Compromised N-Glycosylation Processing of Kv3.1b Correlates with Perturbed Motor Neuron Structure and Locomotor Activity
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Construction of NB_1 Cells with Knockout of GnT-I Stably Expressing Various Forms of Kv3.1b
2.2. Zebrafish
2.3. Kv3.1b Recombinant Vectors
2.4. Confocal Microscopy
2.5. Motor Activity of Kv3.1b Expressing Fish
2.6. Total Membrane Purification and Glycosidase Treatment
2.7. Western Blotting Assay
2.8. TIRF Microscopy
2.9. Whole Cell Recordings and Analysis
2.10. Data Analysis
3. Results
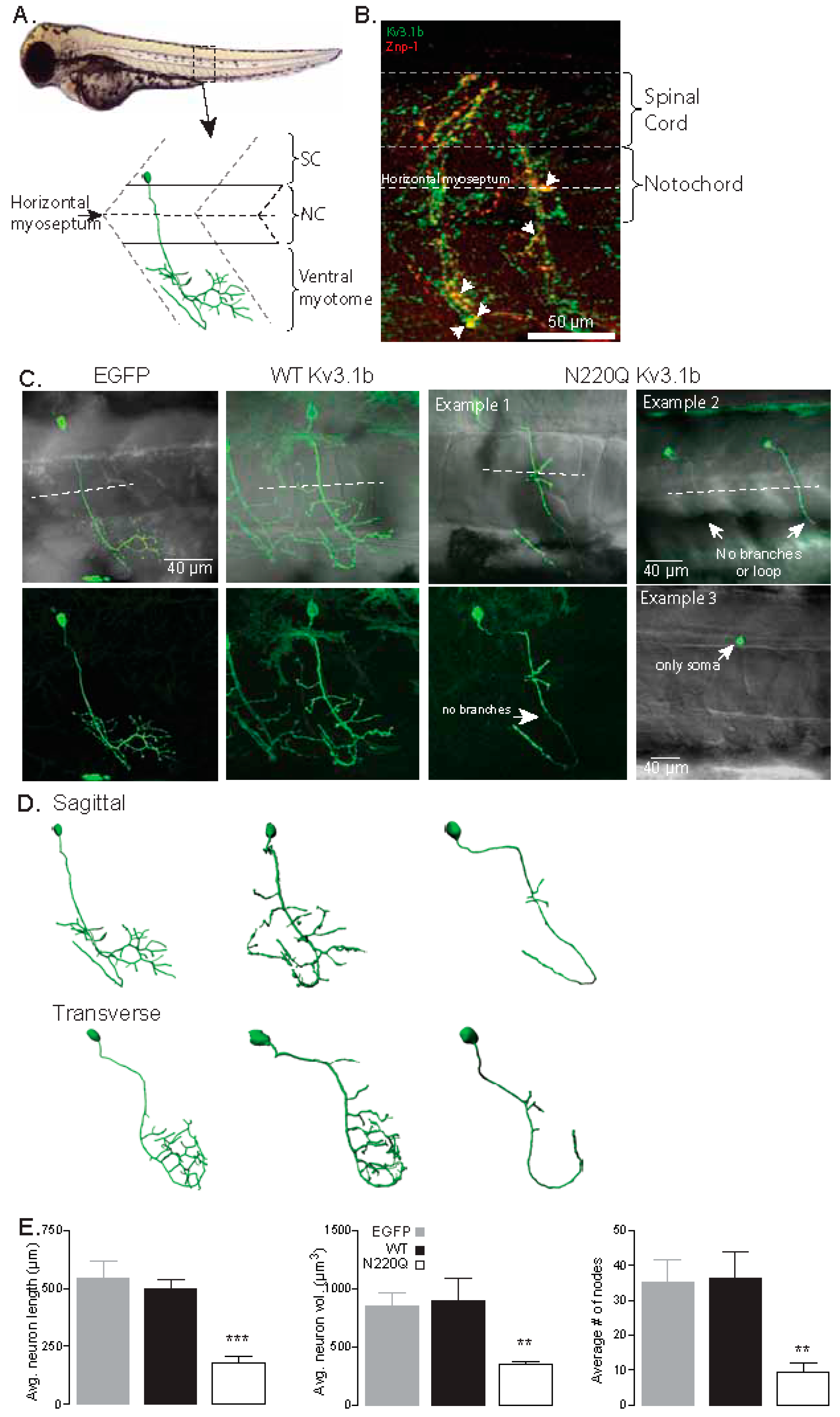
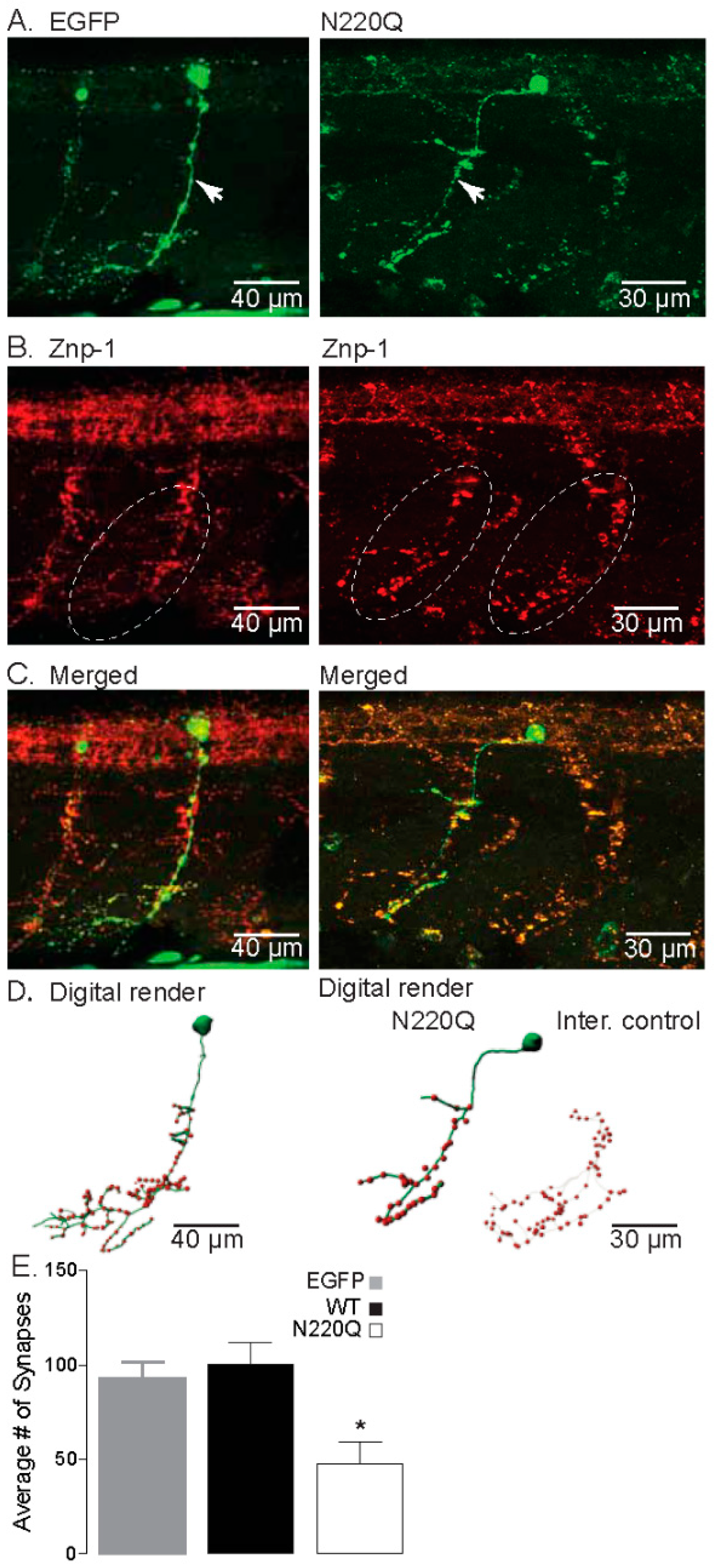
3.1. N-Glycosylation Processing of the Kv3.1b Protein Impacts Motor Neuron Development in Zebrafish
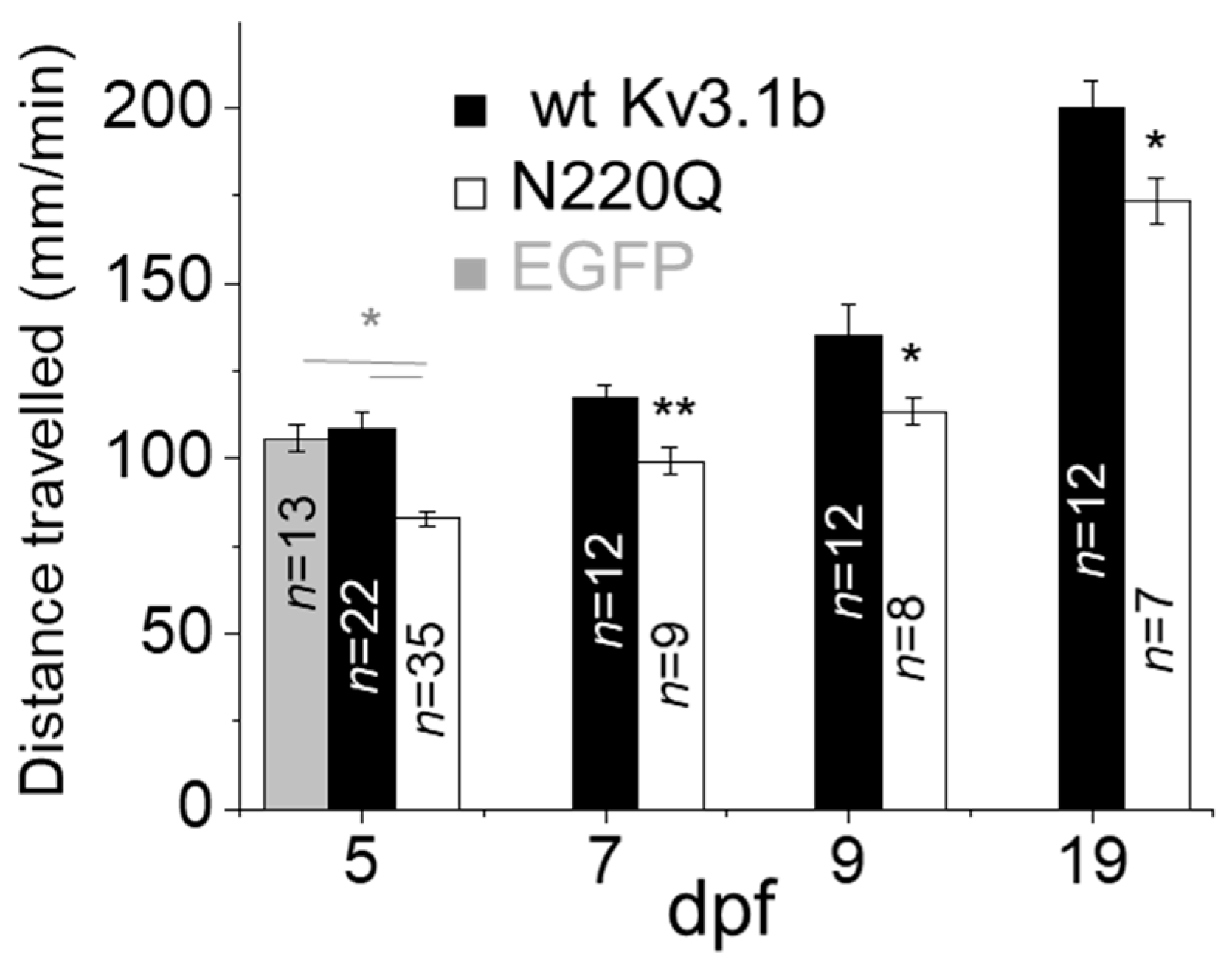
3.2. N-Glycosylation Site Vacancy of Kv3.1b Hamper Motor Activity in Zebrafish Larvae
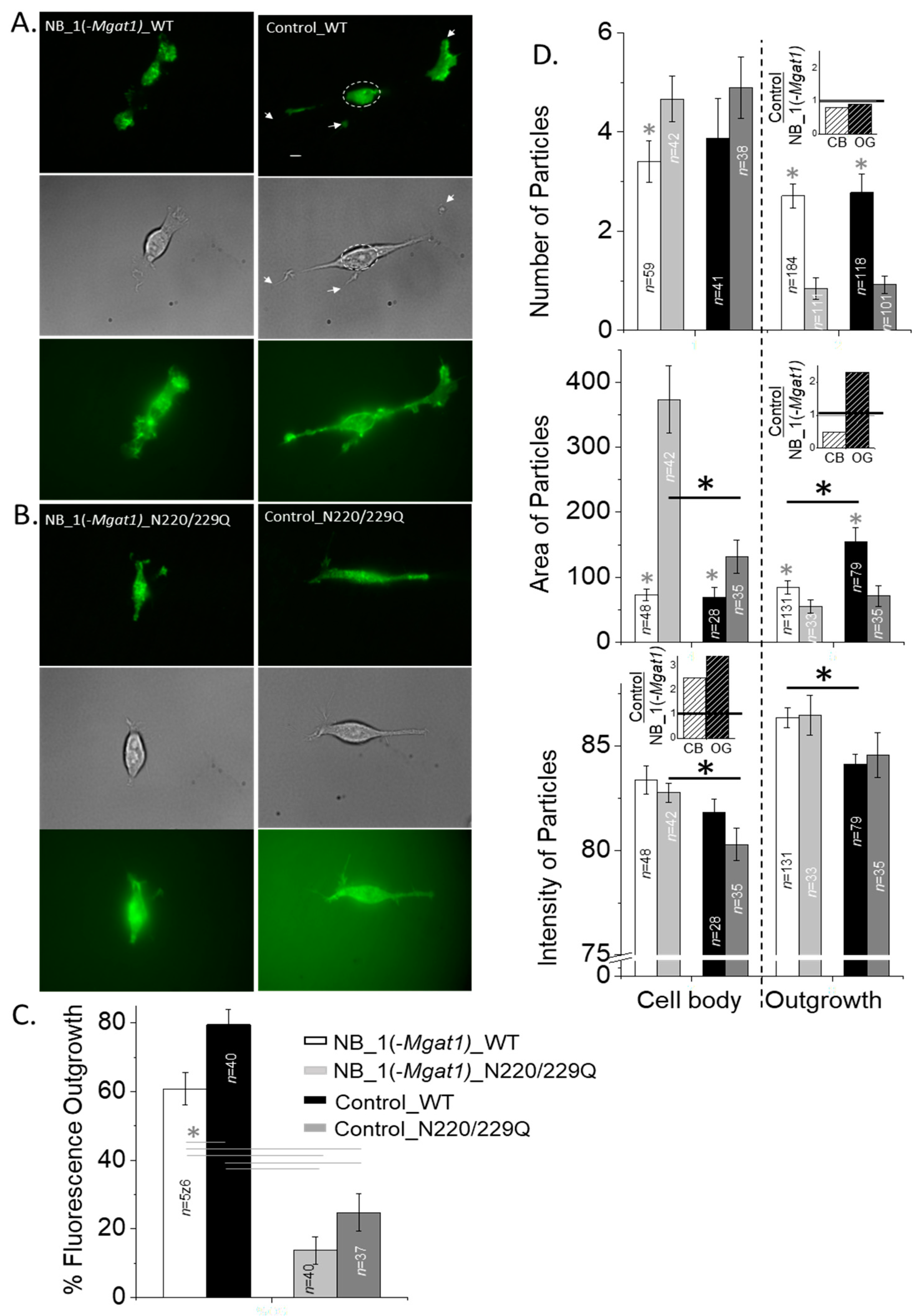
3.3. N-Glycosylation Mutant Neuronal-Derived Cell Model Expressing WT Kv3.1b with and without Oligomannose N-Glycans
3.4. N-Glycosylation Processing Modified the Spatial Arrangement of Kv3.1b Protein-Containing Particles in Subdomains of the Cell Membrane
3.5. Opening and Closing Rate of Outward Ionic Current Decreased as N-Glycan Branching Is Lessened
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ioffe, E.; Stanley, P. Mice lacking N-acetylglucosaminyltransferase I activity die at mid-gestation, revealing an essential role for complex or hybrid N-linked carbohydrates. Proc. Natl. Acad. Sci. USA 1994, 91, 728–732. [Google Scholar] [CrossRef] [Green Version]
- Metzler, M.; Gertz, A.; Sarkar, M.; Schachter, H.; Schrader, J.W.; Marth, J.D. Complex asparagine-linked oligosaccharides are required for morphogenic events during post-implantation development. EMBO J. 1994, 13, 2056–2065. [Google Scholar] [CrossRef]
- Freeze, H.H. Congenital Disorders of Glycosylation: CDG-I., CDG-II, and beyond. Curr. Mol. Med. 2007, 7, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Jaeken, J. Congenital disorders of glycosylation. Ann. N.Y. Acad. Sci. 2010, 1214, 190–198. [Google Scholar] [CrossRef]
- Stanley, P.; Taniguchi, N.; Aebi, M. N-Glycans. In Essentials of Glycobiology; Varki, A., Cummings, R.D., Esko, J.D., Stanley, P., Hart, G.W., Aebi, M., Darvill, A.G., Kinoshita, T., Packer, N.H., Prestegard, J.H., et al., Eds.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2015. [Google Scholar]
- Grissmer, S.; Ghanshani, S.; Dethlefs, B.; McPherson, J.D.; Wasmuth, J.J.; Gutman, G.A.; Cahalan, M.D.; Chandy, K.G. The Shaw-related potassium channel gene, Kv3.1, on human chromosome 11, encodes the type l K+ channel in T cells. J. Biol. Chem. 1992, 267, 20971–20979. [Google Scholar] [CrossRef]
- Kanemasa, T.; Gan, L.; Perney, T.M.; Wang, L.Y.; Kaczmarek, L.K. Electrophysiological and pharmacological characterization of a mammalian Shaw channel expressed in NIH 3T3 fibroblasts. J. Neurophysiol. 1995, 74, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, L.K.; Zhang, Y. Kv3 Channels: Enablers of Rapid Firing, Neurotransmitter Release, and Neuronal Endurance. Physiol. Rev. 2017, 97, 1431–1468. [Google Scholar] [CrossRef] [Green Version]
- Schwalbe, R.A.; Corey, M.J.; Cartwright, T.A. Novel Kv3 glycoforms differentially expressed in adult mammalian brain contain sialylated N-glycans. Biochem. Cell Biol. 2008, 86, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Issa, F.A.; Mazzochi, C.; Mock, A.F.; Papazian, D.M. Spinocerebellar Ataxia Type 13 Mutant Potassium Channel Alters Neuronal Excitability and Causes Locomotor Deficits in Zebrafish. J. Neurosci. 2011, 31, 6831–6841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waters, M.F.; Minassian, N.A.; Stevanin, G.; Figueroa, K.P.; Bannister, J.P.; Nolte, D.; Mock, A.F.; Evidente, V.G.H.; Fee, D.B.; Müller, U.; et al. Mutations in voltage-gated potassium channel KCNC3 cause degenerative and developmental central nervous system phenotypes. Nat. Genet. 2006, 38, 447–451. [Google Scholar] [CrossRef]
- Figueroa, K.P.; Minassian, N.A.; Stevanin, G.; Waters, M.; Garibyan, V.; Forlani, S.; Strzelczyk, A.; Bürk, K.; Brice, A.; Dürr, A.; et al. KCNC3: Phenotype, mutations, channel biophysics-a study of 260 familial ataxia patients. Hum. Mutat. 2010, 31, 191–196. [Google Scholar] [CrossRef] [Green Version]
- Hall, M.K.; Cartwright, T.A.; Fleming, C.M.; Schwalbe, R.A. Importance of Glycosylation on Function of a Potassium Channel in Neuroblastoma Cells. PLoS ONE 2011, 6, e19317. [Google Scholar] [CrossRef]
- Hall, M.; Weidner, D.; Bernetski, C.; Schwalbe, R. N-Linked glycan site occupancy impacts the distribution of a potassium channel in the cell body and outgrowths of neuronal-derived cells. Biochim. et Biophys. Acta BBA Gen. Subj. 2014, 1840, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.K.; Weidner, D.A.; Edwards, M.A.J.; Schwalbe, R.A. Complex N-Glycans Influence the Spatial Arrangement of Voltage Gated Potassium Channels in Membranes of Neuronal-Derived Cells. PLoS ONE 2015, 10, e0137138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murashov, A.K.; Hall, M.K.; Schwalbe, R.A.; Weidner, D.A.; Dayal, S.; Pak, E. Membrane Distribution and Activity of a Neuronal Voltage-Gated K+ Channel is Modified by Replacement of Complex Type N-Glycans with Hybrid Type. J. Glycobiol. 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Kunkel, D.; Schwartzkroin, P.; Tempel, B. Localization of Kv1.1 and Kv1.2, two K channel proteins, to synaptic terminals, somata, and dendrites in the mouse brain. J. Neurosci. 1994, 14, 4588–4599. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, I.; Wang, H.; Sutachan, J.J.; Zhu, J.; Recio-Pinto, E.; Thornhill, W.B. Glycosylation Affects Rat Kv1.1 Potassium Channel Gating by a Combined Surface Potential and Cooperative Subunit Interaction Mechanism. J. Physiol. 2003, 550, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.; Bennett, E.S. Gating of the shaker potassium channel is modulated differentially by N-glycosylation and sialic acids. Pflügers Arch. Eur. J. Physiol. 2007, 456, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Schwetz, T.A.; Norring, S.A.; Bennett, E.S. N-glycans modulate Kv1.5 gating but have no effect on Kv1.4 gating. Biochim. et Biophys. Acta BBA Biomembr. 2010, 1798, 367–375. [Google Scholar] [CrossRef] [Green Version]
- Ednie, A.R.; Bennett, E.S. Modulation of Voltage-Gated Ion Channels by Sialylation. Compr. Physiol. 2012, 2, 1269–1301. [Google Scholar] [CrossRef]
- Eisen, J.S.; Myers, P.Z.; Westerfield, M. Pathway selection by growth cones of identified motoneurones in live zebra fish embryos. Nat. Cell Biol. 1986, 320, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Myers, P.; Eisen, J.; Westerfield, M. Development and axonal outgrowth of identified motoneurons in the zebrafish. J. Neurosci. 1986, 6, 2278–2289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westerfield, M.; McMurray, J.V.; Eisen, J.S. Identified motoneurons and their innervation of axial muscles in the zebrafish. J. Neurosci. 1986, 6, 2267–2277. [Google Scholar] [CrossRef] [Green Version]
- Issa, F.A.; Mock, A.F.; Sagasti, A.; Papazian, D.M. Spinocerebellar ataxia type 13 mutation that is associated with disease onset in infancy disrupts axonal pathfinding during neuronal development. Dis. Model. Mech. 2012, 5, 921–929. [Google Scholar] [CrossRef] [Green Version]
- Hall, M.K.; Burch, A.P.; Schwalbe, R.A. Functional analysis of N-acetylglucosaminyltransferase-I knockdown in 2D and 3D neuroblastoma cell cultures. IJMS. Pending editor decision.
- Hall, M.K.; Whitman, A.A.; Weidner, D.A.; Schwalbe, R.A. Knockdown of N-Acetylglucosaminyltransferase-II Reduces Matrix Metalloproteinase 2 Activity and Suppresses Tumorigenicity in Neuroblastoma Cell Line. Biology 2020, 9, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakano, T.; Windrem, M.; Zappavigna, V.; Goldman, S.A. Identification of a conserved 125 base-pair Hb9 enhancer that specifies gene expression to spinal motor neurons. Dev. Biol. 2005, 283, 474–485. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Xu, R.; Liu, A. IRDL Cloning: A One-Tube, Zero-Background, Easy-to-Use, Directional Cloning Method Improves Throughput in Recombinant DNA Preparation. PLoS ONE 2014, 9, e107907. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.W.; Westerfield, M. Function of identified motoneurones and co-ordination of primary and secondary motor systems during zebra fish swimming. J. Physiol. 1988, 403, 73–89. [Google Scholar] [CrossRef] [PubMed]
- Eaton, R.; Lee, R.; Foreman, M. The Mauthner cell and other identified neurons of the brainstem escape network of fish. Prog. Neurobiol. 2001, 63, 467–485. [Google Scholar] [CrossRef]
- Hall, M.K.; Weidner, D.A.; Whitman, A.A.; Schwalbe, R.A. Lack of complex type N-glycans lessens aberrant neuronal properties. PLoS ONE 2018, 13, e0199202. [Google Scholar] [CrossRef]
- Cartwright, T.A.; Corey, M.J.; Schwalbe, R.A. Complex oligosaccharides are N-linked to Kv3 voltage-gated K+ channels in rat brain. Biochim. et Biophys. Acta BBA Gen. Subj. 2007, 1770, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Cartwright, T.A.; Schwalbe, R.A. Atypical sialylated N-glycan structures are attached to neuronal voltage-gated potassium channels. Biosci. Rep. 2009, 29, 301–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brooks, N.L.; Corey, M.J.; Schwalbe, R.A. Characterization of N-glycosylation consensus sequences in the Kv3.1 channel. FEBS J. 2006, 273, 3287–3300. [Google Scholar] [CrossRef]
- Deuchars, S.A.; Brooke, R.E.; Frater, B.; Deuchars, J. Properties of interneurones in the intermediolateral cell column of the rat spinal cord: Role of the potassium channel subunit Kv3.1. Neuroscience 2001, 106, 433–446. [Google Scholar] [CrossRef]
- Rudy, B.; McBain, C.J. Kv3 channels: Voltage-gated K+ channels designed for high-frequency repetitive firing. Trends Neurosci. 2001, 24, 517–526. [Google Scholar] [CrossRef]
- Wang, L.-Y.; Gan, L.; Perney, T.M.; Schwartz, I.; Kaczmarek, L.K. Activation of Kv3.1 channels in neuronal spine-like structures may induce local potassium ion depletion. Proc. Natl. Acad. Sci. USA 1998, 95, 1882–1887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, C.S.; Grange, R.W.; Joho, R.H. Pleiotropic effects of a disrupted K+ channel gene: Reduced body weight, impaired motor skill and muscle contraction, but no seizures. Proc. Natl. Acad. Sci. USA 1997, 94, 1533–1538. [Google Scholar] [CrossRef] [Green Version]
- Kaczmarek, L.K. Non-conducting functions of voltage-gated ion channels. Nat. Rev. Neurosci. 2006, 7, 761–771. [Google Scholar] [CrossRef]
- Moreno, R.L.; Ribera, A.B. Zebrafish Motor Neuron Subtypes Differ Electrically Prior to Axonal Outgrowth. J. Neurophysiol. 2009, 102, 2477–2484. [Google Scholar] [CrossRef] [Green Version]
- Panzer, J.A.; Song, Y.; Balice-Gordon, R.J. In Vivo Imaging of Preferential Motor Axon Outgrowth to and Synaptogenesis at Prepatterned Acetylcholine Receptor Clusters in Embryonic Zebrafish Skeletal Muscle. J. Neurosci. 2006, 26, 934–947. [Google Scholar] [CrossRef] [PubMed]
- Flanagan-Steet, H.; Fox, M.A.; Meyer, D.; Sanes, J.R. Neuromuscular synapses can form in vivo by incorporation of initially aneural postsynaptic specializations. Development 2005, 132, 4471–4481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsieh, J.-Y.; Ulrich, B.N.; Issa, F.A.; Meng-Chin, A.L.; Brown, B.; Papazian, D.M. Infant and adult SCA13 mutations differentially affect Purkinje cell excitability, maturation, and viability in vivo. Elife 2020, 9. [Google Scholar] [CrossRef]
- Tannous, A.; Pisoni, G.B.; Hebert, D.N.; Molinari, M. N-linked sugar-regulated protein folding and quality control in the ER. Semin. Cell Dev. Biol. 2015, 41, 79–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindholm, D.; Wootz, H.; Korhonen, L. ER stress and neurodegenerative diseases. Cell Death Differ. 2006, 13, 385–392. [Google Scholar] [CrossRef]
- Trimmer, J.S.; Rhodes, K.J. Localization of Voltage-Gated Ion Channels IN Mammalian Brain. Annu. Rev. Physiol. 2004, 66, 477–519. [Google Scholar] [CrossRef]
- Lee, H.S.; Qi, Y.; Im, W. Effects of N-glycosylation on protein conformation and dynamics: Protein Data Bank analysis and molecular dynamics simulation study. Sci. Rep. 2015, 5, 1–7. [Google Scholar] [CrossRef]

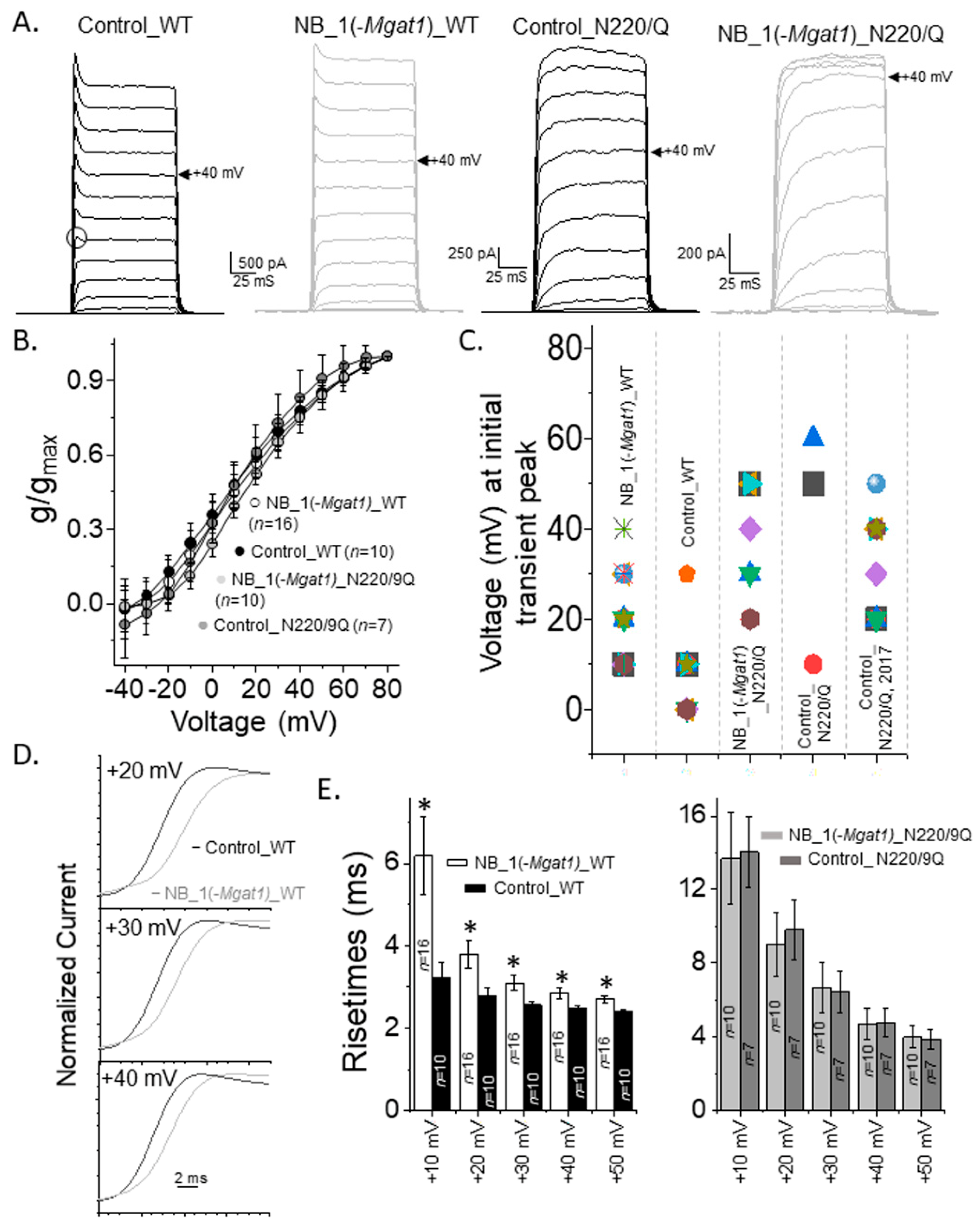
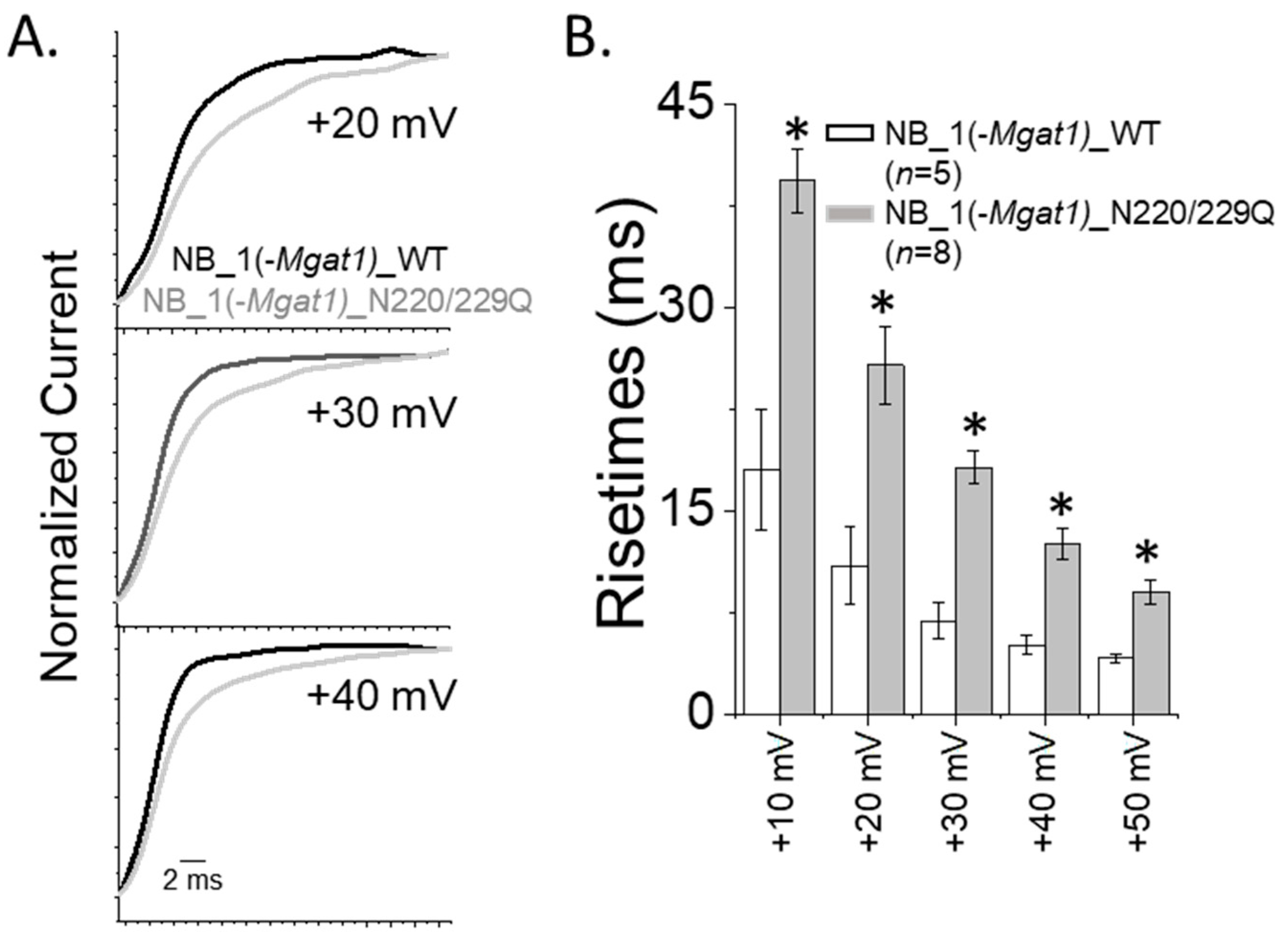
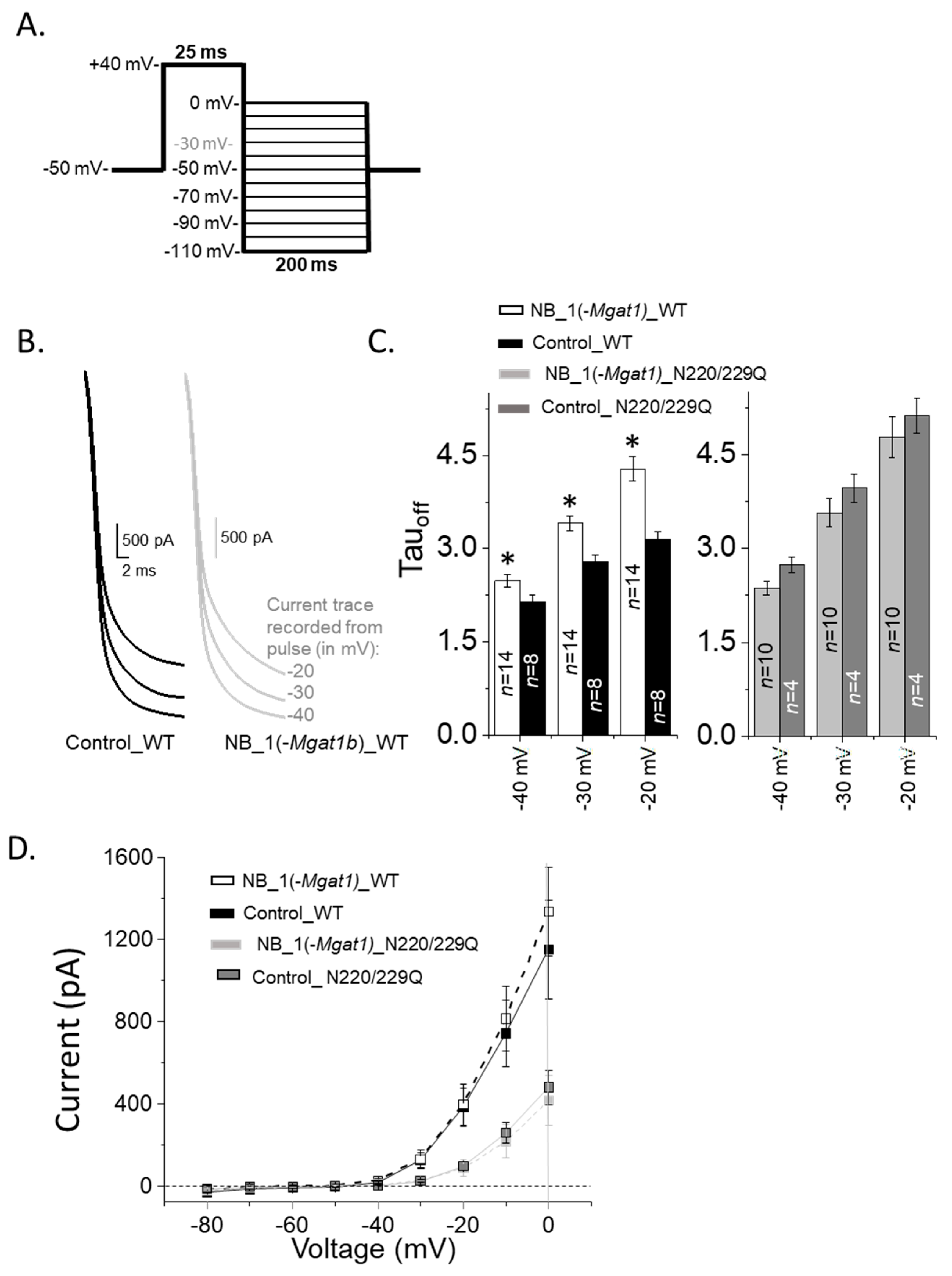
| Cell Line | Non-Inactivating w/Transient Peaks | Non-Inactivating w/o Transient Peaks | Inactivating |
|---|---|---|---|
| NB_1(-Mgat1)_WT | 70% | 13% | 17% |
| Control_WT | 91% | 0% | 9% |
| NB_1(-Mgat1)_N220/229Q | 47% | 12% | 41% |
| Control_N220/229Q | 30% | 40% | 30% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Issa, F.A.; Hall, M.K.; Hatchett, C.J.; Weidner, D.A.; Fiorenza, A.C.; Schwalbe, R.A. Compromised N-Glycosylation Processing of Kv3.1b Correlates with Perturbed Motor Neuron Structure and Locomotor Activity. Biology 2021, 10, 486. https://doi.org/10.3390/biology10060486
Issa FA, Hall MK, Hatchett CJ, Weidner DA, Fiorenza AC, Schwalbe RA. Compromised N-Glycosylation Processing of Kv3.1b Correlates with Perturbed Motor Neuron Structure and Locomotor Activity. Biology. 2021; 10(6):486. https://doi.org/10.3390/biology10060486
Chicago/Turabian StyleIssa, Fadi A., M. Kristen Hall, Cody J. Hatchett, Douglas A. Weidner, Alexandria C. Fiorenza, and Ruth A. Schwalbe. 2021. "Compromised N-Glycosylation Processing of Kv3.1b Correlates with Perturbed Motor Neuron Structure and Locomotor Activity" Biology 10, no. 6: 486. https://doi.org/10.3390/biology10060486






