Consequences of Oviposition Site Choice for Geckos in Changing Environments
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Species
2.2. Site Descriptions and Collection of Adult Females
2.3. Egg Incubation Experiment
2.4. Release and Mark-Recapture
2.5. Statistical Analyses
3. Results
3.1. Hatching Success and Incubation Period
3.2. Hatchling Morphology
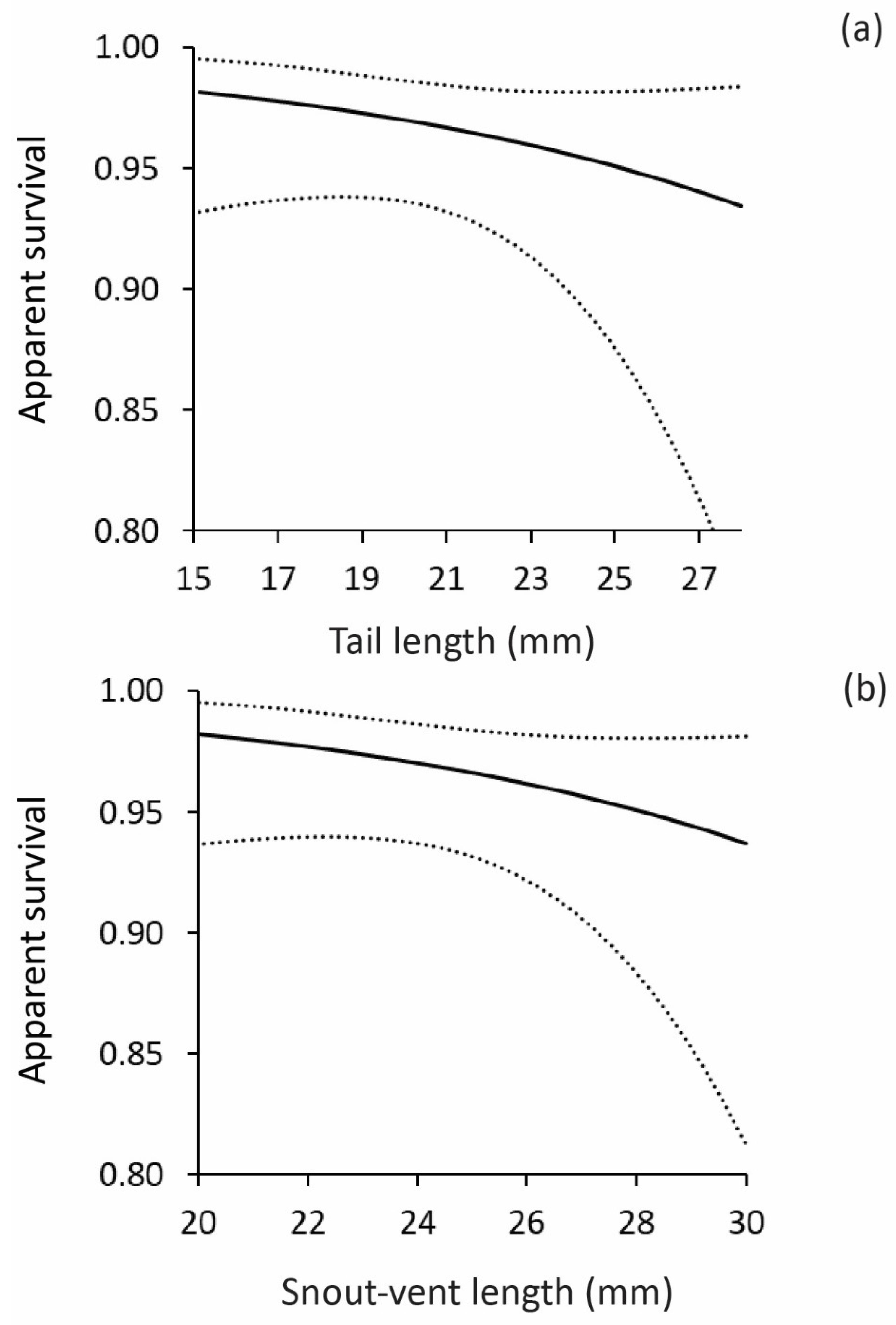
3.3. Effects of Incubation Temperature on Hatchling Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, G.; Shine, R. Maternal nest-site choice and offspring fitness in a tropical snake (Tropidonophis mairii, Colubridae). Ecology 2004, 85, 1627–1634. [Google Scholar] [CrossRef]
- Blouin-Demers, G.; Weatherhead, P.J.; Row, J.R. Phenotypic consequences of nest-site selection in black rat snakes (Elaphe obsoleta). Can. J. Zool. 2004, 82, 449–456. [Google Scholar] [CrossRef]
- Refsnider, J.M.; Janzen, F.J. Putting eggs in one basket: Ecological and evolutionary hypotheses for variation in oviposition-site choice. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 39–57. [Google Scholar] [CrossRef]
- Resetarits, W.J.J. Oviposition site choice and life history evolution. Am. Zool. 1996, 36, 205–215. [Google Scholar] [CrossRef]
- Spencer, R.J. Experimentally testing nest site selection: Fitness trade-offs and predation risk in turtles. Ecology 2002, 83, 2136–2144. [Google Scholar] [CrossRef]
- Webb, J.K.; Brook, B.W.; Shine, R. Collectors endanger Australia’s most threatened snake, the broad-headed snake Hoplocephalus bungaroides. Oryx 2002, 36, 170–181. [Google Scholar] [CrossRef]
- Wolf, A.J.; Hellgren, E.C.; Bogosian, V.; Moody, R.W. Effects of habitat disturbance on Texas horned lizards: An urban case study. Herpetologica 2013, 69, 265–281. [Google Scholar] [CrossRef]
- Jolly, C.J.; Von Takach, B.; Webb, J.K. Slow life history leaves endangered snake vulnerable to illegal collecting. Sci. Rep. 2021, 11, 5380. [Google Scholar] [CrossRef]
- Sinervo, B.; Mendez-de-la-Cruz, F.; Miles, D.B.; Heulin, B.; Bastiaans, E.; Cruz, M.V.S.; Lara-Resendiz, R.; Martinez-Mendez, N.; Calderon-Espinosa, M.L.; Meza-Lazaro, R.N.; et al. Erosion of lizard diversity by climate change and altered thermal niches. Science 2010, 328, 894–899. [Google Scholar] [CrossRef]
- Deeming, D.C. Post-hatching phenotypic effects of incubation in reptiles. In Reptilian Incubation: Environment, Evolution and Behaviour; Deeming, D.C., Ed.; Nottingham University Press: Nottingham, UK, 2004; pp. 229–251. [Google Scholar]
- Warner, D.A.; Andrews, R.M. Nest-site selection in relation to temperature and moisture by the lizard Sceloporus undulatus. Herpetologica 2002, 58, 399–407. [Google Scholar] [CrossRef]
- Shine, R.; Elphick, M.J.; Barrott, E.G. Sunny side up: Lethally high, not low, nest temperatures may prevent oviparous reptiles from reproducing at high elevations. Biol. J. Linn. Soc. 2003, 78, 325–334. [Google Scholar] [CrossRef]
- Hall, J.M.; Warner, D.A. Thermal spikes from the urban heat island increase mortality and alter physiology of lizard embryos. J. Exp. Biol. 2018, 221. [Google Scholar] [CrossRef] [PubMed]
- Packard, G.; Packard, M. The physiological ecology of reptilian eggs and embryos. Biol. Reptil. 1988, 16, 523–605. [Google Scholar]
- Angilletta, M.J.; Zelic, M.H.; Adrian, G.J.; Hurliman, A.M.; Smith, C.D. Heat tolerance during embryonic development has not diverged among populations of a widespread species (Sceloporus undulatus). Conserv. Physiol. 2013, 1, cot018. [Google Scholar] [CrossRef] [PubMed]
- Amiel, J.J.; Bao, S.; Shine, R. The effects of incubation temperature on the development of the cortical forebrain in a lizard. Anim. Cogn. 2016, 19, 1–9. [Google Scholar] [CrossRef]
- Shine, R. Seasonal shifts in nest temperature can modify the phenotypes of hatchling lizards, regardless of overall mean incubation temperature. Funct. Ecol. 2004, 18, 43–49. [Google Scholar] [CrossRef]
- Shine, R. Incubation regimes of cold-climate reptiles: The thermal consequences of nest-site choice, viviparity and maternal basking. Biol. J. Linn. Soc. 2004, 83, 145–155. [Google Scholar] [CrossRef]
- Warner, D.A.; Shine, R. Interactions among thermal parameters determine offspring sex under temperature-dependent sex determination. Proc. R. Soc. B-Biol. Sci. 2011, 278, 256–265. [Google Scholar] [CrossRef]
- Abayarathna, T.; Murray, B.R.; Webb, J.K. Higher incubation temperatures produce long-lasting upward shifts in cold tolerance, but not heat tolerance, of hatchling geckos. Biol. Open 2019, 8, bio042564. [Google Scholar] [CrossRef]
- Abayarathna, T.; Webb, J.K. Effects of incubation temperatures on learning abilities of hatchling velvet geckos. Anim. Cogn. 2020, 23, 613–620. [Google Scholar] [CrossRef]
- Perkins-Kirkpatrick, S.E.; Lewis, S.C. Increasing trends in regional heatwaves. Nat. Commun. 2020, 11, 3357. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, N.J.; Janzen, F.J. Temperature-dependent sex determination and contemporary climate change. Sex. Dev. 2010, 4, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Levy, O.; Buckley, L.B.; Keitt, T.H.; Smith, C.D.; Boateng, K.O.; Kumar, D.S.; Angilletta, M.J. Resolving the life cycle alters expected impacts of climate change. Proc. R. Soc. B-Biol. Sci. 2015, 282, 20150837. [Google Scholar] [CrossRef]
- Telemeco, R.S.; Fletcher, B.; Levy, O.; Riley, A.; Rodriguez-Sanchez, Y.; Smith, C.; Teague, C.; Waters, A.; Angilletta, M.J.; Buckley, L.B. Lizards fail to plastically adjust nesting behavior or thermal tolerance as needed to buffer populations from climate warming. Glob. Chang. Biol. 2017, 23, 1075–1084. [Google Scholar] [CrossRef] [PubMed]
- Noble, D.W.A.; Stenhouse, V.; Schwanz, L.E. Developmental temperatures and phenotypic plasticity in reptiles: A systematic review and meta-analysis. Biol. Rev. 2018, 93, 72–97. [Google Scholar] [CrossRef]
- While, G.M.; Noble, D.W.A.; Uller, T.; Warner, D.A.; Riley, J.L.; Du, W.G.; Schwanz, L.E. Patterns of developmental plasticity in response to incubation temperature in reptiles. J. Exp. Zool. 2018, 329, 162–176. [Google Scholar] [CrossRef]
- Dayananda, B.; Gray, S.; Pike, D.; Webb, J.K. Communal nesting under climate change: Fitness consequences of higher nest temperatures for a nocturnal lizard. Glob. Chang. Biol. 2016, 22, 2405–2414. [Google Scholar] [CrossRef]
- Han, Q.; Sun, S.; Liu, Z.; Xu, W.; Shi, P. Accelerated exacerbation of global extreme heatwaves under warming scenarios. Int. J. Climatol. 2022, 1–12. [Google Scholar] [CrossRef]
- Pike, D.A.; Webb, J.K.; Shine, R. Nesting in a thermally challenging environment: Nest-site selection in a rock-dwelling gecko, Oedura lesueurii (Reptilia: Gekkonidae). Biol. J. Linn. Soc. 2010, 99, 250–259. [Google Scholar] [CrossRef]
- Cuartas-Villa, S.; Webb, J.K. Nest site selection in a southern and northern population of the velvet gecko (Amalosia lesueurii). J. Therm. Biol. 2021, 102, 103121. [Google Scholar] [CrossRef]
- Webb, J.K.; Pike, D.A.; Shine, R. Population ecology of the velvet gecko, Oedura lesueurii in south eastern Australia: Implications for the persistence of an endangered snake. Austral Ecol. 2008, 33, 839–847. [Google Scholar] [CrossRef]
- Webb, J.K. Effects of tail autotomy on survival, growth and territory occupation in free-ranging juvenile geckos (Oedura lesueurii). Austral Ecol. 2006, 31, 432–440. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Inference: A Practical Information-Theoretic Approach; Springer: New York, NY, USA, 1998. [Google Scholar]
- Warner, D.A.; Shine, R. Fitness of juvenile lizards depends on seasonal timing of hatching, not offspring body size. Oecologia 2007, 154, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Shine, R. Reptilian viviparity in cold climates—Testing the assumptions of an evolutionary hypothesis. Oecologia 1983, 57, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Bauwens, D. Survivorship during hibernation in the European common lizard, Lacerta vivipara. Copeia 1981, 1981, 741–744. [Google Scholar] [CrossRef]
- Andrews, R.M.; Mathies, T.; Warner, D.A. Effect of incubation temperature on morphology, growth, and survival of juvenile Sceloporus undulatus. Herpetol. Monogr. 2000, 14, 420–431. [Google Scholar] [CrossRef]
- Sinervo, B.; Adolph, S.C. Thermal sensitivity of growth rate in hatchling Sceloporus lizards: Environmental, behavioral and genetic aspects. Oecologia 1989, 78, 411–419. [Google Scholar] [CrossRef]
- Christian, K.A.; Tracy, C.R. The effect of the thermal envrionment on the ability of hatchling land iguanas to avoid predation during dispersal. Oecologia 1981, 49, 218–223. [Google Scholar] [CrossRef]
- Booth, D.T. Influence of incubation temperature on hatchling phenotype in reptiles. Physiol. Biochem. Zool. 2006, 79, 274–281. [Google Scholar] [CrossRef]
- Janzen, F.J. An experimental analysis of natural selection on body size of hatchling turtles. Ecology 1993, 74, 332–341. [Google Scholar] [CrossRef]
- Ferguson, G.W.; Fox, S.F. Annual variation of survival advantage of large juvenile side-blotched lizards, Uta stansburiana—Its causes and evolutionary significance. Evolution 1984, 38, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Sorci, G.; Clobert, J. Natural selection on hatchling body size and mass in two environments in the common lizard (Lacerta vivipara). Evol. Ecol. Res. 1999, 1, 303–316. [Google Scholar]
- Sorci, G.; Clobert, J.; Belichon, S. Phenotypic plasticity of growth and survival in the common lizard Lacerta vivipara. J. Anim. Ecol. 1996, 65, 781–790. [Google Scholar] [CrossRef]
- Olsson, M.; Madsen, T. Between-year variation in determinants of offspring survival in the Sand Lizard, Lacerta agilis. Funct. Ecol. 2001, 15, 443–450. [Google Scholar] [CrossRef]
- Meylan, S.; Belliure, J.; Clobert, J.; de Fraipont, M. Stress and body condition as prenatal and postnatal determinants of dispersal in the common lizard (Lacerta vivipara). Horm. Behav. 2002, 42, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Dowdy, A.; Abbs, D.; Bhend, J.; Chiew, F.; Church, J.; Ekström, M.; Kirono, D.; Lenton, A.; Lucas, C.; McInnes, K. East coast cluster report. In Climate Change in Australia Projections for Australia’s Natural Resource Management Regions: Cluster Reports; CSIRO: Canberra, Australia; Bureau of Meteorology: Melbourne, Australia, 2015. [Google Scholar]
- Hall, J.M.; Sun, B.-j. Heat tolerance of reptile embryos: Current knowledge, methodological considerations, and future directions. J. Exp. Zool. Part A—Ecol. Integr. Physiol. 2021, 335, 45–58. [Google Scholar] [CrossRef]
- Webb, J.K.; Shine, R. Ecological characteristics of a threatened snake species, Hoplocephalus bungaroides (Serpentes, Elapidae). Anim. Conserv. 1998, 1, 185–193. [Google Scholar] [CrossRef]
| Cold Incubation | Warm Incubation | |
|---|---|---|
| (n = 56) | (n = 54) | |
| Hatching success (%) | 70.9 | 70.1 |
| Incubation period (d) | 101.83 (8.25) | 86.24 (8.67) |
| Snout-vent length (mm) | 27.05 (1.94) | 25.78 (1.69) |
| Tail length (mm) | 23.06 (3.01) | 21.25 (4.25) |
| Wet body mass (g) | 0.48 (0.06) | 0.46 (0.06) |
| Model | AICc | Delta AICc | AICc Weights | Likelihood | N | Deviance |
|---|---|---|---|---|---|---|
| s (time + TL) p (constant) | 283.2314 | 0 | 0.51811 | 1 | 5 | 272.6489 |
| s (time + SVL) p (constant) | 285.2356 | 2.0042 | 0.1902 | 0.3671 | 6 | 272.4121 |
| s (time + mass) p (constant) | 286.38 | 3.1486 | 0.10733 | 0.2072 | 6 | 273.5565 |
| s (constant) p (time) | 288.074 | 4.8426 | 0.04601 | 0.0888 | 8 | 270.634 |
| s (constant) p (constant) | 289.0449 | 5.8135 | 0.02832 | 0.0547 | 2 | 284.9317 |
| s (SVL) p (constant) | 289.0882 | 5.8568 | 0.02771 | 0.0535 | 3 | 282.8596 |
| s (TL) p (constant) | 289.6272 | 6.3958 | 0.02116 | 0.0408 | 3 | 283.3986 |
| s (incubation) p (time) | 290.3492 | 7.1178 | 0.01475 | 0.0285 | 9 | 270.531 |
| Model | AICc | Delta AICc | AICc Weights | Likelihood | N | Deviance |
|---|---|---|---|---|---|---|
| s (time + mass) p (incubation) | 226.2744 | 0 | 0.39835 | 1 | 7 | 211.1431 |
| s (time) p (incubation) | 227.9026 | 1.6282 | 0.17648 | 0.443 | 6 | 215.0626 |
| s (time + mass) p (constant) | 228.0635 | 1.7891 | 0.16284 | 0.4088 | 5 | 217.4695 |
| s (time + SVL) p (incubation) | 228.3592 | 2.0848 | 0.14046 | 0.3526 | 7 | 213.2279 |
| s (time + SVL) p (constant) | 230.0278 | 3.7534 | 0.06098 | 0.1531 | 5 | 219.4337 |
| s (time) p (constant | 231.179 | 4.9046 | 0.0343 | 0.0861 | 5 | 220.5849 |
| s (time + TL) p (incubation) | 233.5323 | 7.2579 | 0.01057 | 0.0265 | 7 | 218.401 |
| s (time + TL) p (constant) | 234.5871 | 8.3127 | 0.00624 | 0.0157 | 5 | 223.993 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abayarathna, T.; Webb, J.K. Consequences of Oviposition Site Choice for Geckos in Changing Environments. Biology 2022, 11, 1281. https://doi.org/10.3390/biology11091281
Abayarathna T, Webb JK. Consequences of Oviposition Site Choice for Geckos in Changing Environments. Biology. 2022; 11(9):1281. https://doi.org/10.3390/biology11091281
Chicago/Turabian StyleAbayarathna, Theja, and Jonathan K. Webb. 2022. "Consequences of Oviposition Site Choice for Geckos in Changing Environments" Biology 11, no. 9: 1281. https://doi.org/10.3390/biology11091281





