz, O.; Hansson, K.A.; Solbra, A.V.; Bruusgaard, J.C.; Joch, B.; Sartori, R.; Giallourou, N.; et al. Enhanced exercise and regenerative capacity in a mouse model that violates size constraints of oxidative muscle fibres. eLife 2016, 5. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morash, T.; Collins-Hooper, H.; Mitchell, R.; Patel, K. Mammalian Skeletal Muscle Fibres Promote Non-Muscle Stem Cells and Non-Stem Cells to Adopt Myogenic Characteristics. Fibers 2017, 5, 5. https://doi.org/10.3390/fib5010005
Morash T, Collins-Hooper H, Mitchell R, Patel K. Mammalian Skeletal Muscle Fibres Promote Non-Muscle Stem Cells and Non-Stem Cells to Adopt Myogenic Characteristics. Fibers. 2017; 5(1):5. https://doi.org/10.3390/fib5010005
Chicago/Turabian StyleMorash, Taryn, Henry Collins-Hooper, Robert Mitchell, and Ketan Patel. 2017. "Mammalian Skeletal Muscle Fibres Promote Non-Muscle Stem Cells and Non-Stem Cells to Adopt Myogenic Characteristics" Fibers 5, no. 1: 5. https://doi.org/10.3390/fib5010005
APA StyleMorash, T., Collins-Hooper, H., Mitchell, R., & Patel, K. (2017). Mammalian Skeletal Muscle Fibres Promote Non-Muscle Stem Cells and Non-Stem Cells to Adopt Myogenic Characteristics. Fibers, 5(1), 5. https://doi.org/10.3390/fib5010005




