Managing Artificially Drained Low-Gradient Agricultural Headwaters for Enhanced Ecosystem Functions
Abstract
:1. Overview and Scope
2. Impacts of Agricultural Expansion
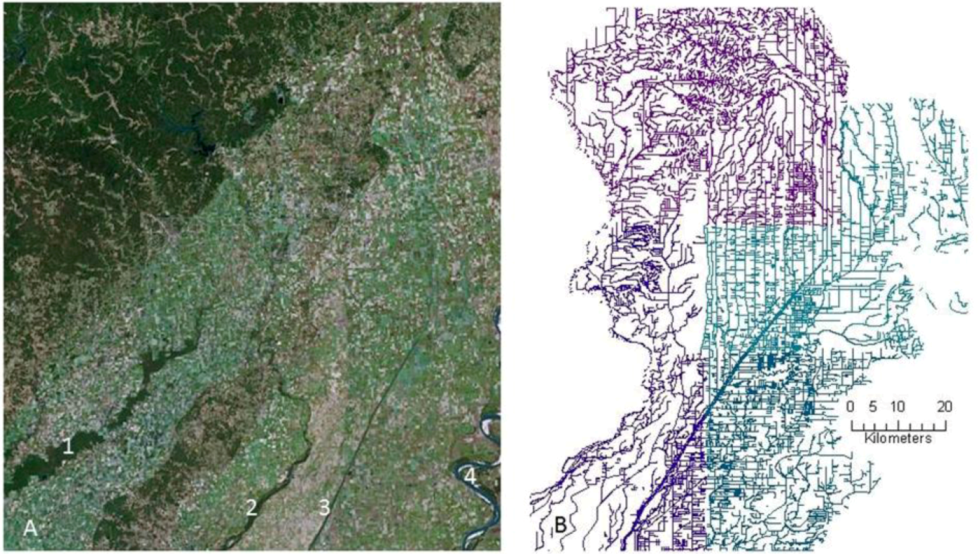
2.1. History of Wetland and Stream Losses to Agriculture
| Header? | Rank of Cause of Impairment | Rank of Source of Impairment | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Water type | quantity assessed | % assessed | Nutrients | Sediment/ Siltation | Turbidity | Organic Enrichment /Low D.O. | Unknown | Agriculture | % Agriculture Impairment | Hydro-modification | Unknown |
| Rivers, Stream, Creeks ** | 1 x 106 km | 18.83 | 5 | 1 | * | 7 | 9 | 1 | 16 | 3 | 2 |
| Lakes, Ponds, Reservoirs*** | 60,000 km2 | 36.53 | 1 | 4 | 10 | 3 | * | 2 | 14 | 5 | 1 |
| Bays & estuaries | 79,000 km2 | 34.85 | 2 | * | 7 | 3 | 5 | * | * | 8 | 1 |
| Coastal shoreline | 4,000 km | 4.39 | 5 | 9 | 7 | 10 | 2 | 5 | 4.50 | * | 2 |
| Oceans, Near coastal waters | 12,800 km2 | 9.15 | 7 | 10 | 5 | 4 | * | 10 | < 1 | 6 | 2 |
| Wetlands | 5000 km2 | 1.19 | 8 | 3 | 5 | 2 | * | 3 | 18 | 5 | 1 |
2.2. Hydrologic Alteration and Habitat Destruction and Impairment

2.3 Nutrient Enrichment
3. Ecological Restoration
3.1. Wetland Restoration
3.2. Riparian Buffers
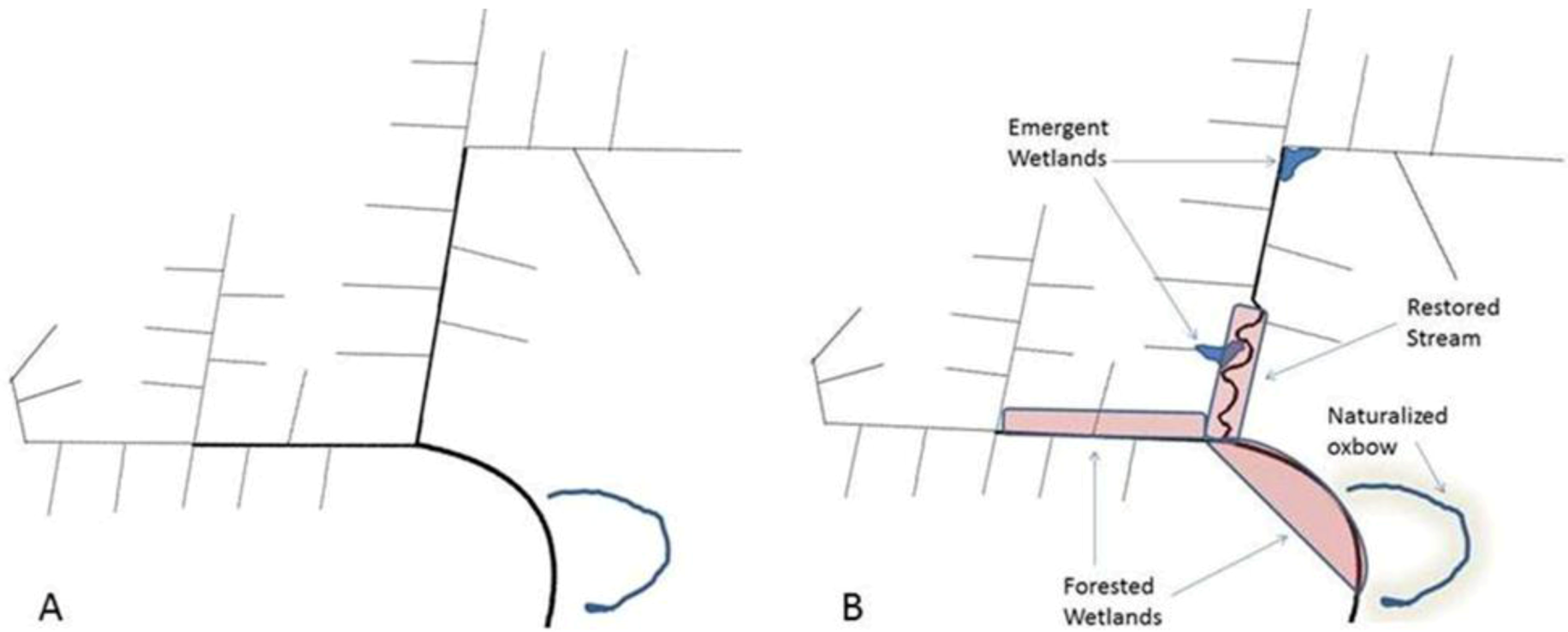
3.3. Limitations to Restoration of Agricultural Headwaters
4. Instream Drainage Management

4.1. Two-stage Ditches
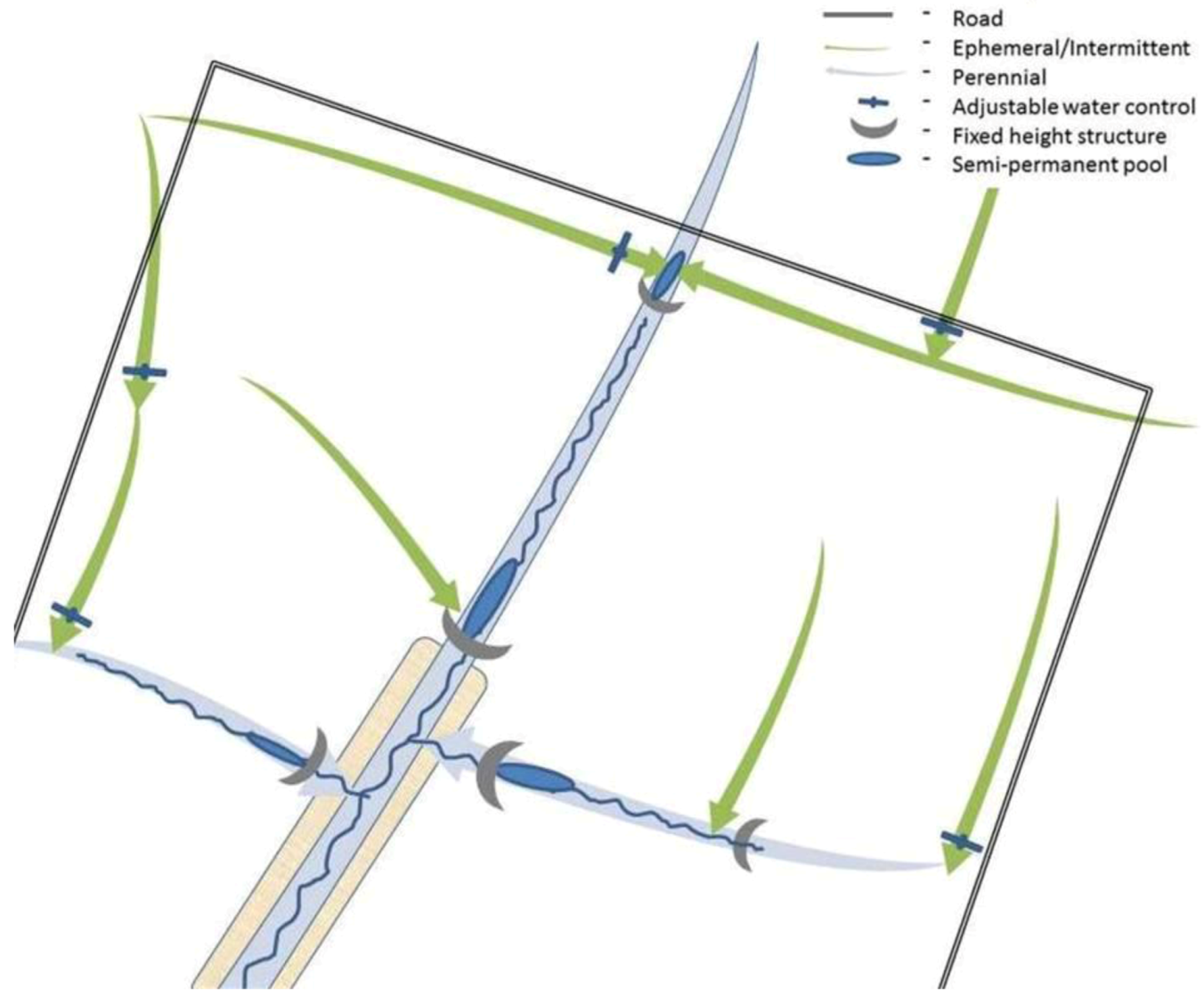
4.2. Controlled Drainage

| Location and basin or plot size | System type | Structure | *Nutrient Elemental Concentration (mg/L) | *Load (kg ha−1 yr−1) | **% load Decrease | Reference | Duration | |
|---|---|---|---|---|---|---|---|---|
| Ontario, Canada; 3.5 ha basin | corn/soybean subsurface drainage | Riser pipes at 25 & 50 cm above free flow | Nitrate | >8–16 | >0.8 | 62%–95% | [231] | 2 years |
| North Carolina, USA; each plot 3–16 ha | Corn subsurface drainage | Flashboard riser ~30–50 cm from soil surface | Nitrate | >2–17 (upper range) | >25–40 | 50%–85% | [232] | 3 years |
| North Carolina, USA; multiple studies | surface drainage | Controlled drainage | TN | >14 kg/ha−1 | 45%** | [233,234] | ||
| TP | >.05 kg/ha−1 | 42%** | ||||||
| subsurface drainage | Controlled drainage | TN | >31 kg/ha−1 | 44%** | ||||
| TP | >0.2 kg/ha−1 | 20%** | ||||||
| Chesapeake Bay, USA.; 80–90 ha basin | 1’-2’ lowland suburban streams | Step-pools and riffles | TN | 0.6–2.5 | at low flow: 0.6 kg m−1 yr−1 | At low flow: 23% | [228] | 3 years |
| Arkansas, USA; 35 ha basin (simulation) | Experimental vegetated surface drainages 60 m length | “rice spill” weirs | TIP | 10 | 0.02 kg m−1 | 86% | ***[235] | 7 days x 2 trials |
| Nitrate | 2–15 | 0.4–0.6 kg m−1 | 97% | |||||
| Open flow Riser pipes | TIP | 10 | 0.02 kg m−1 | 88% | ||||
| Nitrate | 2–15 | 0.4–0.6 kg m−1 | 79% | |||||
| Low-grade weirs | Nitrate | 3–4 | >1.2 kg m−1 yr-1 | 79% | [236] | 8 hours | ||
| Southwest Sweden; Each plot 0.2 ha | Subsurface, experimental plots of potatoes | enclosed riser pipes, ~ 90–130 cm above free flow | TP | - | >0.028 | 58–85%** | [209] | 22 months |
| Nitrate | >11–19 | >30–38 | 78–94%** | |||||
| Controlled drainage 20-70 cm below surface | TP | > 0.02 | >0.026–0.138 | 56–95%** | [237] | 4 years | ||
| Nitrate | > 9–10 | >26–37 | 69%–94%** | |||||
| Northeast Italy; 0.001 ha plots | Subsurface, experimental plots of beets, maize, or wetland plants | Controlled drainage 0–60 cm from surface | Nitrate | >8–77 (upper range) | 3–11.8 g m−2 | 46%–63%** | [225] | 31 months |
| wetland | 96%** | |||||||
| Ontario, Canada; 1.9 ha plots | Subsurface, maize | Riser <60cm from surface | Nitrate | >19.2 | 58 | 46%** | [238] | 1 year |
| Ontario, Canada; 4 ha plots | Subsurface, soybeans | INNOTAG controlled drainage units <65 cm from surface | Nitrate | 12–15 | >16.9 | 14%–25% | [239] | 2 years |
| Ontario, Canada 0.1 ha plots | Subsurface corn/soybean | Riser 30cm above free flow | Nitrate | >4–8 | >1.7–19 | 31%–44% | [240] | 4 years |
| Subsurface + subirrigation, corn/soybean | 62%–66% | |||||||
| Lithuania; 4.9 and 5.4 ha plots | Subsurface; barley, winter wheat, summer wheat, rape | Riser 68cm above free flow | Nitrate | 5–25 (total range) | >14 | 22% | [241] | 7 years |
| Ohio, USA; 12 plots, each 0.066 ha | Subsurface; corn/soybean | Riser 30 cm below surface; | Nitrate | >9.5–16 | >14–24 | 45% | [226] | 4 years |
| Subsurface +subirrigation | 30% | |||||||
5. Ecosystems and Communities
5.1 Spatiotemporal effects on macrophytes

5.2 Microhabitat Effects on Macrophytes
| Causes | Effects |
|---|---|
| Lack of canopy | High temp, increased primary production |
| Increased peak discharge | Mechanical stress, temperature variability |
| Increased hydrologic variability | Drought/anoxia stress |
| Increased slope of ditch bank | Burying, sharp gradient limits area for establishment, shading |
| Suspended sediment | Turbidity, scouring |
| Elevated N and P | Turbidity, increased primary production |
| Substratum | Less variability, compaction, unconsolidated fine particulates |
| Herbicides/Pesticides | Plant toxicity |
5.3 Ecosystem Functions of Macrophytes
5.4 Potential Impact of Aquatic Macroinvertebrates on Nutrient Export
6. Conclusions
6.1. Recommendations
6.2. Future Directions
Acknowledgments
References and Notes
- Allan, J.D. Landscapes and riverscapes: The influence of land use on stream ecosystems. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 257–284. [Google Scholar] [CrossRef]
- Blann, K.L.; Anderson, J.L.; Sands, G.R.; Vondracek, B. Effects of agricultural drainage on aquatic ecosystems: A review. Crit. Revi. Environ. Sci. Technol. 2009, 39, 909–1001. [Google Scholar]
- Carpenter, S.R.; Stanley, E.H.; Vander Zanden, M.J. State of the world’s freshwater ecosystems: Physical, chemical, and biological changes. Annu. Rev. Environ. Res. 2011, 36, 75–99. [Google Scholar] [CrossRef]
- Rabalais, N.N.; Turner, R.E.; Wiseman, W.J., Jr. Hypoxia in the Gulf of Mexico. J. Environ. Qual. 2001, 30, 320–329. [Google Scholar] [CrossRef]
- Breitburg, D.L.; Hondorp, D.W.; Davias, L.A.; Diaz, R.J. Hypoxia, nitrogen, and fisheries: Integrating effects across local and global landscapes. Ann. Rev. Mar. Sci. 2009, 1, 329–349. [Google Scholar] [CrossRef]
- Mander, Ü.; Kuusemets, V.; Hayakawa, Y. Purification processes, ecological functions, planning and design of riparian buffer zones in agricultural watersheds (Editorial). Ecol. Eng. 2005, 24, 421–432. [Google Scholar] [CrossRef]
- Strock, J.S.; Kleinman, P.J.A.; King, K.W.; Delgado, J.A. Drainage water management for water quality protection. J. Soil Water Conserv. 2010, 65, 131A–136A. [Google Scholar] [CrossRef]
- Kröger, R.; Thornton, K.W.; Moore, M.T.; Farris, J.L.; Prevost, J.D.; Pierce, S.C. Tiered collaborative strategies for reducing hypoxia and restoring the Gulf of Mexico. J. Soil Water Conserv. 2012, 67, 70A–73A. [Google Scholar] [CrossRef]
- Kröger, R.; Moore, M.T.; Thornton, K.W.; Farris, J.L.; Prevost, J.D.; Pierce, S.C. Tiered on-the-ground implementation projects for Gulf of Mexico water quality improvements. J. Soil Water Conserv. 2012, 67, 94A–99A. [Google Scholar]
- Day J.W., Jr.; Arancibia, A.Y.; Mitsch, W.J.; Lara-Dominguez, A.L.; Day, J.N.; Ko, J.; Lane, R.; Lindsey, J; Lomeli, D.Z. Using ecotechnology to address water quality and wetland habitat loss problems in the Mississippi basin: A hierarchical approach. Biotechnol. Adv. 2003, 22, 135–159. [Google Scholar]
- Evans, R.; Bass, K.; Burchell, M.; Hinson, D.; Johnson, R.; Doxey, M. Management alternatives to enhance water quality function of channelized streams and drainage canals. J. Soil Water Conserv. 2007, 62, 308–320. [Google Scholar]
- Mitsch, W.J.; Day, J.W., Jr. Restoration of wetlands in the Mississippi–Ohio–Missouri (MOM) River Basin: Experience and needed research. Ecol. Eng. 2006, 26, 55–69. [Google Scholar] [CrossRef]
- Ranalli, A.J.; Macalady, D.L. The importance of the riparian zone and in-stream processes in nitrate attenuation in undisturbed and agricultural watersheds—A review of the scientific literature. J. Hydrol. 2010, 389, 406–415. [Google Scholar] [CrossRef]
- Brinson, M.M.; Eckles, D.S.U.S. Department of Agriculture conservation program and practice effects on wetland ecosystem services: A synthesis. Ecol. Appl. 2011, 21, 116–127. [Google Scholar]
- Moore, M.T.; Kröger, R. Agricultural Drainage Ditches: Mitigation Wetland for the 21st Century; Moore, M.T., Kröger, R., Eds.; Research Signpost: Kerala, India, 2010; p. 259. [Google Scholar]
- Davies, B.D.; Biggs, J.; Williams, P.; Thompson, S. Making agricultural landscapes more sustainable for freshwater biodiversity: A case study from southern England. Aqua. Conserv. Mar. Freshwater Ecosyst. 2009, 19, 439–447. [Google Scholar] [CrossRef]
- Bouldin, J.L.; Farris, J.L.; Moore, M.T.; Cooper, C.M. Vegetative and structural characteristics of agricultural drainages in the Mississippi Delta landscapes. Environ. Pollut. 2004, 132, 403–411. [Google Scholar] [CrossRef]
- Moore, M.T.; Cooper, C.M.; Farris, J.L. Drainage ditches. In Water encyclopedia: Surface and Agricultural Water; Lehr, J., Keeley, J., Eds.; Wiley: New York, NY, USA, 2005; pp. 87–92. [Google Scholar]
- Strahler, A.N. Quantitative analysis of watershed geomorphology. Trans. Am. Geophys. Union 1957, 38, 913–920. [Google Scholar]
- Beauchamp, K.H. A history of drainage and drainage methods. In Farm Drainage in the United States—History, Status, and Prospects; Pavelis, G.A., Ed.; Economic Research Service, U.S. Department of Agriculture: Washington, DC, USA, 1987; pp. 13–29. [Google Scholar]
- van Schilfgaarde, J. Drainage yesterday, today, and tomorrow. In Proceedings of the American Society of Agricultural Engineers National Drainage Symposium; American Society of Agricultural Engineers: St. Joseph, MI, USA, 1971. [Google Scholar]
- Allen, J. Prehistoric Agricultural Systems in the Waghi Valley—A further note. Mankind 1970, 7, 177–183. [Google Scholar]
- Ballard, C. Wetland drainage and agricultural transformations in the Southern Highlands of Papua New Guinea. Asia Pac. Viewpoint 2001, 42, 287–304. [Google Scholar]
- Muke, J.; Mandui, H. In the shadows of Kuk: Evidence for prehistoric agriculture at Kana, Wahgi Valley, Papua New Guinea. Archaeol. Oceania 2003, 38, 177–185. [Google Scholar]
- Denham, T. Archaeological evidence for mid-Holocene agriculture in the interior of Papua New Guinea: A critical review. Archaeol. Oceania 2003, 38, 159–176. [Google Scholar]
- Pavelis, G.A. Farm Drainage in the United States: History, Status, and Prospects 1987; U.S. Department of Agriculture: Washington, DC, USA, 1987; p. 170. [Google Scholar]
- Blomqvist, M.M.; Vos, P.; Klinkhamer, G.L.; ter Keurs, W.J. Declining plant species richness of grassland ditch banks—A problem of colonisation or extinction? Biol. Conser. 2003, 109, 391–406. [Google Scholar]
- Hietala-Koivu, R.; Lankoski, J.; Tarmi, S. Loss of biodiversity and its social cost in an agricultural landscape. Agri. Ecosyst. Environ. 2004, 103, 75–83. [Google Scholar] [CrossRef]
- Helm, A.; Hanski, I.; Portel, M. Slow response of plant species richness to habitat loss and fragmentation. Ecol. Lett. 2006, 9, 72–77. [Google Scholar]
- Herzon, I.; Helenius, J. Agricultural drainage ditches, their biological importance and functioning. Biol. Conser. 2008, 141, 1171–1183. [Google Scholar] [CrossRef]
- USEPA. National Water Quality Inventory: Report to Congress, 2004 Reporting Cycle (EPA 841-R-08–001) 2009. Available online: http://water.epa.gov/lawsregs/guidance/cwa/305b/2004report_index.cfm (accessed on 31 October 2012).
- USEPA National Assessment Database. Available online: http://iaspub.epa.gov/waters10/w305b_report_v2.nation (accessed on 31 October 2012).
- de Wit, M.; Behrendt, H.; Bendoricchio, G.; Bleuten, W.; van Gaans, P. The Contribution of Agriculture to Nutrient Pollution in Three European Rivers, with Reference to the European Nitrates Directive; European Water Management Online; European Water Association: Hennef, Germany, 2002. [Google Scholar]
- Mourad, D.S.J.; Van Der Perk, M.; Piirimäe, K. Changes in nutrient emissions, fluxes and retention in a north-eastern European lowland drainage basin. Environ. Monit. Assess. 2006, 120, 415–448. [Google Scholar]
- Ongley, E.D.; Xiaolan, Z.; Tao, Y. Current status of agricultural and rural non-point source pollution assessment in China. Environ. Pollut. 2010, 158, 1159–1168. [Google Scholar]
- Qu, H.J.; Kroeze, C. Nutrient export by rivers to the coastal waters of China: management strategies and future trends. Reg. Environ. Change 2012, 12, 153–167. [Google Scholar]
- Khaleel, R.; Reddy, K.R.; Overcash, M.R. Transport of potential pollutants in runoff water from land areas receiving animal wastes: a review. Water Res. 1980, 14, 421–436. [Google Scholar]
- Smukler, S.M.; O'Geen, A.T.; Jackson, L.E. Assessment of best management practices for nutrient cycling: a case study on an organic farm in Mediterranean-type climate. J. Soil Water Conserv. 2012, 67, 16–31. [Google Scholar]
- Kideys, A.E. Fall and rise of the Black Sea ecosystem. Science 2002, 297, 1482–1484. [Google Scholar]
- Hefner, J.M.; Brown, J.D. Wetland trends in the southeastern United States. Wetlands 1985, 4, 1–11. [Google Scholar]
- Dahl, T.E. Wetland losses in the United States, 1780’s to 1980’s; U.S. Fish and Wildlife Service: Washington, DC, USA, 1990. [Google Scholar]
- Foote, A.L.; Pandey, S.; Krogman, N.T. Processes of wetland loss in India. Environ. Conserv. 1996, 1, 45–54. [Google Scholar]
- Davis, J.A.; Froend, R. Loss and degradation of wetlands in southwestern Australia: underlying causes, consequences and solutions. Wetl. Ecol. Manag. 1999, 7, 13–23. [Google Scholar]
- Coleman, J.M.; Huh, O.K.; Braud, D., Jr. Wetland loss in world deltas. J. Coastal Res. 2008, 24, 1–14. [Google Scholar]
- Zhang, J.; Ma, K.; Fu, B. Wetland loss under the impact of agricultural development in the Sanjiang Plain, NE China. Environ. Monit. Assess. 2010, 166, 139–148. [Google Scholar]
- Rudis, V.A. Regional forest fragmentation effects on bottomland hardwood community types and resource values. Landscape Ecol. 1995, 10, 291–307. [Google Scholar]
- Twedt, D.J.; Loesch, C.R. Forest area and the distribution in the Mississippi Alluvial Valley: Implications for breeding bird conservation. J. Biogeogr. 1999, 26, 1215–1224. [Google Scholar]
- MacDonald, P.O.; Frayer, W.E.; Clauser, J.K. Documentation, chronology, and future projections of bottomland hardwood habitat loss in the Lower Mississippi Alluvial Plain, Volume 1. In Technical Report for U.S. Department of the Interior; Fish and Wildlife Service: Washington, DC, USA, 1979. [Google Scholar]
- Brown, R.G. Effects of wetland channelization on runoff and loading. Wetlands 1998, 8, 123–133. [Google Scholar]
- Hey, D.L.; Philippi, N.S. Flood reduction through wetland restoration: the Upper Mississippi River Basin as a case history. Restor. Ecol. 1995, 3, 4–17. [Google Scholar]
- Shankman, D.; Pugh, T.B. Discharge response to channelization of a coastal plain stream. Wetlands 1992, 12, 157–162. [Google Scholar] [CrossRef]
- Criss, R.E.; Shock, E.L. Flood enhancement through flood control. Geology 2001, 29, 875–878. [Google Scholar] [CrossRef]
- Steiger, J.; Tabacchi, E.; Dufour, S.; Corenblit, D.; Peiry, J.L. Hydrogeomorphic processes affecting riparian habitat within alluvial channel-floodplain river systems: a review for the temperate zone. River Res. Appl. 2005, 21, 719–737. [Google Scholar] [CrossRef]
- Sophocleous, M. Interactions between groundwater and surface water: the state of the science. Hydrogeol. J. 2002, 10, 52–67. [Google Scholar] [CrossRef]
- Skaggs, R.W.; Chescheir, G.M.; Phillips, B.D. Methods to determine lateral effect of a drainage ditch on wetland hydrology. Trans. ASAE 2005, 48, 577–584. [Google Scholar]
- Hill, A.R. The environmental impacts of agricultural land drainage. J. Environ. Manage. 1976, 4, 251–274. [Google Scholar]
- Schlosser, I.J.; Karr, J.R. Riparian vegetation and channel morphology impact on spatial patterns of water quality in agricultural watersheds. Environ. Manage. 1981, 5, 233–243. [Google Scholar]
- Peterjohn, W.T.; Correll, D.L. Nutrient dynamics in an agricultural watershed: observations on the role of a riparian forest. Ecology 1984, 65, 1466–1475. [Google Scholar]
- Skaggs, R.W.; Breve, M.A.; Gilliam, J.W. Hydrologic and water quality impacts of agricultural drainage. Critical Rev. Environ. Sci. Technol. 1994, 24, 1–32. [Google Scholar]
- Thomas, D.L.; Perry, C.D.; Evans, R.O.; Izuno, F.T.; Stone, K.C.; Gilliam, J.W. Agricultural drainage effects on water quality in Southeastern U.S. J. Irrig. Drain. E.-ASCE 1995, 121, 277–282. [Google Scholar]
- Mainstone, C.P.; Schofield, K. Agricultural management for nonpoint pollution control, with particular reference to the UK. Eur. Water Pollut. Contr. 1996, 6, 21–30. [Google Scholar]
- Magner, J.; Steffen, L. Stream morphological response to climate and land-use in the Minnesota River Basin. In Proceedings of the American Society of Civil. Engineers Joint Water Resources Engineering, Planning and Management Conference, Minneapolis, MI, USA, 30 July –2 August 2000; ASCE.
- Shields, F.D.; Knight, S.S.; Cooper, C.M. Effects of channel incision on base flow stream habitats and fishes. Environ. Manage. 1994, 18, 43–57. [Google Scholar]
- Shields, F.D., Jr.; Knight, S.S.; Cooper, C.M. Rehabilitation of aquatic habitats in warmwater streams damaged by channel incision in Mississippi. Hydrobiologia. 1998, 382, 63–86. [Google Scholar]
- Hrody, P.J.; Sutton, T.M. Fish community responses to half-log additions in warmwater streams. N. Am. J. Fish. Manage. 2008, 28, 70–80. [Google Scholar]
- Smiley, P.C., Jr.; Gillespie, R.B. Influence of physical habitat and agricultural contaminants on fishes within agricultural drainage ditches. In Agricultural Drainage Ditches: Mitigation Wetlands for the 21st Century; Moore, M.T., Kröger, R., Eds.; Research Signpost: Kerala, India, 2010; pp. 37–73. [Google Scholar]
- McRae, S.E.; Allan, J.D.; Burch, J.D. Reach- and catchment-scale determinants of the distribution of freshwater mussels (Bivalvia: Unionidae) in south-eastern Michigan, USA. Freshwater Biol. 2004, 49, 127–142. [Google Scholar]
- Pool, K.E.; Downing, J.A. Relationship of declining mussel biodiversity to stream-reach and watershed characteristics in an agricultural landscape. J. N. Am. Benthol. Soc. 2004, 23, 114–125. [Google Scholar]
- Downing, J.A.; Van Meter, P.; Woolnough, D.A. Suspects and evidence: A review of the causes of extirpation and decline in freshwater mussels. Anim. Biodivers. Conserv. 2010, 33, 151–185. [Google Scholar]
- Robinson, M.; Rycroft, D.W. Chapter 23: The impact of drainage on streamflow. In Agricultural Drainage; Skaggs, R.W., van Schilfgaarde, J., Eds.; American Society of Agronomy Madison: Madison, WI, USA, 1999; pp. 767–800. [Google Scholar]
- Knox, J.C. Agricultural influence on landscape sensitivity in the Upper Mississippi River Valley. Catena 2001, 42, 193–224. [Google Scholar]
- Knox, J.C. Floodplain sedimentation in the Upper Mississippi Valley: Natural versus human accelerated. Geomorphology 2006, 79, 286–310. [Google Scholar]
- Poff, N.L.; Allan, J.D.; Bain, M.B.; Karr, J.R.; Prestegaard, K.L.; Richter, B.D.; Sparks, R.E.; Stromberg, J.C. The natural flow regime. BioScience 1997, 47, 769–784. [Google Scholar]
- Zaimes, G.N.; Schultz, R.C.; Isenhart, T.M. Stream bank erosion adjacent to riparian forest buffers, row-crop fields, and continuously-grazed pastures along Bear Creek in central Iowa. J. Soil Water Conserv. 2004, 59, 19–27. [Google Scholar]
- Magner, J.A.; Payne, G.A.; Steffen, L.J. Drainage effects on stream nitrate-N and hydrology in south-central Minnesota (USA). Environ. Monit. Assess. 2004, 91, 183–198. [Google Scholar]
- King, K.W.; Smiley, P.C., Jr.; Fausey, N.R. Hydrology of channelized and natural headwater streams. Hydrol. Sci. J. 2009, 54, 929–948. [Google Scholar]
- Simon, A. The discharge of sediment in channelized alluvial streams. J. Am. Water Resour. A 1989, 25, 1177–1188. [Google Scholar]
- Bengtson, R.L.; Carter, C.E.; Morris, H.F.; Bartkiewicz, S.A. Nitrogen and phosphorus losses under subsurface drainage practices in southern Louisiana. Proc. ASAE 1988, 31, 729–733. [Google Scholar]
- Woltemade, C.J. Ability of restored wetlands to restore nitrogen and phosphorous concentrations in agricultural drainage water. J. Soil Water Conserv. 2000, 3, 303–309. [Google Scholar]
- Sims, J.T.; Simard, R.R.; Joern, B.C. Phosphorus losses in agricultural drainage: historical perspective and current research. J. Environ. Qual. 1998, 27, 277–293. [Google Scholar]
- Gentry, L.E.; David, M.B.; Royer, T.V.; Mitchell, C.A.; Starks, K.M. Phosphorus transport pathways to streams in tile-drained agricultural watersheds. J. Environ. Qual. 2007, 36, 408–415. [Google Scholar]
- The Ohio State University Extension, Agricultural drainage: Water quality impacts and subsurface drainage studies in the Midwest. In Ohio State University Extension Bulletin 871; Zucker, L.A.; Brown, L.C. (Eds.) The Ohio State University Extension: Columbus, Ohio, USA, 1988.
- Sugg, Z. Assessing U.S. Farm Drainage: Can GIS Lead to Better Estimates of Subsurface Drainage Extent? World Resources Institute: Washington, DC, USA, 2007. [Google Scholar]
- Vought, L.B.-M.; Lacoursière, J.O. Restoration of streams in the agricultural landscape. In Restoration of Lakes, Streams, Floodplains, and Bogs in Europe; Principles and Case Studies; Eiseltová, M., Ed.; Springer: Prague, Czech Republic, 2010; pp. 225–242. [Google Scholar]
- Siebert, S.; Burke, J.; Faures, J.M.; Frenken, K.; Hoogeveen, J.; Döll, P.; Portmann, F.T. Groundwater use for irrigation—A global inventory. Hydrol. Earth Syst. Sci. Discuss. 2010, 7, 1863–1880. [Google Scholar]
- Wen, F.; Chen, X. Evaluation of the impact of groundwater irrigation on streamflow in Nebraska. J. Hydrol. 2006, 327, 603–617. [Google Scholar]
- Rugel, K.; Jackson, C.R.; Romeis, J.J.; Golladay, S.W.; Hicks, D.W.; Dowd, J.F. Effects of irrigation withdrawals on streamflows in a karst environment: lower Flint River Basin, Georgia, USA. Hydrol. Process. 2012, 26, 523–534. [Google Scholar]
- Horton, J.L.; Kolb, T.E.; Hart, S.C. Physiological response to groundwater depth varies among species and with river flow regulation. Eco. Appl. 2001, 11, 1046–1059. [Google Scholar]
- Vitousek, P.M.; Moonery, H.A.; Lubchencho, J.; Melillo, J.M. Human domination of Earth’s ecosystems. Science 1997, 277, 494–499. [Google Scholar]
- Caraco, N.F.; Cole, J.J. Human impact on nitrate export: an analysis using major world rivers. AMBIO. 1999, 28, 167–170. [Google Scholar]
- Birgand, F.; Skaggs, R.W.; Chescher, G.M.; Gilliam, J.W. Nitrogen removal in streams in agricultural catchments—A literature review. Critical Reviews in Environ. Sci. Technol. 2007, 37, 381–487. [Google Scholar]
- Turner, R.E.; Rabalais, N.N. Changes in Mississippi River water quality this century. BioScience 1991, 41, 140–147. [Google Scholar]
- Shields, F.D., Jr.; Lizotte, R.E., Jr.; Knight, S.S.; Cooper, C.M.; Wilcox, D. The stream channel incision syndrome and water quality. Eco. Eng. 2010, 36, 78–90. [Google Scholar]
- Howarth, R.W.; Jensen, H.S.; Marino, R.; Postma, H. Transport to and processing of P in near-shore and oceanic waters. In Phosphorus in the Global Environment: Transfers, Cycles, and Management; Tiessen, H., Ed.; John Wiley and Sons: New York, NY, USA, 1995; pp. 323–345. [Google Scholar]
- Schilling, K.E.; Li, Z.; Zhang, Y. Groundwater-surface water interaction in the riparian zone of an incised channel, Walnut Creek, Iowa. J. Hydrol. 2006, 327, 140–150. [Google Scholar]
- Camargo, J.A.; Alonso, A.; Salamanca, A. Nitrate toxicity to aquatic animals: A review with new data for freshwater invertebrates. Chemosphere 2005, 58, 1255–1267. [Google Scholar]
- Haywood, G.P. Ammonia toxicity in teleost fish: A review. Technical Report for Department of Fisheries Research Branch: Nanaimo, Columbia, 1983. [Google Scholar]
- Arthur, J.W.; West, C.W.; Allen, K.N.; Hedtke, S.F. Seasonal toxicity of ammonia to five fish and nine invertebrate species. B. Environ. Contam. Tox. 1987, 38, 324–331. [Google Scholar]
- Jofre, M.B.; Karasov, W.H. Direct effect of ammonia on three species of North American anuran amphibians. Environ. Toxicol. Chem. 1999, 18, 1806–1812. [Google Scholar]
- Ortiz, M.E.; Marco, A.; Saiz, N.; Lizana, M. Impact of ammonium nitrate on growth and survival of six European amphibians. Arch. of Environ. Contam. and Toxicol. 2004, 47, 234–239. [Google Scholar]
- Smith, G.R.; Temple, K.G.; Vaala, D.A.; Dingfelder, H.A. Effects of nitrate on the tadpoles of two ranids (Rana. catesbeiana and R. clamitans). Arch. Env. Contam. Toxicol. 2005, 49, 559–562. [Google Scholar]
- Britto, D.T.; Kronzucker, H.J. NH4+ toxicity in higher plants: A critical review. J. Plant. Physiol. 2002, 159, 567–584. [Google Scholar] [CrossRef]
- USEPA Draft 2009 update. Aquatic Life ambient water quality criteria for ammonia—freshwater. EPA-82-D-09–001 2009. Available online: http://water.epa.gov/scitech/swguidance/standards/criteria/aqlife/ammonia/upload/2009_12_23_criteria_ammonia_2009update.pdf (accessed on 6 December 2012).
- Carpenter, S.R.; Caraco, N.F.; Correll, D.L.; Sharpley, A.N.; Smith, V.H. Nonpoint pollution of surface waters with phosphorus and nitrogen. Eco. Appl. 1998, 8, 559–568. [Google Scholar] [CrossRef]
- Bennett, E.M.; Carpenter, S.R.; Caraco, N.F. Human impact on erodible phosphorus and eutrophication: A global perspective. BioScience 2001, 51, 227–234. [Google Scholar] [CrossRef]
- Evans-White, M.A.; Dodds, W.K.; Huggins, D.G.; Baker, D.S. Thresholds in macroinvertebrate biodiversity and stoichiometry across water-quality gradients in Central Plains (USA) streams. J. N. Am. Benthol. Soc. 2009, 28, 855–868. [Google Scholar]
- Jeppesen, E.; Søndergaard, M.; Jensen, J.P.; Havens, K.E.; Anneville, O.; Carvalho, L.; Coveney, M.F.; Deneke, R.; Dokulil, M.T.; Foy, B.; et al. Lake responses to reduced nutrient loading—An analysis of contemporary long-term data from 35 case studies. Freshwater Biol. 2005, 50, 1747. [Google Scholar] [CrossRef]
- Thomaz, S.M.; Dibble, E.D.; Evangelista, L.R.; Higuti, J.; Bini, L.M. Influence of aquatic macrophyte habitat complexity on invertebrate abundance and richness in tropical lagoons. Freshwater Biol. 2008, 53, 358–367. [Google Scholar]
- Portielje, R.; Roijackers, R.M.M. Primary succession of aquatic macrophytes in experimental ditches in relation to nutrient input. Aquat. Bot. 1995, 50, 127–140. [Google Scholar]
- Newman, S.; Grace, J.B.; Koebel, J.W. Effects of nutrients and hydroperiod on Typha., Cladium. and Eleocharis.: Implications for Everglades restoration. Eco. Appl. 1996, 6, 774–783. [Google Scholar]
- Lorenzen, B.; Brix, H.; Mendelssohn, I.A.; McKee, K.L.; Miao, S.L. Growth, biomass allocation and nutrient use efficiency in Cladium. jamaicense and Typha. domingensis as affected by phosphorus and oxygen availability. Aquat. Bot. 2001, 70, 117–133. [Google Scholar] [CrossRef]
- Diaz, R.J. Overview of hypoxia around the world. J. Environ. Qual. 2001, 30, 275–281. [Google Scholar] [CrossRef]
- Diaz, R.J.; Rosenberg, R. Spreading dead zones and consequences for marine ecosystems. Science 2008, 321, 926–929. [Google Scholar]
- Nixon, S.W.; Oviatt, C.A.; Frithsen, J.; Sullivan, B. Nutrients and the productivity of estuarine and coastal marine ecosystems. J. Limnol. Soc. S. Afr. 1986, 12, 43–71. [Google Scholar]
- Micheli, F. Eutrophication, fisheries, and consumer-resource dynamics in marine pelagic ecosystems. Science 1999, 285, 1396–1398. [Google Scholar] [CrossRef]
- Baird, D.; Christian, R.R.; Peterson, C.H.; Johnson, G.A. Consequences of hypoxia on estuarine ecosystem function: energy diversion from consumers to microbes. Eco. Appl. 2004, 14, 805–822. [Google Scholar] [CrossRef]
- Heisler, J.; Glibert, P.M.; Burkholder, J.M.; Anderson, D.M.; Cochlan, W.; Dennison, W.C.; Dortch, Q.; Gobler, C.J.; Heil, C.A.; Humphries, E.; et al. Eutrophication and harmful algal blooms: a scientific consensus. Harmful Algae 2008, 8, 1, 3–13. [Google Scholar]
- Rabalais, N.N. Nitrogen in aquatic ecosystems. AMBIO 2002, 31, 102–112. [Google Scholar]
- Seitzinger, S.P.; Kroeze, C.; Bouwman, A.F.; Caraco, N.; Dentener, F.; Styles, R.V. Global patterns of dissolved inorganic and particulate nitrogen inputs to coastal system: Recent conditions and future projections. Estuaries Coasts 2002, 25, 640–655. [Google Scholar]
- Bagge, O.; Nielsen, E.; Mellergaard, S.; Dalsgaard, I. Hypoxia and the demersal fish stock in the Kattegat (IIIa) and Subdivision 22. In Proceedings of ICES Council Meeting 1990, ICES, Copenhagen, Denmark, 19–21 March 1990; p. 52.
- Mee, L.D. The Black Sea in crisis: A need for concerted international action. AMBIO 1992, 21, 278–286. [Google Scholar]
- Österblom, H.; Hansson, S.; Larsson, U.; Hjerne, O.; Wulff, F.; Elmgren, R.; Folke, C. Human-induced trophic cascades and ecological regime shifts in the Baltic Sea. Ecosystems 2007, 10, 877–889. [Google Scholar] [CrossRef]
- Chesney, E.J.; Baltz, D.M. The effects of hypoxia on the northern Gulf of Mexico coastal ecosystem: A fisheries perspective. In Coastal Hypoxia: Consequences for Living Resources and Ecosystems; Rabalais, N.N., Turner, R.E., Eds.; American Geophysical Union: Washington, DC, USA, 2001; pp. 321–354. [Google Scholar]
- Craig, J.K.; Crowder, L.B. Hypoxia-induced habitat shifts and energetic consequences in Atlantic croaker and brown shrimp on the Gulf of Mexico shelf. Mar. Ecol. Prog. Ser. 2005, 294, 79–94. [Google Scholar]
- O’Connor, T.; Whitall, D. Linking hypoxia to shrimp catch in the northern Gulf of Mexico. Mar. Pollut. Bull. 2007, 54, 460–463. [Google Scholar] [CrossRef]
- Mississippi River/Gulf of Mexico Watershed Nutrient Task Force. In Action Plan for Reducing, Mitigating, and Controlling Hypoxia in the Northern Gulf of Mexico; United States of America Environmental Protection Agency: Washington, DC, USA, 2001.
- USEPA. Gulf Hypoxia Action Plan 2008 for reducing, mitigating and controlling hypoxia in the Northern Gulf of Mexico and improving water quality in the Mississippi River Basin. Mississippi River Gulf of Mexico Watershed Nutrient Task Force. Available online: http://water.epa.gov/type/watersheds/named/msbasin/upload/2008_8_28_msbasin_ghap2008_update082608.pdf (accessed on 31 October 2012).
- GOMA (Gulf of Mexico Alliance). Governors’ Action Plan II: For Healthy and Resilient Coasts. Gulf of Mexico Alliance, 2009. Available online: http://gulfofmexicoaliance.org/pdfs/ap2_final2.pdf (accessed on 31 October 2012).
- Mitsch, W.J.; Gosselink, J.G. Wetlands, 3rd ed; John Wiley and Sons: New York, NY, USA, 2000; p. 920. [Google Scholar]
- Reddy, K.R.; DeLaune, R.D. Biogeochemistry of Wetlands; CRC Press: Boca Raton, FL, USA, 2008; p. 816. [Google Scholar]
- Kadlec, R.H.; Wallace, S.D. Treatment Wetlands, 2nd ed; Taylor and Frances Group: Boca Raton, FL, USA, 2009; p. 1016. [Google Scholar]
- Hilderbrand, R.H.; Watts, A.C.; Randle, A.M. The myths of restoration ecology. Eco. Soc. 2005, 10, 19. [Google Scholar]
- Moreno-Mateos, D.; Power, M.E.; Comın, F.A.; Yockteng, R. Structural and functional loss in restored wetland ecosystems. PLOS Biol. 2012, 10, 1–8. [Google Scholar]
- Faulkner, S.; Barrow, W., Jr.; Keeland, B.; Walls, S.; Telesco, D. Effects of conservation practices on wetland ecosystem services in the Mississippi Alluvial Valley. Eco. Appl. 2011, 21, 31–48. [Google Scholar] [CrossRef]
- Maltby, E.; Acreman, M.C. Ecosystem services of wetlands: Pathfinder for a new paradigm. Hydrol. Sci. J. 2011, 56, 1341–1359. [Google Scholar] [CrossRef]
- Breukelaar, A.W.; Lammens, E.H.R.R.; Klein Breteler, J.G.P.; Tatrai, I. Effects of benthivorous bream (Abramis. brama) and carp (Cyprinus. carpio) on sediment resuspension and concentrations of nutrients and chlorophyll a. Freshwater Biol. 1994, 32, 113–121. [Google Scholar]
- Post, D.M.; Taylor, J.P.; Kitchell, J.F.; Olsen, M.H.; Schindler, D.E.; Herwig, B.R. The role of migratory waterfowl as nutrient vectors in a managed wetland. Conserv. Biol. 1998, 4, 910–920. [Google Scholar]
- Kitchell, J.F.; Schindler, D.R.; Herwig, B.R.; Post, D.M.; Olson, M.H. Nutrient cycling at the landscape scale: The role of diel foraging migrations by geese at the Bosque del Apache National Wildlife Refuge, New Mexico. Limnol. Oceanogr. 1999, 44, 828–836. [Google Scholar] [CrossRef]
- Tomer, M.D.; Locke, M.A. The challenge of documenting water quality benefits of conservation practices: A review of USDA-ARS’s conservation effects assessment project watershed studies. Water Sci. Technol. 2011, 64, 300–310. [Google Scholar] [CrossRef]
- Liira, J.; Schmidt, T.; Aavik, T.; Arens, P.; Augenstein, I.; Bailey, D.; Billeter, R.; Bukacek, R.; Burel, F.; De Blust, G.; et al. Plant functional group composition and large-scale species richness in European agricultural landscapes. J. Veg. Sci. 2008, 19, 3–14. [Google Scholar] [CrossRef]
- Manhoudt, A.G.E.; Visser, A.J.; de Snoo, G.R. Management regimes and farming practices enhancing plant species richness on ditch banks. Agri. Ecosyst. Environ. 2007, 119, 353–358. [Google Scholar] [CrossRef]
- de Snoo, G.R.; van der Poll, R.J. Effect of herbicide drift on adjacent boundary vegetation. Agri. Ecosyst. Environ. 1999, 73, 1–6. [Google Scholar]
- TerHaar, M.J.; Herricks, E.E. Management and development of aquatic habitat in agricultural drainage systems, Technical Report for Water Resources Center. University of Illinois: Urbana, IL, USA, 1989; Volume 145. [Google Scholar]
- Smiley, P.C., Jr.; King, K.W.; Fausey, N.R. Influence of herbaceous riparian buffers on physical habitat, water chemistry, and stream communities within channelized agricultural headwater streams. Eco. Eng. 2011, 37, 1314–1323. [Google Scholar]
- Tomer, M.D.; Dosskey, M.G.; Burkart, M.R.; James, D.E.; Helmers, M.J.; Eisenhauer, D.E. Methods to prioritize placement of riparian buffers for improved water quality. Agroforesty. Systems 2009, 75, 17–25. [Google Scholar] [CrossRef]
- Schultz, R.C.; Isenhart, T.M.; Simpkins, W.W.; Colletti, J.P. Riparian forest buffers in agroecosystems—lessons learned from the Bear Creek Watershed, central Iowa, USA. Agroforest. Syst. 2004, 61, 35–50. [Google Scholar] [CrossRef]
- Bentrup, G. Conservation Buffers: Design Guidelines for Buffers, Corridors, and Greenways. Gen. Tech. Rep. SRS-109; U.S. Forest Service: Ashville, NC, USA, 2008; p. 110. [Google Scholar]
- Haycock, N.E.; Muscutt, A.D. Landscape management strategies for the control of diffuse pollution. Landscape Urban. Plan. 1995, 31, 313–321. [Google Scholar] [CrossRef]
- Lyons, J.; Trimble, S.W.; Pain, L.K. Grass versus trees: managing riparian areas to benefit streams of central North America. J. Am. Water Resour. A 2000, 36, 919–930. [Google Scholar]
- Parkyn, S. Review of Riparian Buffer Zone Effectiveness. Technical Paper for Ministry of Agriculture and Forestry, Wellington, NZ, USA, 2004. [Google Scholar]
- Pankau, R.C.; Schoonover, J.E.; Williard, K.W.J.; Edwards, P.J. Concentrated flow paths in riparian buffer zones of southern Illinois. Agroforestry. Systems 2012, 84, 191–205. [Google Scholar] [CrossRef]
- Vought, L.B.-M.; Pinay, G.; Fuglsang, A.; Ruffioni, C. Structure and function of buffer strips from a water quality perspective in agricultural landscapes. Landscape Urban Plann. 1995, 31, 323–331. [Google Scholar] [CrossRef]
- Blackwell, M.S.A.; Hogan, D.V.; Pinay, G.; Maltby, E. The role of buffer zones for agricultural runoff. In The Wetlands Handbook; Maltby, E., Barker, T., Eds.; Wiley-Blackwell: Chichester, UK, 2009; pp. 417–439. [Google Scholar]
- Parkyn, S.M.; Davies-Colley, R.J.; Cooper, A.B.; Stroud, M.J. Predictions of stream nutrient and sediment yield changes following restoration of forested riparian buffers. Eco. Eng. 2005, 24, 551–558. [Google Scholar] [CrossRef]
- Sarriquet, P.E.; Delettre, Y.R.; Marmonier, P. Effects of catchment disturbance on stream invertebrates: Comparison of different habitats (vegetation, benthic, and interstitial) using bio-ecological groups. Ann. Limnol.-Int. J. Limn. 2006, 42, 205–219. [Google Scholar]
- Haycock, N.E.; Pinay, G. Groundwater nitrate dynamics in grass and poplar vegetated riparian buffers during the winter. J. Environ. Qual. 1993, 22, 273–278. [Google Scholar] [CrossRef]
- Hefting, M.M.; Clement, J.C.; Bienkowski, P.; Dowrick, D.; Guenat, C.; Butturini, A.; Topa, S.T.; Pinay, G.; Verhoeven, J.T.A. The role of vegetation and litter in the nitrogen dynamics of riparian buffer zones in Europe. Eco. Eng. 2005, 24, 465–482. [Google Scholar] [CrossRef]
- Sweeney, B.W.; Bott, T.L.; Jackson, J.K.; Kaplan, L.A.; Newbold, J.D.; Standley, L.J.; Hession, W.C.; Horwitz, R.J. Riparian deforestation, stream narrowing, and loss of stream ecosystem services. Proc. Nat. Acad. Sci. USA 2004, 101, 14132–14137. [Google Scholar]
- Clément, J.C.; Pinay, G.; Marmonier, P. Seasonal Dynamics of Denitrification along Topohydrosequences in Three Different Riparian Wetlands. J. Environ. Qual. 2002, 31, 1025–1037. [Google Scholar] [CrossRef]
- Kuusemets, V.; Mander, U.; Lohmus, K.; Ivask, M. Nitrogen and phosphorus variation in shallow groundwater and assimilation in plants in complex riparian buffer zones. Water Sci. Technol. 2001, 44, 615–622. [Google Scholar]
- Bunn, S.E.; Davies, P.M.; Kellaway, D.M.; Prosser, I.P. Influence of invasive macrophytes on channel morphology and hydrology in an open tropical lowland stream, and potential control by riparian shading. Freshwater Biol. 1998, 39, 171–178. [Google Scholar] [CrossRef]
- Paine, L.K.; Ribic, C.A. Comparison of riparian plant communities under four land management systems in southwestern Wisconsin. Agri. Ecosyst. Environ. 2002, 92, 93–105. [Google Scholar] [CrossRef]
- Parkyn, S.M.; Davies-Colley, R.J.; Halliday, N.J.; Costley, K.J.; Croker, G.F. Planted Riparian Buffer Zones in New Zealand: Do They Live Up to Expectations? Restor. Ecol. 2003, 11, 436–447. [Google Scholar]
- Dodds, W.K.; Whiles, M.R. Freshwater Ecology: Concepts & Environmental Applications of Limnology, 2nd ed; Academic Press: Burlington, MA, USA, 2010; p. 811. [Google Scholar]
- Shankman, D. Stream channelization and changing vegetation patterns in the U.S. Coastal Plain. Geogr. Rev. 1996, 86, 216–232. [Google Scholar] [CrossRef]
- Smiley, P.C., Jr.; Shields, D.F., Jr.; Knight, S.S. Designing impact assessments for evaluating ecological effects of agricultural conservation practices on streams. J. Am. Water Resour. A. 2009, 45, 867–878. [Google Scholar] [CrossRef]
- Zedler, J.B. Wetlands at your service: Reducing impacts of agriculture at the watershed scale. Front. Ecol. Environ. 2003, 1, 65–72. [Google Scholar] [CrossRef]
- Kleinman, P.J.A.; Sharpley, A.N.; McDowell, R.W.; Flaten, D.N.; Buda, A.R.; Tao, L.; Bergstrom, L.; Zhu, Q. Managing agricultural phosphorus for water quality protection: Principles for progress. Plant Soil 2011, 349, 169–182. [Google Scholar]
- Alexander, R.B.; Smith, R.A.; Schwarz, G.E. Effect of stream channel size on the delivery of nitrogen to the Gulf of Mexico. Nature 2000, 403, 758–761. [Google Scholar]
- Esselman, P.C.; Infante, D.M.; Wang, L.; Wu, D.; Cooper, A.R; Taylor, W.W. An index of cumulative disturbance to river fish habitats of the conterminous United States from landscape anthropogenic activities. Eco. Res. 2011, 29, 133–151. [Google Scholar] [CrossRef]
- Vannote, R.L.; Minshall, G.W.; Cummins, K.W.; Sedell, J.R.; Cushing, C.E. The river continuum concept. Can. J. Fish Aquat. Sci. 1980, 37, 130–137. [Google Scholar] [CrossRef]
- Arango, C.P.; Tank, J.L. Land use influences the spatiotemporal controls on nitrification and denitrification in headwater streams. J. N. Am. Benthol. Soc. 2008, 27, 90–107. [Google Scholar] [CrossRef]
- Benke, A.C.; Henry III, R.L.; Gillespie, D.M.; Hunter, R.J. Importance of snag habitat for animal production in southeastern streams. Fisheries 1985, 10, 8–13. [Google Scholar]
- Julian, J.P.; Seegert, S.Z.; Powers, S.M.; Stanley, E.H.; Doyle, M.W. Light as a first-order control on ecosystem structure in a temperate stream. Ecohydrology. 2011, 4, 422–432. [Google Scholar] [CrossRef]
- Wilcock, R.J.; Scarsbrook, M.R.; Costley, K.J.; Nagels, J.W. Controlled release experiments to determine the effects of shade and plants on nutrient retention in a lowland stream. Hydrobiologia. 2002, 485, 153–162. [Google Scholar]
- Wilcock, R.J.; Scarsbrook, M.R.; Cooke, J.G.; Costley, K.J.; Nagels, J.W. Shade and flow effects on ammonia retention in macrophyte-rich streams: Implications for water quality. Environ. Pollut. 2004, 132, 95–100. [Google Scholar]
- Collier, K.J.; Cooper, A.B.; Davies-Colley, R.J.; Rutherford, J.C.; Smith, C.M.; Williamson, R.B. Managing Riparian Zones: A Contribution to Protecting New Zealand’s Rivers and Streams, Volume 2: Guidelines; New Zealand Department of Conservation: Wellington, New Zealand, 1995; p. 20. [Google Scholar]
- Boutin, C.; Jobin, J.; Bélanger, L. Importance of riparian habitats to flora conservation in farming landscape of southern Québec, Canada. Agri. Ecosyst. Environ. 2003, 94, 73–87. [Google Scholar]
- Ryan, R.L.; Erickson, D.L.; De Young, R. Farmers' Motivations for Adopting Conservation Practices along Riparian Zones in a Mid-western Agricultural Watershed. J. Environ. Plann. Manage. 2003, 46, 19–37. [Google Scholar] [CrossRef]
- Soomers, H.; Winkel, D.N.; Wassen, Y.; Wassen, M.J. The dispersal and deposition of hydrochorous plant seeds in drainage ditches. Freshwater Biol. 2010, 55, 2032–2046. [Google Scholar] [CrossRef]
- Simon, T.N.; Travis, J. The contribution of man-made ditches to the regional stream biodiversity of the new river watershed in the Florida panhandle. Hydrobiologia. 2011, 661, 163–177. [Google Scholar] [CrossRef]
- Shields, F.D. Jr.; Cooper, C.M. Riparian wetlands and flood stages. In Hydraulic Engineering; Cotroneo, G.V., Rumer, R.R., Eds.; American Society of Civil Engineers Publications: Reston, VA, USA, 1994; Volume 2, pp. 351–355. [Google Scholar]
- Williams, D.D.; Hynes, H.B.N. The ecology of temporary streams II: General remarks on temporary streams. Int. Rev. Ges. Hydrobio. 1977, 62, 53–61. [Google Scholar] [CrossRef]
- Wilde, S.A.; Steinbrenner, E.C.; Pierce, R.S.; Dosen, R.C.; Pronin, D.T. Influence of forest cover on the state of the ground water table. Soil Sci. Soc. Am. J. 1953, 17, 65–67. [Google Scholar] [CrossRef]
- Borg, H.; Stoneman, G.L.; Ward, C.G. The effect of logging and regeneration on groundwater, streamflow and stream salinity in the southern forest of Western Australia. J. Hydrol. 1987, 99, 253–270. [Google Scholar]
- Mulholland, P.J.; Helton, A.M.; Poole, G.C.; Hall, R.O., Jr.; Hamilton, S.K.; Peterson, B.J.; Tank, J.L.; Ashkenas, L.R.; Cooper, L.W.; Dahm, C.N.; et al. Stream denitrification across biomes and its response to anthropogenic nitrate loading. Nature 2008, 452, 202–205. [Google Scholar]
- Royer, T.V.; Tank, J.L.; David, M.D. Transport and fate of nitrate in headwater agricultural streams in Illinois. J. Environ. Qual. 2004, 33, 1296–1304. [Google Scholar] [CrossRef]
- Burt, T.; Pinay, G.; Sabater, S. Ecohydrology Bearings—Invited Commentary. What do we still need to know about the ecohydrology of riparian zones? Ecohydrology. 2010, 3, 373–377. [Google Scholar]
- Samani, J.M.V.; Kouwen, N. Stability and erosion in grassed channels. J. Hydraul. Eng.-ASCE 2002, 128, 40–45. [Google Scholar]
- Shields, D.F., Jr.; Smiley, P.C. Jr.; Cooper, C.M.; Borselli, L. Modifying erosion control structures for ecological benefits. J. Soil Water Conserv. 2007, 62, 157. [Google Scholar]
- Shields, F.D., Jr.; Smiley, P.C., Jr.; Cooper, C.M. Design and management of edge-of-field water control structures for ecological benefits. J. Soil Water Conserv. 2002, 57, 151–157. [Google Scholar]
- Smiley, P.C., Jr.; Knight, S.S.; Shields, F.D., Jr.; Cooper, C.M. Influence of gully erosion control on amphibian and reptile communities within riparian zones of channelized streams. Ecohydrology 2009, 2, 303–312. [Google Scholar] [CrossRef]
- Manley, S.W.; Kaminski, R.M.; Rodrigue, P.B.; Dewey, J.C.; Schoenholtz, S.H.; Gerard, P.D.; Reinecke, K.J. Soil and nutrient retention in winter-flooded rice fields with implications for watershed management. J. Soil Water Conserv. 2009, 64, 173–182. [Google Scholar] [CrossRef]
- Rosgen, D.L. River restoration utilizing natural stability concepts. Land Water 1994, 38, 6–41. [Google Scholar]
- Ward, A.; Mecklenburg, D.; Powell, G.E.; Brown, L.; Jayakaran, A. Two-Stage Channel Design Procedures. In Proceedings of the Self-Sustaining Solutions for Streams, Wetlands, and Watersheds Conference, 12–15 September 2004; American Society of Agricultural Engineers: St. Paul, MN, USA.
- Powell, G.E.; Ward, A.D.; Mecklenburg, D.E.; Jayakaran, A.D. Two-stage channel systems: Part 1, a practical approach for sizing agricultural ditches. J. Soil Water Conserv. 2007, 62, 277–286. [Google Scholar]
- Kröger, R.; Holland, M.M.; Moore, M.T.; Cooper, C.M. Plant senescence: a mechanism for nutrient release in temperate agricultural wetlands. Environ. Pollut. 2007, 146, 114–119. [Google Scholar] [CrossRef]
- Kröger, R.; Cooper, C.M.; Moore, M.T. A preliminary study of alternative controlled drainage strategy in surface drainage ditches: Low grade weirs. Agr. Water Manage. 2008, 95, 678–684. [Google Scholar] [CrossRef]
- Powell, K.L.; Bouchard, V. Is denitrification enhanced by the development of natural fluvial morphology in agricultural headwater ditches? J. N. Am. Benthol. Soc. 2010, 29, 761–772. [Google Scholar] [CrossRef]
- Roley, S.S.; Tank, J.L.; Stephen, M.L.; Johnson, L.T.; Beaulieu, J.J.; Witter, J.D. Floodplain restoration enhances denitrification and reach-scale nitrogen removal in an agricultural stream. Eco. Appl. 2012, 22, 281–297. [Google Scholar] [CrossRef]
- Roley, S.S.; Tank, J.L.; Williams, W.L. Hydrologic connectivity increases denitrification in the hyporheic zone and restored floodplains of an agricultural stream. J. Geophys. Res. 2012, 117, 16. [Google Scholar]
- Landwehr, K.; Rhoads, B.L. Depositional response of a headwater stream to channelization, east central Illinois, USA. River Res. Appl. 2003, 19, 77–100. [Google Scholar] [CrossRef]
- D’Ambrosio, J.L.; Ward, A.; Witter, J.D.; Tank, J.L. Ecological services of constructed two-stage agricultural ditches. In proceedings of 21st Century Watershed Technology Conference and Workshop Improving Water Quality and the Environment, Bari, Italy, 27 May–1 June 2012; p. 8.
- Kramer, G. Design, Construction, and Assessment of a self-sustaining drainage ditch. Master’s Thesis, University of Minnesota, Minneapolis, MN, USA, 2011. [Google Scholar]
- Janssen, J.R. Environmental and Management Influences on Fish and Invertebrate Communities in Agricultural Headwater Systems. Master’s Thesis, University of Michigan, Ann Arbor, MI, USA, 2008. [Google Scholar]
- Sharpley, A.N.; Krogstad, T.; Kleinman, P.J.A.; Haggard, B.E.; Shigaki, F.; Saporito, L. Managing Natural Processes in drainage ditches for non-point source phosphorus control. J. Soil Water Conserv. 2007, 62, 197–206. [Google Scholar]
- Shields, F.D. Jr.; Pezeshki, S.R.; Wilson, G.V.; Wu, W.; Dabney, S.M. Rehabilitation of an incised stream with plant materials: the dominance of geomorphic processes. Eco. Soc. 2008, 13, 54. [Google Scholar]
- Gilliam, J.W.; Skaggs, R.W. Controlled agricultural drainage to maintain water quality. J. Irrig. Drain. E. ASCE 1986, 112, 254–263. [Google Scholar] [CrossRef]
- Wesström, I.; Messing, I.; Linner, H.; Lindstrom, J. Controlled drainage—Effects on drain outflow and water quality. Agr. Water Manage. 2001, 47, 85–100. [Google Scholar] [CrossRef]
- Simon, A.; Darby, S.E. Effectiveness of grade-control structures in reducing erosion along incised river channels: the case of Hotophia Creek, Mississippi. Geomorphology 2002, 42, 229–254. [Google Scholar]
- Needelman, B.A.; Ruppert, D.E.; Vaughan, R.E. The role of ditch soil formation and redox biogeochemistry in mitigating nutrient and pollutant losses from agriculture. J. Soil Water Conserv. 2007, 62, 207–215. [Google Scholar]
- Needelman, B.A.; Kleinman, P.J.A.; Strock, J.S.; Allen, A.L. Improved management of agriculture drainage ditches for water quality protection: An overview. J. Soil Water Conserv. 2007, 62, 171–178. [Google Scholar]
- Woli, K.P.; David, M.B.; Cooke, R.A.; McIsaac, G.F.; Mitchell, C.A. Nitrogen balance in and export from agricultural fields associated with controlled drainage systems and denitrfying bioreactors. Eco. Eng. 2010, 36, 1558–1566. [Google Scholar] [CrossRef]
- Penn, C.J.; Bryant, R.B; Kleinman, P.J.A.; Allen, A.L. Removing dissolved phosphorus from drainage ditch water with phosphorus sorbing materials. J. Soil Water Conserv. 2007, 62, 269–276. [Google Scholar]
- Penn, C.J.; McGrath, J.M.; Bryant, R.B. Ditch Drainage Management for Water Quality Improvement. In Agricultural Drainage Ditches: Mitigation Wetlands for the 21st Century; Moore, M.T., Kröger, R., Eds.; Research Signpost: Kerala, India, 2010; pp. 151–173. [Google Scholar]
- Van der Hoven, S.J.; Fromm, N.J.; Peterson, E.W. Quantifying nitrogen cycling beneath a meander of a low gradient, N-impacted, agricultural stream using tracers and numerical modeling. Hydrol. Process. 2008, 22, 1206–1215. [Google Scholar]
- Boulton, A.J. Hyporheic rehabilitation in rivers: Restoring vertical connectivity. Freshwater Biol. 2007, 52, 632–659. [Google Scholar] [CrossRef]
- Peterson, E.W.; Benning, C. Factors influencing nitrate within a low-gradient agricultural stream. Environ. Earth Sci. 2012. [Google Scholar] [CrossRef]
- Wondzell, S.M.; LaNier, J.; Haggerty, R.; Woodsmith, R.D.; Edwards, R.T. Changes in hyporheic exchange flow following experimental wood removal in a small, low-gradient stream. Water Resour. Res. 2009. [Google Scholar] [CrossRef]
- Grimaldi, C.; Chaplot, V. Nitrate depletion during within-stream transport: Effects of exchange processes between streamwater, the hyporheic and riparian zones. Water Air Soil Poll. 2000, 124, 95–112. [Google Scholar] [CrossRef]
- Lefebvre, S.; Marmonier, P., Pinay; Bour, O.; Aqulina, L.; Baudy, J. Nutrient dynamics in interstitial low-order rural streams with different bedrock geology. Arch. Hydrol. 2005, 164, 169–191. [Google Scholar]
- Kasahara, T.; Hill, A.R. Effects of riffle–step restoration on hyporheic zone chemistry in N-rich lowland streams. Can. J. Fish. Aquat. Sci. 2006, 63, 120–133. [Google Scholar] [CrossRef]
- Kasahara, T.; Hill, A.R. Instream restoration: its effects on lateral stream-subsurface water exchange in urban and agricultural streams in southern Ontario. River Res. Appl. 2007, 23, 801–814. [Google Scholar] [CrossRef]
- Sawyer, A.H.; Cardenas, M.B.; Buttles, J. Hyporheic exchange due to channel-spanning logs. Water Resour. Res. 2011. [Google Scholar] [CrossRef]
- Borin, M.; Bonaiti, G.; Giardini, L. Controlled drainage and wetlands to reduce agricultural pollution: A lysimetric study. J. Environ. Qual. 2001, 30, 1330–1340. [Google Scholar] [CrossRef]
- Fausey, N.R. Drainage management for humid regions. Int. Agr. Eng. J. 2005, 14, 209–214. [Google Scholar]
- Groffman, P.M.; Dorsey, A.M.; Mayer, P.M. N processing within geomorphic structures in urban streams. J. N. Am. Benthol. Soc. 2005, 24, 613–625. [Google Scholar]
- Filoso, S.; Palmer, M.A. Assessing stream restoration effectiveness at reducing nitrogen export to downstream waters. Eco. Appl. 2011, 21, 1989–2006. [Google Scholar] [CrossRef]
- Lautz, L.K.; Fanelli, R.M. Seasonal biogeochemical hotspots in the streambed around restoration structures. Biogeochemistry 2008, 91, 85–104. [Google Scholar] [CrossRef]
- Robertson, W.D.; Merkley, L.C. In-stream bioreactor for agricultural nitrate treatment. J. Environ. Qual. 2009, 38, 230–237. [Google Scholar] [CrossRef]
- Lalonde, V.; Madramootoo, C.A.; Trenhold, L.; Broughton, R.S. Effects of controlled drainage on nitrate concentrations in subsurface drain discharge. Agr. Water Manage. 1996, 29, 187–199. [Google Scholar]
- Gilliam, J.W.; Skaggs, R.W.; Weed, S.B. Drainage control to diminish nitrate loss from agricultural fields. J. Environ. Qual. 1979, 8, 137–142. [Google Scholar]
- Evans, R.O.; Gilliam, J.W.; Skaggs, R.W. Controlled Drainage Management Guidelines For Improving Water Quality; Technical Report for Cooperative Extension Service: Raleigh, NC, USA, 1991; p. 16. [Google Scholar]
- Evans, R.O.; Skaggs, R.W.; Gilliam, J.W. Controlled versus conventional drainage effects on water quality. J. Irrig. Drain. E. ASCE 1995, 121, 271–276. [Google Scholar] [CrossRef]
- Kröger, R.; Moore, M.T.; Jerry, L.; Farris, J.L.; Gopalan, M. Evidence for the Use of Low-Grade Weirs in Drainage Ditches to Improve Nutrient Reductions from Agriculture. Water Air Soil Poll. 2011, 221, 223–234. [Google Scholar]
- Kröger, R.; Pierce, S.C.; Littlejohn, K.A.; Moore, M.T.; Farris, J.L. Decreasing nitrate-N loads to coastal ecosystems with innovative drainage management strategies in agricultural landscapes: An experimental approach. Agr. Water Manage. 2012, 103, 162–166. [Google Scholar]
- Wesström, I.; Messing, I. Effects of controlled drainage on N and P losses and N dynamics in a loamy sand with spring crops. Agr. Water Manage. 2007, 87, 229–240. [Google Scholar] [CrossRef]
- Ng, H.Y.F.; Tan, C.S.; Drury, C.F.; Gaynor, J.D. Controlled drainage and subirrigation influences tile nitrate loss and corn yields in a sandy loam soil in Southwestern Ontario. Agri. Ecosyst. Environ. 2001, 1758, 1–8. [Google Scholar]
- Tan, C.S.; Drury, C.F.; Soultani, M.; vanWesenbeeck, I.J.; Ng, H.Y.F.; Gaynor, J.D.; Welacky, T.W. Effect of controlled drainage and tillage on soil structure and tile drainage nitrate loss at the field scale. Water Sci. Technol. 1998, 38, 103–110. [Google Scholar]
- Drury, C.F.; Tan, C.S.; Reynolds, W.D.; Welacky, T.W.; Oloya, T.O.; Gaynor, J.D. Managing Tile Drainage, Subirrigation, and Nitrogen Fertilization to Enhance Crop Yields and Reduce Nitrate Loss. J. Environ. Qual. 2009, 38, 1193–1204. [Google Scholar]
- Bastienė, N.; Šaulienė. The impact of controlled drainage on water quality. Research for Rural Development 2009. In Proceedings of Annual 15th International Scientific Conference, Jelgava, Latvia, 20–22 May 2009; pp. 271–278.
- Pierce, S.C.; Kröger, R. Low-grade weirs in agricultural ditches for sediment retention and nutrient reduction create in-stream wetlands. Wetland Sci. Pract. 2011, 28, 33–39. [Google Scholar]
- Blackwell, M.S.A.; Pilgrim, E.S. Ecosystem services delivered by small-scale wetlands. Hydrol. Sci. J. 2011, 56, 1467–1484. [Google Scholar]
- Hunt, P.G.; Stone, K.C.; Humenik, F.J.; Matheny, T.A.; Johnson, M.H. In-stream wetland mitigation of nitrogen contamination in a USA coastal plain stream. J. Environ. Qual. 1999, 28, 249–256. [Google Scholar]
- O’Geen, A.T.; Maynard, J.J.; Dahlgren, R.A. Efficacy of constructed wetlands to mitigate non-point source pollution from irrigation tailwaters in the San Joaquin Valley, California, USA. Water Sci. Technol. 2007, 55, 55–61. [Google Scholar]
- Tanner, C.C.; Nguyen, M.L.; Sukias, J.P.S. Nutrient removal by a constructed wetland treating subsurface drainage from grazed dairy pasture. Agri. Ecosyst. Environ. 2005, 105, 145–162. [Google Scholar]
- Sukias, J.; Tanner, C. Surface flow constructed wetland as a drainage management tool—long term performance. In Adding to the Knowledge Base for the Nutrient Manager, Fertilizer & Lime Research Centre, Occasional Report No. 24; Currie, L.D., Christensen, C.L., Eds.; Massey University: Palmerston North, New Zealand, 2011; pp. 1–16. [Google Scholar]
- Koskiaho, J.; Ekholm, P.; Räty, M.; Riihimäki, J.; Puustinen, M. Retaining agricultural nutrients in constructed wetlands—Experiences under boreal conditions. Eco. Eng. 2003, 20, 89–103. [Google Scholar]
- Kovacic, D.A.; Twait, R.M.; Wallace, M.P.; Bowling, J.M. Use of created wetlands to improve water quality in the Midwest—Lake Bloomington case study. Eco. Eng. 2006, 28, 258–270. [Google Scholar]
- Braskerud, B.C. Factors affecting nitrogen retention in small constructed wetlands treating agricultural non-point source pollution. Eco. Eng. 2002, 18, 351–370. [Google Scholar]
- Braskerud, B.C. Factors affecting phosphorus retention in small constructed wetlands treating agricultural non-point source pollution. Eco. Eng. 2002, 19, 41–61. [Google Scholar]
- Tomer, M.; Tanner, C.; Howard-Williams, C. Discussing wetlands, agriculture, and ecosystem services. Wetland Sci. Pract. 2009, 26, 26–29. [Google Scholar]
- Pierce, S.C.; Kröger, R.; Prevost, D.; Poganski, B.; Flora, C.; Pierce, T. Field-scale monitoring of agricultural ditches as conduits of nitrogen, phosphorus, and suspended sediment in response to storm events and low-input drainage management: A case-study of the Tchula Lake Farm. In Proceedings of the Mississippi Water Resources Conference, Jackson, MS, USA, 3–5 April 2012.
- Shields, F.D., Jr.; Knight, S.S.; Cooper, C.M. Can warmwater streams be rehabilitated using watershed-scale standard erosion control measures alone? Environ. Manage. 2007, 40, 62–79. [Google Scholar]
- Litvan, M.E.; Stewart, T.W.; Pierce, C.L.; Larson, C.J. Effects of grade control structures on the macroinvertebrate assemblage of an agriculturally-impacted stream. River Res. Appl. 2008, 24, 218–233. [Google Scholar]
- Litvan, M.E.; Stewart, T.W.; Pierce, C.L.; Larson, C.J. Fish Assemblages in a Western Iowa Stream Modified by Grade Control Structures. N. Am. J. Fish. Manage. 2008, 28, 1398–1413. [Google Scholar]
- Santucci, V.J., Jr.; Gephard, S.R.; Pescitelli, S.M. Effects of Multiple Low-Head Dams on Fish, Macroinvertebrates, Habitat, and Water Quality in the Fox River, Illinois. N. Am. J. Fish. Manage. 2005, 25, 975–992. [Google Scholar]
- Swift, M.J.; Izac, A.M.N.; van Noordwijk, M. Biodiversity and ecosystem services in agricultural landscapes—Are we asking the right questions? Agri. Ecosys. Environ. 2004, 104, 113–134. [Google Scholar]
- MacArthur, R.H. Fluctuations of animal populations and a measure of community stability. Ecology 1955, 36, 533–536. [Google Scholar]
- Griffin, J.N.; O’Gorman, E.J.; Emmerson, M.C.; Jenkins, S.R.; Klein, A.M.; Loreau, M.; Symstad, A. Biodiversity and the stability of ecosystem functioning. In Biodiversity, Ecosystem Functioning, and Human Wellbeing—An Ecological and Economic Perspective; Naeem, S., Bunker, D.E., Hector, A., Loreau, M., Perrings, C., Eds.; Oxford University Press: New York, NY, USA, 2009; pp. 78–93. [Google Scholar]
- Doak, D.F.; Bigger, D.; Harding, E.K.; Marvier, M.A.; O’Malley, R.E.; Thomson, D. The statistical inevitability of stability–diversity relationships in community ecology. Am. Nat. 1998, 151, 264–276. [Google Scholar]
- Vandewalle, M.; de Bello, F.; Berg, M.P.; Bolger, T.; Dolédec, S.; Dubs, F.; Feld, C.K.; Harrington, R.; Harrison, P.A.; Lavorel, S.; et al. Functional traits as indicators of biodiversity response to land use changes across ecosystems and organisms. Biodivers. Conserv. 2010, 19, 2921–2947. [Google Scholar] [Green Version]
- MacArthur, R.H.; MacArthur, J.W. On bird species diversity. Ecology 1961, 42, 594–598. [Google Scholar]
- Armitage, P.D.; Szoszkiewicz, K.; Blackburn, J.H.; Nesbitt, I. Ditch communities: A major contributor to floodplain biodiversity. Aqu. Conserv. Mar. Freshwater Ecosyst. 2003, 13, 165–185. [Google Scholar]
- Hanski, I. Metapopulation Ecology; Oxford University Press: Oxford, UK, 1998. [Google Scholar]
- Knapp, C.W.; Dodds, W.K.; Wilson, K.C.; O’Brien, J.M.; Graham, D.W. Spatial heterogeneity of denitrification genes in a highly homogenous urban stream. Environ. Sci. Technol. 2009, 43, 4273–4279. [Google Scholar]
- Palmer, M.A.; Menninger, H.L.; Bernhardt, E. River restoration, habitat heterogeneity and biodiversity: a failure of theory or practice? Freshwater Biol. 2010, 55, 205–222. [Google Scholar]
- Dimitrakopoulous, P.G.; Schmid, B. Biodiversity effects increase linearly with biotope space. Eco. Lett. 2004, 7, 574–583. [Google Scholar]
- Pedersen, T.C.M.; Baattrup-Pedersen, A.; Madsen, T.V. Effects of stream restoration and management on plant communities in lowland streams. Freshwater Biol. 2006, 51, 161–179. [Google Scholar]
- Vivian-Smith, G. Microtopographic heterogeneity and floristic diversity in experimental wetland communities. J. Ecol. 1997, 85, 71–82. [Google Scholar]
- Lundholm, J.T. Plant Species diversity and environmental heterogeneity: Spatial scale and competing hypotheses. J. Veg. Sci. 2009, 20, 377–391. [Google Scholar]
- Pezeshki, S.R. Wetland plant responses to soil flooding. Environ. Exp. Bot. 2001, 46, 299–312. [Google Scholar]
- Miller, R.C.; Zedler, J.B. Responses of native and invasive wetland plants to hydroperiod and water depth. Plant Ecol. 2003, 167, 57–69. [Google Scholar]
- Fraser, L.H.; Karnezis, J.P. A comparative assessment of seedling survival and biomass accumulation for fourteen wetland plant species grown under minor water-depth differences. Wetlands 2005, 25, 520–530. [Google Scholar] [CrossRef]
- Franklin, P.; Dunbar, M.; Whitehead, P. Flow controls on lowland river macrophytes: A review. Scie. Total Environ. 2008, 400, 369–378. [Google Scholar] [CrossRef]
- Bornette, G.; Puijalon, S. Response of aquatic plants to abiotic factors: A review. Aquat. Sci. 2011, 73, 1–14. [Google Scholar] [CrossRef]
- Lorenz, A.W.; Korte, T.; Sundermann, A.; Januschke, K.; Haase, P. Macrophytes respond to reach-scale river restorations. J. Appl. Ecol. 2012, 49, 202–212. [Google Scholar] [CrossRef]
- Davies, B.; Biggs, J.; Williams, P.; Whitfield, M.; Nicolet, P.; Sear, D.; Bray, S.; Maund, S. Comparative biodiversity of aquatic habitats in the European agricultural landscape. Agri. Ecosysts. Environ. 2008, 125, 1–8. [Google Scholar] [CrossRef]
- Davies, B.R.; Biggs, J.; Williams, P.J.; Lee, J.T.; Thompson, S. A comparison of the catchment sizes of rivers, streams, ponds, ditches and lakes: implications for protecting aquatic biodiversity in an agricultural landscape. Hydrobiologia. 2008, 597, 7–17. [Google Scholar] [CrossRef]
- de Snoo, G.R.; Naus, N.; Verhulst, J.; van Ruijven, J.; Schaffers, A.P. Long-term changes in plant diversity of grasslands under agricultural and conservation management. Appl. Veg. Sci. 2012, 15, 299–306. [Google Scholar]
- Leng, X.; Musters, C.J.M.; de Snoo, G.R. Restoration of plant diversity on ditch banks: Seed and site limitation in response to agri-environment schemes. Biol. Conserv. 2009, 142, 1340–1349. [Google Scholar] [CrossRef]
- Geertsema, W.; Opdam, P.; Kropff, M.J. Plant strategies and agricultural landscapes: Survival in spatially and temporally fragmented habitat. Landscape Ecol. 2002, 17, 263–279. [Google Scholar] [CrossRef]
- Milsom, T.P.; Sherwood, A.J.; Rose, S.C.; Town, S.J.; Runham, S.R. Dynamics and management of plant communities in ditches bordering arable fenland in eastern England. Agri. Ecosys. Environ. 2004, 103, 85–99. [Google Scholar] [CrossRef]
- Biggs, J.; Williams, P.; Whitfield, M.; Nicolet, P.; Brown, C.; Hollis, J.; Arnold, D.; Pepper, T. The freshwater biota of British agricultural landscapes and their sensitivity to pesticides. Agri. Ecosyst. Environ. 2007, 122, 137–148. [Google Scholar] [CrossRef]
- Beltman, B. Effects of weed control on species composition of aquatic plants and bank plants and macrofauna in ditches. Hydrol. Bull. 1987, 21, 171–179. [Google Scholar] [CrossRef]
- Best, E.P.H. The impact of mechanical harvesting regimes on the species composition of Dutch ditch vegetation: A quantitative approach. J. Aquat. Plant Manage. 1993, 31, 148–154. [Google Scholar]
- Blomqvist, M.M.; Tamis, W.L.M.; Bakker, J.P.; Van der Meijden, E. Seed and (micro) site limitation in ditch banks: Germination, establishment and survival under different management regimes. J. Nat. Conserv. 2006, 14, 16–33. [Google Scholar] [CrossRef]
- Geertsema, W.; Sprangers, J.T.C.M. Plant distribution patterns related to species characteristics and spatial and temporal habitat heterogeneity in a network of ditch banks. Plant Ecol. 2002, 162, 91–108. [Google Scholar] [CrossRef]
- van Zuidam, J.P.; Raaphorst, E.P.; Peeters, E.T.H.M. The role of propagule banks from drainage ditches dominated by free-floating or submerged plants in vegetation restoration. Restor. Ecol. 2012, 20, 416–425. [Google Scholar] [CrossRef]
- Mountford, J.O. The vegetation of artificial drainage channels within grazing marshes in the UK: How does its composition correspond with described communities? Biol. Environ. 2006, 106, 277–286. [Google Scholar] [CrossRef]
- Leng, X.; Musters, C.J.M.; de Snoo, G.R. Spatial variation in ditch bank plant species composition at the regional level: the role of environment and dispersal. J. Veg. Sci. 2010, 21, 868–875. [Google Scholar] [CrossRef]
- Lenssen, J.; Menting, F.; van der Putten, W.; Blom, K. Control of plant species richness and zonation of functional groups along a freshwater flooding gradient. OIKOS 1999, 86, 523–534. [Google Scholar] [CrossRef]
- Silvertown, J.; Dodd, M.E.; Gowing, D.J.G.; Mountford, J.O. Hydrologically defined niches reveal a basis for species richness in plant communities. Nature 1999, 400, 61–63. [Google Scholar] [CrossRef]
- Casanova, M.T.; Brock, M.A. How do depth, duration and frequency of flooding influence the establishment of wetland plant communities. Plant Ecol. 2000, 147, 237–250. [Google Scholar] [CrossRef]
- Best, E.P.H.; van der Schaaf, S.; Oomes, M.J.M. Responses of restored grassland ditch vegetation to hydrological changes, 1989–1992. Plant Ecol. 1995, 116, 107–122. [Google Scholar] [CrossRef]
- Pezeshki, S.R.; Anderson, P.H.; Shields, F.D., Jr. Effects of soil moisture regimes on growth and survival of black willow (Salix nigra) posts (cuttings). Wetlands 1998, 18, 460–470. [Google Scholar] [CrossRef]
- Li, S.; Pezeshki, S.R.; Goodwin, S. Effects of soil moisture regimes on photosynthesis and growth in cattail (Typha. latifolia). Acta Oecol. 2004, 25, 17–22. [Google Scholar]
- Pezeshki, S.R.; Shields, F.D., Jr. Black willow cutting survival in streambank plantings, southeastern United States. J. Am. Water Resour. A. 2006, 42, 191–200. [Google Scholar]
- Twisk, W.; Noordervliet, M.A.W.; ter Keurs, W.J. The nature value of the ditch vegetation in peat areas in relation to farm management. Aquat. Ecol. 2003, 37, 191–209. [Google Scholar] [CrossRef]
- Madsen, R.V.; Chambers, P.A.; James, W.F.; Koch, E.W.; Westlake, D.F. The interaction between water movement, sediment dynamics and submersed macrophytes. Hydrobiologia. 2001, 444, 71–84. [Google Scholar] [CrossRef]
- Schaller, J.L.; Royer, T.V.; David, M.B.; Tank, J.L. Denitrification associated with plants and sediments in an agricultural stream. J. N. Am. Benthol. Soc. 2004, 23, 667–676. [Google Scholar] [CrossRef]
- Janse, J.H.; van Puijenbroek, P.J.T.M. Effects of eutrophication in drainage ditches. Environ. Pollut. 1998, 102, 547–552. [Google Scholar]
- Kočić, A.; Hengl, T.; Horvatić, J. Water nutrient concentrations in channels in relation to occurrence of aquatic plants: a case study in eastern Croatia. Hydrobiologia 2008, 603, 253–266. [Google Scholar] [CrossRef]
- Goulder, R. Conservation of aquatic plants in artificial watercourses: are main drains a substitute for vulnerable navigation canals? Aqu. Conserv. Mar. Freshwater Ecosyst. 2008, 18, 163–174. [Google Scholar] [CrossRef]
- Syzmura, M.; Syzmura, T.; Dunajski, A.; Wolski, K. Grasses (Poaceae.) in riparian vegetation of watercourses in agriculture landscape. Pol. J. Environ. Stud. 2009, 18, 1217–1223. [Google Scholar]
- Lu, T.; Keming, M.A.; Bojie, F.U.; Jieyu, Z.; Lu, Q.; Hudson, S. Diversity and composition of wetland communities along an agricultural drainage ditch density gradient. Polish J. Ecol. 2009, 57, 113–123. [Google Scholar]
- Pywell, R.F.; Bullock, J.M.; Roy, D.B.; Warman, L.; Walker, K.J.; Rothery, P. Plant traits as predictors of performance in ecological restoration. J. Appl. Ecol. 2003, 40, 65–77. [Google Scholar] [CrossRef]
- Boutin, C.; Keddy, P.A. A functional classification of wetland plants. J. Veg. Sci. 1993, 4, 591–600. [Google Scholar] [CrossRef]
- Ervin, G.N. Spatio-temporally variable effects of a dominant macrophyte on vascular plant neighbors. Wetlands 2005, 25, 317–325. [Google Scholar] [CrossRef]
- Pierce, S.C.; Pezeshki, S.R. Vegetation in agricultural ditches: limitations to establishment, productivity, and ecosystem functioning. In Agricultural Drainage Ditches: Mitigation Wetlands of the 21st Century; Moore, M.T., Kröger, R., Eds.; Research Signpost: Trivandrum, India, 2010; pp. 75–106. [Google Scholar]
- Shupryt, M.P.; Stelzer, R.S. Macrophyte beds contribute disproportionately to benthic invertebrate abundance and biomass in a sand plains stream. Hydrobiologia. 2009, 632, 329–339. [Google Scholar]
- Pederson, M.L.; Friberg, N. Influence of disturbance on habitats and biological communities in lowland streams. Fundam. Appl. Limnol. 2009, 174, 27–41. [Google Scholar]
- Brix, H. Do macrophytes play a role in constructed treatment wetlands? Water Sci. Technol. 1997, 35, 11–17. [Google Scholar]
- Toet, S.; Huibers, L.H.F.A.; Logtestijn, R.S.P.V.L.; Verhoeven, J.T.A. Denitrification in the periphyton associated with plant shoots and in the sediment of a wetland system supplied with sewage treatment plant effluent. Hydrobiologia 2003, 501, 29–44. [Google Scholar]
- Wu, Q.T.; Gao, T.; Zeng, S.; Chua, H. Plant biofilm oxidation ditch for in situ treatment of polluted waters. Eco. Eng. 2006, 28, 124–130. [Google Scholar]
- Thomaz, S.M.; da Cunha, E.R. The role of macrophytes in habitat structuring in aquatic ecosystems: methods of measurement, causes and consequences on animal assemblages’ composition and biodiversity. Acta. Limnol. Bras. 2010, 22, 218–236. [Google Scholar]
- Dibble, E.D. Use of fractal dimension to assess habitat complexity and its influence on dominant invertebrates inhabiting tropical and temperate macrophytes. J. Freshwater Ecol. 2009, 24, 93,102. [Google Scholar]
- Weisner, S.E.B.; Thiere, G. Effects of vegetation state on biodiversity and nitrogen retention in created wetlands: A test of the biodiversity–ecosystem functioning hypothesis. Freshwater Biol. 2010, 55, 387–396. [Google Scholar]
- Read, J.; Wevill, T.; Fletcher, T.; Deletic, A. Variation among plant species in pollutant removal from stormwater in biofiltration systems. Water Res. 2008, 42, 893–902. [Google Scholar]
- Srivastava, J.; Gupta, A.; Chandra, H. Managing water quality with aquatic macrophytes. Rev. Environ. Sci. Biotechnol. 2008, 7, 255–266. [Google Scholar]
- Brisson, J.; Chazarenc, F. Maximizing pollutant removal in constructed wetlands: Should we pay more attention to macrophyte species selection? Sci. Total Environ. 2009, 407, 3923–3930. [Google Scholar]
- Vymazal, J. Plants used in constructed wetlands with horizontal subsurface flow: A review. Hydrobiologia 2011, 674, 133–156. [Google Scholar]
- Sand-Jensen, K. Influence of submerged macrophytes on sediment composition and near-bed flow in lowland streams. Freshwater Biol. 1998, 39, 663–679. [Google Scholar]
- Kröger, R.; Moore, M.T.; Locke, M.A.; Cullum, R.F.; Steinriede, R.W., Jr.; Testa, S., III; Bryant, C.T.; Cooper, C.M. Evaluating the influence of wetland vegetation on chemical residence time in Mississippi Delta drainage ditches. Agr. Water Manage. 2009, 96, 1175–1179. [Google Scholar]
- Asaeda, T.; Rajapakse, L.; Kanoh, M. Fine sediment retention as affected by annual shoot collapse: Sparganium. erectum as an ecosystem engineer in a lowland stream. River Res. Appl. 2010, 26, 1153–1169. [Google Scholar]
- Braskerud, B.C. The influence of vegetation on sedimentation and resuspension of soil particles in small constructed wetlands. J. Environ. Qual. 2001, 30, 1447–1457. [Google Scholar] [CrossRef]
- Stringfellow, W.; Graham, J.; Rogers, M.; Borglin, S.; Brunell, M.; Hanlon, J.; Spier, C.; Nguyen, K. Water quality changes occurring in agricultural drains of varying riparian function. In Agricultural Drainage Ditches: Mitigation Wetlands for the 21st Century; Moore, M.T., Kröger, R., Eds.; Research Signpost: Kerala, India, 2010; pp. 173–194. [Google Scholar]
- Horppila, J.; Nurminen, L. The effect of an emergent macrophyte (Typha. augustifolia) on sediment resuspension in a shallow north temperate lake. Freshwater Biol. 2001, 46, 1447–1455. [Google Scholar]
- Shields, F.D. Jr.; Bowie, A.J.; Cooper, C.M. Control of streambank erosion due to bed degradation with vegetation and structure. Water Resour. Bull. 1995, 31, 475–489. [Google Scholar]
- Blom, C.W.P.M. Adaptations to flooding stress: From plant community to molecule. Plant Biol. 1999, 1, 261–273. [Google Scholar] [CrossRef]
- Moore, M.T.; Kröger, R.; Locke, M.A.; Cullum, R.F.; Steinriede, R.W., Jr.; Testa, S., III; Lizotte, R.E., Jr.; Bryant, C.T.; Cooper, C.M. Nutrient mitigation capacity in Mississippi Delta, USA drainage ditches. Environ. Pollut. 2010, 158, 175–184. [Google Scholar]
- Jiang, C.; Fan, X.; Cui, G.; Zhang, Y. Removal of agricultural non-point source pollutants by ditch wetlands: implications for lake eutrophication control. Hydrobiologia 2007, 581, 319–327. [Google Scholar] [CrossRef]
- Güsewell, S.; Koerselman, W. Variation in nitrogen and phosphorus concentrations of wetland plants. Perspect. Plant. Ecol. 2002, 5, 37–61. [Google Scholar] [CrossRef]
- Shields, F.D., Jr.; Cooper, C.M.; Testa, S., III; Ursic, M.E. Nutrient Transport in the Yazoo River Basin; U.S. Department of Agriculture, Agricultural Research Service, Water Quality, Ecology Research Unit, National Sedimentation Laboratory: Oxford, MS, USA, 2008. [Google Scholar]
- DeBusk, T.A.; Peterson, J.E.; Reddy, K.R.; Graetz, D.A.; Clough, K.S. Optimization of the vegetative uptake of phosphorus from dairy wastewater. Technical Report for South Florida Water Management District: West Palm Beach, FL, 1989; p. 250, No.88–009–0625. [Google Scholar]
- Barko, J.W.; Gunnison, D.; Carpenter, S.R. Sediment interactions with submersed macrophyte growth and community dynamics. Aquat. Bot. 1991, 41, 41–65. [Google Scholar]
- Chen, R.L.; Barko, J.W. Effects of freshwater macrophytes on sediment chemistry. J. Freshwater Ecol. 1988, 4, 279–289. [Google Scholar]
- Jeperson, D.N.; Sorrell, B.K.; Brix, H. Growth and root oxygen release by Typha latifolia and its effects on sediment methanogenesis. Aquat. Bot. 1998, 61, 165–180. [Google Scholar]
- Neuman, G.; Römhel, V. Root-induced changes in the availability of nutrients in the rhizosphere. In Plant Roots: The Hidden Half, 3rd; Waisel, Y., Eshel, A., Kafkafi, U., Eds.; Marcel Dekker: New York, NY, USA, 2002; pp. 617–649. [Google Scholar]
- Ehrenfeld, J.G.; Ravit, B.; Elgersma, K. Feedback in the plant-soil system. Annu. Rev. Environ. Resour. 2005, 30, 75–115. [Google Scholar]
- Rubio, G.; Oesterheld, M.; Alvarez, C.R.; Lavado, R.S. Mechanisms for the increase in phosphorus uptake of water-logged plants: Soil phosphorus availability, root morphology and uptake kinetics. Oecologia 1997, 112, 150–155. [Google Scholar]
- Pierce, S.C.; Moore, M.T.; Larsen, D.; Pezeshki, S.R. Macronutrient (N,P,K) and redoximorphic metal (Fe, Mn) allocation in Leersia. oryzoides (Rice cutgrass) grown under different flood regimes. Water Air Soil Poll. 2010, 207, 73–84. [Google Scholar]
- Bostic, E.M.; White, J.R. Soil phosphorus and vegetation influence on wetland phosphorus release after simulated drought. Soil Sci. Soc. Am. J. 2007, 71, 238–244. [Google Scholar]
- Richardson, C.J. Mechanisms controlling phosphorus retention capacity in freshwater wetlands. Science 1985, 228, 1424–1427. [Google Scholar]
- Smith, D.R.; Pappas, E.A. Effect of ditch dredging on the fate of nutrients in deep drainage ditches of the Midwestern United States. J. Soil Water Conserv. 2007, 62, 252–261. [Google Scholar]
- Smith, D.R.; Huang, C. Assessing nutrient transport following dredging of agricultural drainage ditches. Trans. ASABE 2010, 53, 429–436. [Google Scholar]
- Arango, C.P.; Tank, J.L.; Schaller, J.L.; Royer, T.V.; Bernot, M.J.; David, M.B. Benthic organic carbon influences denitrification in streams with high nitrate concentration. Freshwater Biol. 2007, 52, 1210–1222. [Google Scholar]
- Forshay, K.J.; Dodson, S.I. Macrophyte presence is an indicator of enhanced denitrification and nitrification in sediments of a temperate restored agricultural stream. Hydrobiologia. 2011, 668, 21–34. [Google Scholar]
- Ullah, S.; Faulkner, S.P. Denitrification potential of different land-use types in an agricultural watershed, lower Mississippi valley. Eco. Eng. 2006, 28, 131–140. [Google Scholar] [Green Version]
- Pierce, S.C.; Pezeshki, S.R.; Larsen, D.; Moore, M.T. Hydrology and species-specific effects of Bacopa. monnieri and Leersia. oryzoides on soil and water chemistry. Ecohydrology 2009, 2, 279–286. [Google Scholar]
- Veraart, A.J.; de Bruijne, W.J.J.; de Klein, J.J.M.; Peeters, E.T.H.M.; Scheffer, M. Effects of aquatic vegetation type on denitrification. Biogeochemistry 2011, 104, 267–274. [Google Scholar]
- DeBusk, T.A.; Peterson, J.E.; Reddy, K.R. Use of aquatic and terrestrial plants for removing phosphorus from dairy wastewaters. Eco. Eng. 1995, 5, 371–390. [Google Scholar]
- Power, M.E.; Rainey, W.E.; Parker, M.S.; Sabo, J.L.; Smyth, A.; Khandwala, S.; Finlay, J.C.; McNeely, F.C.; Marsee, K.; Anderson, C. River to watershed subsidies in an old-growth conifer forest. In Food Webs at the Landscape Level; Polis, G.A., Power, M.E., Huxel, G., Eds.; University of Chicago Press: Chicago, IL, USA, 2004; pp. 217–240. [Google Scholar]
- Jackson, J.K.; Fisher, S.G. Secondary production, emergence, and export of aquatic insects of a Sonoran Desert stream. Ecology 1986, 67, 629–638. [Google Scholar]
- Richardson, J.S.; Zhang, Y.; Marczak, L.B. Resource Subsides across the land-freshwater interface and responses in recipient communities. River Res. Appl. 2010, 26, 55–66. [Google Scholar]
- Nakano, S.; Murakami, M. Reciprocal subsides: Dynamic interdependence between terrestrial and aquatic food webs. Proc. Nat. Acad. Sci. USA 2001, 98, 166–170. [Google Scholar]
- Gratton, C.; Vander Zanden, M.J. Flux of aquatic insect productivity to land: comparison of lentic and lotic ecosystems. Ecology 2009, 90, 2689–2699. [Google Scholar]
- Lamberti, G.A.; Chaloner, D.T.; Hershey, A.E. Linkages among aquatic ecosystems. J. N. Am. Benthol. Soc. 2010, 29, 245–263. [Google Scholar]
- Woods, H.A.; Fagan, W.F.; Elser, J.J.; Harrison, J.F. Allometric and phylogenetic variation in insect phosphorus content. Funct. Ecol. 2004, 18, 103–109. [Google Scholar]
- Fagan, W.F.; Siemann, E.; Mitter, C.; Denno, R.F.; Huberty, A.F.; Woods, H.A.; Elser, J.J. Nitrogen in insects: Implications for trophic complexity and species diversification. Am. Nat. 2002, 160, 784–802. [Google Scholar]
- Werner, I.; Markiewicz, D.A.; Goding, K.; Reece, K. Benthic macroinvertebrate communities in ephemeral agricultural drainage ditches of California’s Central Valley. In Agricultural Drainage Ditches: Mitigation Wetlands for the 21st Century; Moore, M.T., Kröger, R., Eds.; Research Signpost: Kerala, India, 2010; pp. 1–15. [Google Scholar]
- Blackburn, M.; Mazzacano, C. Using Aquatic Macroinvertebrates As Indicators of Streamflow Duration: Washington and Idaho Indicators; The Xerces Society for Invertebrate Conservation: Portland, OR, USA, 2012. [Google Scholar]
- Rabini, C.F.; Wallace, G.S. The influence of flow variation on the ability to evaluate the biological health of headwater streams. Hydrology, Water Resources and Ecology in Headwaters. In Proceedings of the HeadWater 1998 Conference, Meran/Merano, Italy, 20–23 April 1998.
- Williams, D.D.; Hynes, H.B.N. The ecology of temporary streams I: The faunas of two Canadian streams. Int. Rev. Ges. Hydrobio. 1976, 61, 761–787. [Google Scholar]
- Feldman, D.L.; Farris, J.L.; Moore, M.T.; Cooper, C.M. A characterization of benthic macroinvertebrate communities in agricultural drainage ditches of the northeast Arkansas Delta, USA. In Agricultural Drainage Ditches: Mitigation Wetlands for the 21st Century; Moore, M.T., Kroger, R., Eds.; Research Signpost: Kerala, India, 2010; pp. 17–35. [Google Scholar]
- Shieh, S.; Ward, J.V.; Kondratieff, B.C. Energy flow through macroinvertebrates in a polluted plains stream. J. N. Am. Benthol. Soc. 2002, 21, 660–675. [Google Scholar]
- Verdonschot, R.C.M.; Keizer-Vlek, H.E.; Verdonschot, P.F.M. Biodiversity value of agricultural drainage ditches: a comparative analysis of the aquatic invertebrate fauna of ditches and small lakes. Aquat. Conserv. Mar. Freshwater Ecosys. 2011, 21, 715–727. [Google Scholar]
- Seale, D. Influence of amphibian larvae on primary production, nutrient flux, and competition in a pond ecosystem. Ecology 1980, 61, 1531–1550. [Google Scholar]
- Regester, K.J.; Whiles, M.R.; Lips, K.R. Variation in the trophic basis of production and energy flow associated with emergence of larval salamander assemblages from forest ponds. Freshwater Biol. 2008, 53, 1754–1767. [Google Scholar]
- Regester, K.J.; Lips, K.R.; Whiles, M.R. Energy flow and subsidies associated with the complex life cycle of ambystomatid salamanders in ponds and adjacent forest in southern Illinois. Oceologia 2006, 147, 303–314. [Google Scholar]
- Relyea, R.A. The impact of insecticides and herbicides on the biodiversity and productivity of aquatic communities. Eco. Appl. 2005, 15, 618–627. [Google Scholar]
- Manna, R.M; Hyne, R.V.; Choung, C.B.; Wilson, S.P. Amphibians and agricultural chemicals: Review of the risks in a complex environment. Environ. Pollut. 2009, 157, 2903–2927. [Google Scholar]
- Vanni, M.J. Nutrient cycling by animals in freshwater ecosystems. Annu. Rev. Ecol. Syst. 2002, 33, 341–370. [Google Scholar]
- Small, G.E.; Helton, A.M.; Kazanci, C. Can consumer stoichiometric regulation control nutrient spiraling in streams? J. N. Am. Benthol. Soc. 2009, 28, 747–765. [Google Scholar]
- Nahlik, A.M.; Kentula, M.E.; Fennessy, M.S.; Landers, D.H. Where is the consensus? A proposed foundation for moving ecosystem service concepts into practice. Eco. Econ. 2012, 77, 27–35. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Pierce, S.C.; Kröger, R.; Pezeshki, R. Managing Artificially Drained Low-Gradient Agricultural Headwaters for Enhanced Ecosystem Functions . Biology 2012, 1, 794-856. https://doi.org/10.3390/biology1030794
Pierce SC, Kröger R, Pezeshki R. Managing Artificially Drained Low-Gradient Agricultural Headwaters for Enhanced Ecosystem Functions . Biology. 2012; 1(3):794-856. https://doi.org/10.3390/biology1030794
Chicago/Turabian StylePierce, Samuel C., Robert Kröger, and Reza Pezeshki. 2012. "Managing Artificially Drained Low-Gradient Agricultural Headwaters for Enhanced Ecosystem Functions " Biology 1, no. 3: 794-856. https://doi.org/10.3390/biology1030794




