Translating Senotherapeutic Interventions into the Clinic with Emerging Proteomic Technologies
Abstract
:Simple Summary
Abstract
1. Introduction
2. Targeting Senescent Cells with Senotherapeutics
2.1. Natural Compounds: Flavonoids
2.2. Dasatinib + Quercetin
2.3. Metformin
2.4. Rapamycin
2.5. Nutlin
2.6. Bcl-2 Family Member Inhibitors
2.7. Hsp90 Inhibitors
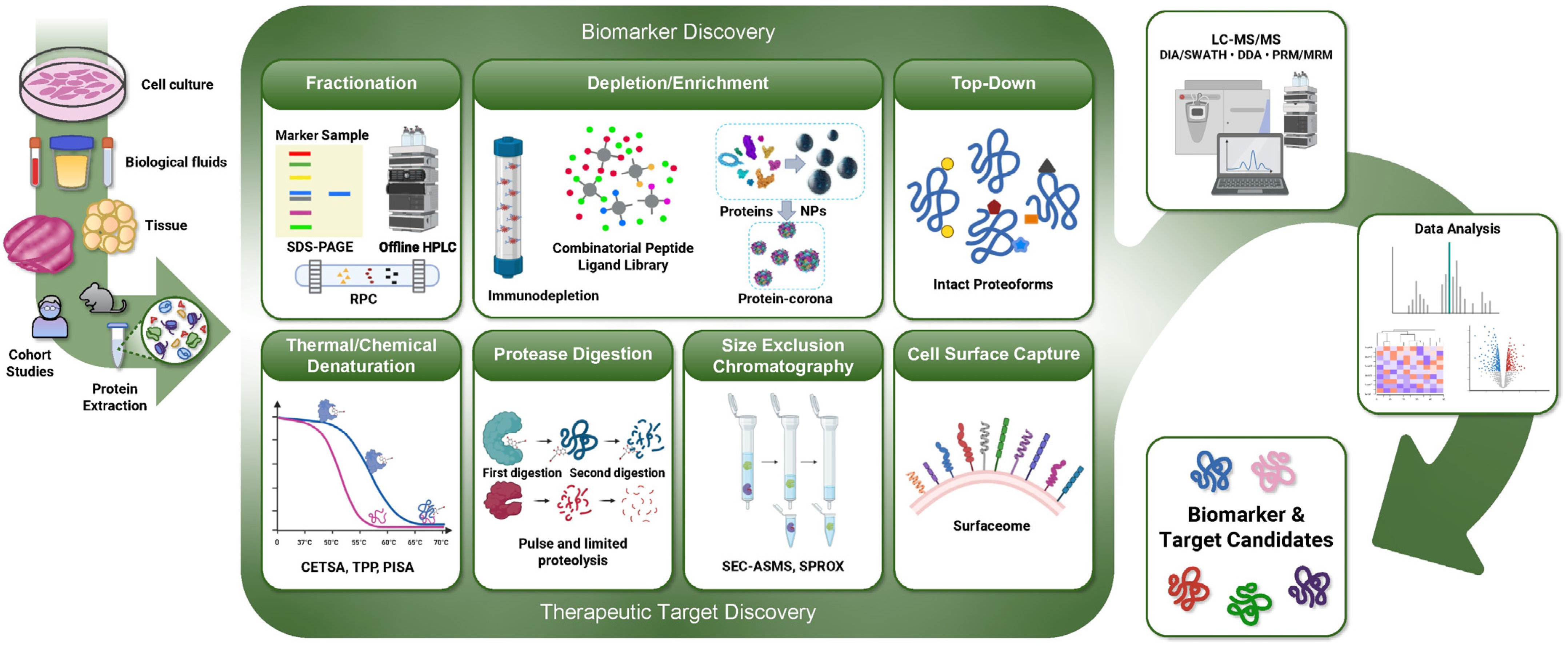
3. Development of Senescence-Associated Biomarkers and Therapeutic Targets for Clinical Translation
3.1. SASP in Circulating Plasma
3.2. Senescence-Associated Markers in Tissues and Biofluids
3.3. Senolytic Clinical Trials
4. Emerging Proteomic Technologies for Accelerating the Development of Senotherapeutics
4.1. Emerging MS-Based Approaches for Biomarker Discovery
4.1.1. Data Acquisition and MS Instrumentation
4.1.2. Identification of ‘Proteoform’-Level Biomarkers with Top-Down Proteomics
4.1.3. Sample Preparation Workflows to Overcome Challenges in Blood Biomarker Discovery
4.2. MS-Based Approaches for the Discovery of Novel Therapeutic Targets
4.2.1. Limited Proteolysis Coupled to Mass Spectrometry (LiP-MS)
4.2.2. Pulse Proteolysis (PP)
4.2.3. Stability of Proteins from Rates of Oxidation (SPROX)
4.2.4. Thermal Denaturation-Based Target Discovery: TPP, CETSA, and PISA
4.2.5. Size-Exclusion Chromatography and Affinity Selection Mass Spectrometry (SEC-ASMS)
4.2.6. Targeting and Quantifying Senescent Cells through the ‘Surfaceome’
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Basisty, N.; Kale, A.; Patel, S.; Campisi, J.; Schilling, B. The power of proteomics to monitor senescence-associated secretory phenotypes and beyond: Toward clinical applications. Expert Rev. Proteom. 2020, 17, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Basisty, N.; Kale, A.; Jeon, O.H.; Kuehnemann, C.; Payne, T.; Rao, C.; Holtz, A.; Shah, S.; Sharma, V.; Ferrucci, L.; et al. A proteomic atlas of senescence-associated secretomes for aging biomarker development. PLoS Biol. 2020, 18, e3000599. [Google Scholar] [CrossRef]
- Chaib, S.; Tchkonia, T.; Kirkland, J.L. Cellular senescence and senolytics: The path to the clinic. Nat. Med. 2022, 28, 1556–1568. [Google Scholar] [CrossRef] [PubMed]
- Neri, F.; Basisty, N.; Desprez, P.Y.; Campisi, J.; Schilling, B. Quantitative Proteomic Analysis of the Senescence-Associated Secretory Phenotype by Data-Independent Acquisition. Curr. Protoc. 2021, 1, e32. [Google Scholar] [CrossRef]
- Childs, B.G.; Durik, M.; Baker, D.J.; van Deursen, J.M. Cellular senescence in aging and age-related disease: From mechanisms to therapy. Nat. Med. 2015, 21, 1424–1435. [Google Scholar] [CrossRef]
- Tanaka, T.; Basisty, N.; Fantoni, G.; Candia, J.; Moore, A.Z.; Biancotto, A.; Schilling, B.; Bandinelli, S.; Ferrucci, L. Plasma proteomic biomarker signature of age predicts health and life span. Elife 2020, 9, e61073. [Google Scholar] [CrossRef]
- Jeon, O.H.; Kim, C.; Laberge, R.M.; Demaria, M.; Rathod, S.; Vasserot, A.P.; Chung, J.W.; Kim, D.H.; Poon, Y.; David, N.; et al. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat. Med. 2017, 23, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.C.; Kim, J.R. Senotherapeutics: Emerging strategy for healthy aging and age-related disease. BMB Rep. 2019, 52, 47–55. [Google Scholar] [CrossRef]
- Beerman, I.; Basisty, N.; de Cabo, R. Short-term senolytic treatment: A paradigm to promote fracture repair during aging. J. Clin. Investig. 2022, 132, e158871. [Google Scholar] [CrossRef]
- Niedernhofer, L.J.; Robbins, P.D. Senotherapeutics for healthy ageing. Nat. Rev. Drug Discov. 2018, 17, 377. [Google Scholar] [CrossRef]
- Rossi, M.; Abdelmohsen, K. The Emergence of Senescent Surface Biomarkers as Senotherapeutic Targets. Cells 2021, 10, 1740. [Google Scholar] [CrossRef]
- Suda, M.; Shimizu, I.; Katsuumi, G.; Yoshida, Y.; Hayashi, Y.; Ikegami, R.; Matsumoto, N.; Yoshida, Y.; Mikawa, R.; Katayama, A.; et al. Senolytic vaccination improves normal and pathological age-related phenotypes and increases lifespan in progeroid mice. Nat. Aging 2021, 1, 1117–1126. [Google Scholar] [CrossRef]
- Poblocka, M.; Bassey, A.L.; Smith, V.M.; Falcicchio, M.; Manso, A.S.; Althubiti, M.; Sheng, X.; Kyle, A.; Barber, R.; Frigerio, M.; et al. Targeted clearance of senescent cells using an antibody-drug conjugate against a specific membrane marker. Sci. Rep. 2021, 11, 20358. [Google Scholar] [CrossRef] [PubMed]
- Yousefzadeh, M.J.; Flores, R.R.; Zhu, Y.; Schmiechen, Z.C.; Brooks, R.W.; Trussoni, C.E.; Cui, Y.; Angelini, L.; Lee, K.A.; McGowan, S.J.; et al. An aged immune system drives senescence and ageing of solid organs. Nature 2021, 594, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Pitcher, L.E.; Yousefzadeh, M.J.; Niedernhofer, L.J.; Robbins, P.D.; Zhu, Y. Cellular senescence: A key therapeutic target in aging and diseases. J. Clin. Investig. 2022, 132, e158450. [Google Scholar] [CrossRef]
- Luis, C.; Maduro, A.T.; Pereira, P.; Mendes, J.J.; Soares, R.; Ramalho, R. Nutritional senolytics and senomorphics: Implications to immune cells metabolism and aging—From theory to practice. Front. Nutr. 2022, 9, 958563. [Google Scholar] [CrossRef]
- Romashkan, S.; Chang, H.; Hadley, E.C. National Institute on Aging Workshop: Repurposing Drugs or Dietary Supplements for Their Senolytic or Senomorphic Effects: Considerations for Clinical Trials. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 1144–1152. [Google Scholar] [CrossRef]
- Walker, K.A.; Basisty, N.; Wilson, D.M., 3rd; Ferrucci, L. Connecting aging biology and inflammation in the omics era. J. Clin. Investig. 2022, 132, e158448. [Google Scholar] [CrossRef]
- Hawkridge, A.M.; Muddiman, D.C. Mass spectrometry-based biomarker discovery: Toward a global proteome index of individuality. Annu. Rev. Anal. Chem. 2009, 2, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, J.L.; Tchkonia, T. Senolytic drugs: From discovery to translation. J. Intern. Med. 2020, 288, 518–536. [Google Scholar] [CrossRef]
- Vun, J.; Iqbal, N.; Jones, E.; Ganguly, P. Anti-Aging Potential of Platelet Rich Plasma (PRP): Evidence from Osteoarthritis (OA) and Applications in Senescence and Inflammaging. Bioengineering 2023, 10, 987. [Google Scholar] [CrossRef]
- Robbins, P.D.; Jurk, D.; Khosla, S.; Kirkland, J.L.; LeBrasseur, N.K.; Miller, J.D.; Passos, J.F.; Pignolo, R.J.; Tchkonia, T.; Niedernhofer, L.J. Senolytic Drugs: Reducing Senescent Cell Viability to Extend Health Span. Annu. Rev. Pharmacol. Toxicol. 2021, 61, 779–803. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Pitcher, L.E.; Prahalad, V.; Niedernhofer, L.J.; Robbins, P.D. Targeting cellular senescence with senotherapeutics: Senolytics and senomorphics. FEBS J. 2023, 290, 1362–1383. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.S.; Kook, S.H.; Son, Y.O.; Kim, J.G.; Jeon, Y.M.; Jang, Y.S.; Choi, K.C.; Kim, J.; Han, S.K.; Lee, K.Y.; et al. Flavonoids purified from Rhus verniciflua Stokes actively inhibit cell growth and induce apoptosis in human osteosarcoma cells. Biochim. Biophys. Acta 2005, 1726, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Yousefzadeh, M.J.; Zhu, Y.; McGowan, S.J.; Angelini, L.; Fuhrmann-Stroissnigg, H.; Xu, M.; Ling, Y.Y.; Melos, K.I.; Pirtskhalava, T.; Inman, C.L.; et al. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine 2018, 36, 18–28. [Google Scholar] [CrossRef]
- Hohmann, M.S.; Habiel, D.M.; Coelho, A.L.; Verri, W.A., Jr.; Hogaboam, C.M. Quercetin Enhances Ligand-induced Apoptosis in Senescent Idiopathic Pulmonary Fibrosis Fibroblasts and Reduces Lung Fibrosis In Vivo. Am. J. Respir. Cell Mol. Biol. 2019, 60, 28–40. [Google Scholar] [CrossRef]
- Yang, W.; Tian, Z.K.; Yang, H.X.; Feng, Z.J.; Sun, J.M.; Jiang, H.; Cheng, C.; Ming, Q.L.; Liu, C.M. Fisetin improves lead-induced neuroinflammation, apoptosis and synaptic dysfunction in mice associated with the AMPK/SIRT1 and autophagy pathway. Food Chem. Toxicol. 2019, 134, 110824. [Google Scholar] [CrossRef]
- Sun, Y.; Qin, H.; Zhang, H.; Feng, X.; Yang, L.; Hou, D.X.; Chen, J. Fisetin inhibits inflammation and induces autophagy by mediating PI3K/AKT/mTOR signaling in LPS-induced RAW264.7 cells. Food Nutr. Res. 2021, 65, 6355. [Google Scholar] [CrossRef]
- Cho, S.Y.; Park, S.J.; Kwon, M.J.; Jeong, T.S.; Bok, S.H.; Choi, W.Y.; Jeong, W.I.; Ryu, S.Y.; Do, S.H.; Lee, C.S.; et al. Quercetin suppresses proinflammatory cytokines production through MAP kinases andNF-kappaB pathway in lipopolysaccharide-stimulated macrophage. Mol. Cell. Biochem. 2003, 243, 153–160. [Google Scholar] [CrossRef]
- Min, Z.; Yangchun, L.; Yuquan, W.; Changying, Z. Quercetin inhibition of myocardial fibrosis through regulating MAPK signaling pathway via ROS. Pak. J. Pharm. Sci. 2019, 32, 1355–1359. [Google Scholar]
- Kang, N.J.; Lee, K.W.; Lee, D.E.; Rogozin, E.A.; Bode, A.M.; Lee, H.J.; Dong, Z. Cocoa procyanidins suppress transformation by inhibiting mitogen-activated protein kinase kinase. J. Biol. Chem. 2008, 283, 20664–20673. [Google Scholar] [CrossRef]
- Maurya, A.K.; Vinayak, M. PI-103 and Quercetin Attenuate PI3K-AKT Signaling Pathway in T- Cell Lymphoma Exposed to Hydrogen Peroxide. PLoS ONE 2016, 11, e0160686. [Google Scholar] [CrossRef] [PubMed]
- Kedhari Sundaram, M.; Raina, R.; Afroze, N.; Bajbouj, K.; Hamad, M.; Haque, S.; Hussain, A. Quercetin modulates signaling pathways and induces apoptosis in cervical cancer cells. Biosci. Rep. 2019, 39, BSR20190720. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Pelling, J.C. Targeting the PI3K/Akt/mTOR axis by apigenin for cancer prevention. Anticancer Agents Med. Chem. 2013, 13, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Granato, M.; Gilardini Montani, M.S.; Santarelli, R.; D’Orazi, G.; Faggioni, A.; Cirone, M. Apigenin, by activating p53 and inhibiting STAT3, modulates the balance between pro-apoptotic and pro-survival pathways to induce PEL cell death. J. Exp. Clin. Cancer Res. 2017, 36, 167. [Google Scholar] [CrossRef]
- Li, J.; Cheng, Y.; Qu, W.; Sun, Y.; Wang, Z.; Wang, H.; Tian, B. Fisetin, a dietary flavonoid, induces cell cycle arrest and apoptosis through activation of p53 and inhibition of NF-kappa B pathways in bladder cancer cells. Basic Clin. Pharmacol. Toxicol. 2011, 108, 84–93. [Google Scholar] [CrossRef]
- Kuo, P.C.; Liu, H.F.; Chao, J.I. Survivin and p53 modulate quercetin-induced cell growth inhibition and apoptosis in human lung carcinoma cells. J. Biol. Chem. 2004, 279, 55875–55885. [Google Scholar] [CrossRef]
- Du, B.X.; Lin, P.; Lin, J. EGCG and ECG induce apoptosis and decrease autophagy via the AMPK/mTOR and PI3K/AKT/mTOR pathway in human melanoma cells. Chin. J. Nat. Med. 2022, 20, 290–300. [Google Scholar] [CrossRef]
- Laberge, R.M.; Sun, Y.; Orjalo, A.V.; Patil, C.K.; Freund, A.; Zhou, L.; Curran, S.C.; Davalos, A.R.; Wilson-Edell, K.A.; Liu, S.; et al. MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nat. Cell Biol. 2015, 17, 1049–1061. [Google Scholar] [CrossRef]
- Novais, E.J.; Tran, V.A.; Johnston, S.N.; Darris, K.R.; Roupas, A.J.; Sessions, G.A.; Shapiro, I.M.; Diekman, B.O.; Risbud, M.V. Long-term treatment with senolytic drugs Dasatinib and Quercetin ameliorates age-dependent intervertebral disc degeneration in mice. Nat. Commun. 2021, 12, 5213. [Google Scholar] [CrossRef]
- Zhu, Y.; Tchkonia, T.; Pirtskhalava, T.; Gower, A.C.; Ding, H.; Giorgadze, N.; Palmer, A.K.; Ikeno, Y.; Hubbard, G.B.; Lenburg, M.; et al. The Achilles’ heel of senescent cells: From transcriptome to senolytic drugs. Aging Cell 2015, 14, 644–658. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Diamond, S.L. Src family kinases inhibition by dasatinib blocks initial and subsequent platelet deposition on collagen under flow, but lacks efficacy with thrombin generation. Thromb. Res. 2020, 192, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, R.; Anjum, A.; Kammerer, L.; Loew, Q.; Akhalkatsi, A.; Rossaro, D.; Escaig, R.; Droste Zu Senden, A.; Raude, B.; Lorenz, M.; et al. Mechanosensing via a GpIIb/Src/14-3-3zeta axis critically regulates platelet migration in vascular inflammation. Blood 2023, 141, 2973–2992. [Google Scholar] [CrossRef]
- Munoz-Galvan, S.; Rivero, M.; Peinado-Serrano, J.; Martinez-Perez, J.; Fernandez-Fernandez, M.C.; Ortiz, M.J.; Garcia-Heredia, J.M.; Carnero, A. PAI1 is a Marker of Bad Prognosis in Rectal Cancer but Predicts a Better Response to Treatment with PIM Inhibitor AZD1208. Cells 2020, 9, 1071. [Google Scholar] [CrossRef]
- Chen, Z.G.; Saba, N.F.; Teng, Y. The diverse functions of FAT1 in cancer progression: Good, bad, or ugly? J. Exp. Clin. Cancer Res. 2022, 41, 248. [Google Scholar] [CrossRef]
- Yahata, T.; Ibrahim, A.A.; Hirano, K.I.; Muguruma, Y.; Naka, K.; Hozumi, K.; Vaughan, D.E.; Miyata, T.; Ando, K. Targeting of plasminogen activator inhibitor-1 activity promotes elimination of chronic myeloid leukemia stem cells. Haematologica 2021, 106, 483–494. [Google Scholar] [CrossRef]
- Xue, T.; Luo, P.; Zhu, H.; Zhao, Y.; Wu, H.; Gai, R.; Wu, Y.; Yang, B.; Yang, X.; He, Q. Oxidative stress is involved in Dasatinib-induced apoptosis in rat primary hepatocytes. Toxicol. Appl. Pharmacol. 2012, 261, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Kopetz, S.; Lesslie, D.P.; Dallas, N.A.; Park, S.I.; Johnson, M.; Parikh, N.U.; Kim, M.P.; Abbruzzese, J.L.; Ellis, L.M.; Chandra, J.; et al. Synergistic activity of the SRC family kinase inhibitor dasatinib and oxaliplatin in colon carcinoma cells is mediated by oxidative stress. Cancer Res. 2009, 69, 3842–3849. [Google Scholar] [CrossRef]
- Hickson, L.J.; Langhi Prata, L.G.P.; Bobart, S.A.; Evans, T.K.; Giorgadze, N.; Hashmi, S.K.; Herrmann, S.M.; Jensen, M.D.; Jia, Q.; Jordan, K.L.; et al. Senolytics decrease senescent cells in humans: Preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease. EBioMedicine 2019, 47, 446–456. [Google Scholar] [CrossRef]
- Palmer, A.K.; Xu, M.; Zhu, Y.; Pirtskhalava, T.; Weivoda, M.M.; Hachfeld, C.M.; Prata, L.G.; van Dijk, T.H.; Verkade, E.; Casaclang-Verzosa, G.; et al. Targeting senescent cells alleviates obesity-induced metabolic dysfunction. Aging Cell 2019, 18, e12950. [Google Scholar] [CrossRef]
- Sierra-Ramirez, A.; Lopez-Aceituno, J.L.; Costa-Machado, L.F.; Plaza, A.; Barradas, M.; Fernandez-Marcos, P.J. Transient metabolic improvement in obese mice treated with navitoclax or dasatinib/quercetin. Aging 2020, 12, 11337–11348. [Google Scholar] [CrossRef]
- Bannister, C.A.; Holden, S.E.; Jenkins-Jones, S.; Morgan, C.L.; Halcox, J.P.; Schernthaner, G.; Mukherjee, J.; Currie, C.J. Can people with type 2 diabetes live longer than those without? A comparison of mortality in people initiated with metformin or sulphonylurea monotherapy and matched, non-diabetic controls. Diabetes Obes. Metab. 2014, 16, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Kalender, A.; Selvaraj, A.; Kim, S.Y.; Gulati, P.; Brule, S.; Viollet, B.; Kemp, B.E.; Bardeesy, N.; Dennis, P.; Schlager, J.J.; et al. Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPase-dependent manner. Cell Metab. 2010, 11, 390–401. [Google Scholar] [CrossRef]
- Moiseeva, O.; Deschenes-Simard, X.; St-Germain, E.; Igelmann, S.; Huot, G.; Cadar, A.E.; Bourdeau, V.; Pollak, M.N.; Ferbeyre, G. Metformin inhibits the senescence-associated secretory phenotype by interfering with IKK/NF-kappaB activation. Aging Cell 2013, 12, 489–498. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, W.; Yan, Z.; Zhao, W.; Mi, J.; Li, J.; Yan, H. Metformin induces autophagy and G0/G1 phase cell cycle arrest in myeloma by targeting the AMPK/mTORC1 and mTORC2 pathways. J. Exp. Clin. Cancer Res. 2018, 37, 63. [Google Scholar] [CrossRef]
- Amin, S.; Lux, A.; O’Callaghan, F. The journey of metformin from glycaemic control to mTOR inhibition and the suppression of tumour growth. Br. J. Clin. Pharmacol. 2019, 85, 37–46. [Google Scholar] [CrossRef]
- Saisho, Y. Metformin and Inflammation: Its Potential Beyond Glucose-lowering Effect. Endocr. Metab. Immune Disord. Drug Targets 2015, 15, 196–205. [Google Scholar] [CrossRef]
- Cameron, A.R.; Morrison, V.L.; Levin, D.; Mohan, M.; Forteath, C.; Beall, C.; McNeilly, A.D.; Balfour, D.J.; Savinko, T.; Wong, A.K.; et al. Anti-Inflammatory Effects of Metformin Irrespective of Diabetes Status. Circ. Res. 2016, 119, 652–665. [Google Scholar] [CrossRef] [PubMed]
- Harrison, D.E.; Strong, R.; Sharp, Z.D.; Nelson, J.F.; Astle, C.M.; Flurkey, K.; Nadon, N.L.; Wilkinson, J.E.; Frenkel, K.; Carter, C.S.; et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 2009, 460, 392–395. [Google Scholar] [CrossRef] [PubMed]
- Bitto, A.; Ito, T.K.; Pineda, V.V.; LeTexier, N.J.; Huang, H.Z.; Sutlief, E.; Tung, H.; Vizzini, N.; Chen, B.; Smith, K.; et al. Transient rapamycin treatment can increase lifespan and healthspan in middle-aged mice. eLife 2016, 5, e16351. [Google Scholar] [CrossRef] [PubMed]
- Quarles, E.; Basisty, N.; Chiao, Y.A.; Merrihew, G.; Gu, H.; Sweetwyne, M.T.; Fredrickson, J.; Nguyen, N.H.; Razumova, M.; Kooiker, K.; et al. Rapamycin persistently improves cardiac function in aged, male and female mice, even following cessation of treatment. Aging Cell 2020, 19, e13086. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.F.; Karunadharma, P.P.; Chiao, Y.A.; Basisty, N.; Crispin, D.; Hsieh, E.J.; Chen, T.; Gu, H.; Djukovic, D.; Raftery, D.; et al. Altered proteome turnover and remodeling by short-term caloric restriction or rapamycin rejuvenate the aging heart. Aging Cell 2014, 13, 529–539. [Google Scholar] [CrossRef]
- Anisimov, V.N.; Zabezhinski, M.A.; Popovich, I.G.; Piskunova, T.S.; Semenchenko, A.V.; Tyndyk, M.L.; Yurova, M.N.; Rosenfeld, S.V.; Blagosklonny, M.V. Rapamycin increases lifespan and inhibits spontaneous tumorigenesis in inbred female mice. Cell Cycle 2011, 10, 4230–4236. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.A.; Harrison, D.E.; Astle, C.M.; Fernandez, E.; Flurkey, K.; Han, M.; Javors, M.A.; Li, X.; Nadon, N.L.; Nelson, J.F.; et al. Rapamycin-mediated lifespan increase in mice is dose and sex dependent and metabolically distinct from dietary restriction. Aging Cell 2014, 13, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Lamming, D.W. Inhibition of the Mechanistic Target of Rapamycin (mTOR)-Rapamycin and Beyond. Cold Spring Harb. Perspect. Med. 2016, 6, a025924. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Yu, Z.; Sunchu, B.; Shoaf, J.; Dang, I.; Zhao, S.; Caples, K.; Bradley, L.; Beaver, L.M.; Ho, E.; et al. Rapamycin inhibits the secretory phenotype of senescent cells by a Nrf2-independent mechanism. Aging Cell 2017, 16, 564–574. [Google Scholar] [CrossRef]
- Hoff, U.; Markmann, D.; Thurn-Valassina, D.; Nieminen-Kelha, M.; Erlangga, Z.; Schmitz, J.; Brasen, J.H.; Budde, K.; Melk, A.; Hegner, B. The mTOR inhibitor Rapamycin protects from premature cellular senescence early after experimental kidney transplantation. PLoS ONE 2022, 17, e0266319. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, N.; Itakura, Y.; Toyoda, M. Rapamycin promotes endothelial-mesenchymal transition during stress-induced premature senescence through the activation of autophagy. Cell Commun. Signal. 2020, 18, 43. [Google Scholar] [CrossRef]
- Herranz, N.; Gallage, S.; Mellone, M.; Wuestefeld, T.; Klotz, S.; Hanley, C.J.; Raguz, S.; Acosta, J.C.; Innes, A.J.; Banito, A.; et al. mTOR regulates MAPKAPK2 translation to control the senescence-associated secretory phenotype. Nat. Cell Biol. 2015, 17, 1205–1217. [Google Scholar] [CrossRef]
- Fry, D.C.; Wartchow, C.; Graves, B.; Janson, C.; Lukacs, C.; Kammlott, U.; Belunis, C.; Palme, S.; Klein, C.; Vu, B. Deconstruction of a nutlin: Dissecting the binding determinants of a potent protein-protein interaction inhibitor. ACS Med. Chem. Lett. 2013, 4, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Chae, J.B.; Jang, H.; Son, C.; Park, C.W.; Choi, H.; Jin, S.; Lee, H.Y.; Lee, H.; Ryu, J.H.; Kim, N.; et al. Targeting senescent retinal pigment epithelial cells facilitates retinal regeneration in mouse models of age-related macular degeneration. Geroscience 2021, 43, 2809–2833. [Google Scholar] [CrossRef]
- Toutfaire, M.; Bauwens, E.; Debacq-Chainiaux, F. The impact of cellular senescence in skin ageing: A notion of mosaic and therapeutic strategies. Biochem. Pharmacol. 2017, 142, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kale, J.; Osterlund, E.J.; Andrews, D.W. BCL-2 family proteins: Changing partners in the dance towards death. Cell Death Differ. 2018, 25, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Wang, Y.; Shao, L.; Laberge, R.M.; Demaria, M.; Campisi, J.; Janakiraman, K.; Sharpless, N.E.; Ding, S.; Feng, W.; et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat. Med. 2016, 22, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Tchkonia, T.; Fuhrmann-Stroissnigg, H.; Dai, H.M.; Ling, Y.Y.; Stout, M.B.; Pirtskhalava, T.; Giorgadze, N.; Johnson, K.O.; Giles, C.B.; et al. Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell 2016, 15, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Yosef, R.; Pilpel, N.; Tokarsky-Amiel, R.; Biran, A.; Ovadya, Y.; Cohen, S.; Vadai, E.; Dassa, L.; Shahar, E.; Condiotti, R.; et al. Directed elimination of senescent cells by inhibition of BCL-W and BCL-XL. Nat. Commun. 2016, 7, 11190. [Google Scholar] [CrossRef]
- Zhu, Y.; Doornebal, E.J.; Pirtskhalava, T.; Giorgadze, N.; Wentworth, M.; Fuhrmann-Stroissnigg, H.; Niedernhofer, L.J.; Robbins, P.D.; Tchkonia, T.; Kirkland, J.L. New agents that target senescent cells: The flavone, fisetin, and the BCL-X(L) inhibitors, A1331852 and A1155463. Aging 2017, 9, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Hagymasi, A.T.; Dempsey, J.P.; Srivastava, P.K. Heat-Shock Proteins. Curr. Protoc. 2022, 2, e592. [Google Scholar] [CrossRef] [PubMed]
- Karkoulis, P.K.; Stravopodis, D.J.; Konstantakou, E.G.; Voutsinas, G.E. Targeted inhibition of heat shock protein 90 disrupts multiple oncogenic signaling pathways, thus inducing cell cycle arrest and programmed cell death in human urinary bladder cancer cell lines. Cancer Cell Int. 2013, 13, 11. [Google Scholar] [CrossRef]
- Fuhrmann-Stroissnigg, H.; Ling, Y.Y.; Zhao, J.; McGowan, S.J.; Zhu, Y.; Brooks, R.W.; Grassi, D.; Gregg, S.Q.; Stripay, J.L.; Dorronsoro, A.; et al. Identification of HSP90 inhibitors as a novel class of senolytics. Nat. Commun. 2017, 8, 422. [Google Scholar] [CrossRef] [PubMed]
- Coppe, J.P.; Desprez, P.Y.; Krtolica, A.; Campisi, J. The senescence-associated secretory phenotype: The dark side of tumor suppression. Annu. Rev. Pathol. 2010, 5, 99–118. [Google Scholar] [CrossRef] [PubMed]
- Coppe, J.P.; Patil, C.K.; Rodier, F.; Sun, Y.; Munoz, D.P.; Goldstein, J.; Nelson, P.S.; Desprez, P.Y.; Campisi, J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008, 6, e301. [Google Scholar] [CrossRef] [PubMed]
- Malaquin, N.; Martinez, A.; Rodier, F. Keeping the senescence secretome under control: Molecular reins on the senescence-associated secretory phenotype. Exp. Gerontol. 2016, 82, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Borghesan, M.; Fafian-Labora, J.; Eleftheriadou, O.; Carpintero-Fernandez, P.; Paez-Ribes, M.; Vizcay-Barrena, G.; Swisa, A.; Kolodkin-Gal, D.; Ximenez-Embun, P.; Lowe, R.; et al. Small Extracellular Vesicles Are Key Regulators of Non-cell Autonomous Intercellular Communication in Senescence via the Interferon Protein IFITM3. Cell Rep. 2019, 27, 3956–3971.E6. [Google Scholar] [CrossRef]
- Jeon, O.H.; Wilson, D.R.; Clement, C.C.; Rathod, S.; Cherry, C.; Powell, B.; Lee, Z.; Khalil, A.M.; Green, J.J.; Campisi, J.; et al. Senescence cell-associated extracellular vesicles serve as osteoarthritis disease and therapeutic markers. JCI Insight 2019, 4, e125019. [Google Scholar] [CrossRef]
- Tanaka, T.; Biancotto, A.; Moaddel, R.; Moore, A.Z.; Gonzalez-Freire, M.; Aon, M.A.; Candia, J.; Zhang, P.; Cheung, F.; Fantoni, G.; et al. Plasma proteomic signature of age in healthy humans. Aging Cell 2018, 17, e12799. [Google Scholar] [CrossRef] [PubMed]
- Schafer, M.J.; Zhang, X.; Kumar, A.; Atkinson, E.J.; Zhu, Y.; Jachim, S.; Mazula, D.L.; Brown, A.K.; Berning, M.; Aversa, Z.; et al. The senescence-associated secretome as an indicator of age and medical risk. JCI Insight 2020, 5, e133668. [Google Scholar] [CrossRef]
- Fielding, R.A.; Atkinson, E.J.; Aversa, Z.; White, T.A.; Heeren, A.A.; Achenbach, S.J.; Mielke, M.M.; Cummings, S.R.; Pahor, M.; Leeuwenburgh, C.; et al. Associations between biomarkers of cellular senescence and physical function in humans: Observations from the lifestyle interventions for elders (LIFE) study. Geroscience 2022, 44, 2757–2770. [Google Scholar] [CrossRef]
- D’Agnillo, F.; Walters, K.A.; Xiao, Y.; Sheng, Z.M.; Scherler, K.; Park, J.; Gygli, S.; Rosas, L.A.; Sadtler, K.; Kalish, H.; et al. Lung epithelial and endothelial damage, loss of tissue repair, inhibition of fibrinolysis, and cellular senescence in fatal COVID-19. Sci. Transl. Med. 2021, 13, eabj7790. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Eckert, E.; James, A.; Meier-Girard, D.; Kupczyk, M.; Andersson, L.I.; Bossios, A.; Mikus, M.; Ono, J.; Izuhara, K.; Middelveld, R.; et al. Lung function fluctuation patterns unveil asthma and COPD phenotypes unrelated to type 2 inflammation. J. Allergy Clin. Immunol. 2021, 148, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Bracun, V.; van Essen, B.; Voors, A.A.; van Veldhuisen, D.J.; Dickstein, K.; Zannad, F.; Metra, M.; Anker, S.; Samani, N.J.; Ponikowski, P.; et al. Insulin-like growth factor binding protein 7 (IGFBP7), a link between heart failure and senescence. ESC Heart Fail. 2022, 9, 4167–4176. [Google Scholar] [CrossRef] [PubMed]
- Diniz, B.S.; Mulsant, B.H.; Reynolds, C.F., 3rd; Blumberger, D.M.; Karp, J.F.; Butters, M.A.; Mendes-Silva, A.P.; Vieira, E.L.; Tseng, G.; Lenze, E.J. Association of Molecular Senescence Markers in Late-Life Depression With Clinical Characteristics and Treatment Outcome. JAMA Netw. Open 2022, 5, e2219678. [Google Scholar] [CrossRef] [PubMed]
- Rouault, C.; Marcelin, G.; Adriouch, S.; Rose, C.; Genser, L.; Ambrosini, M.; Bichet, J.C.; Zhang, Y.; Marquet, F.; Aron-Wisnewsky, J.; et al. Senescence-associated beta-galactosidase in subcutaneous adipose tissue associates with altered glycaemic status and truncal fat in severe obesity. Diabetologia 2021, 64, 240–254. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Singh, H.; Kwon, K.; Tsitrin, T.; Petrini, J.; Nelson, K.E.; Pieper, R. Protein signatures from blood plasma and urine suggest changes in vascular function and IL-12 signaling in elderly with a history of chronic diseases compared with an age-matched healthy cohort. Geroscience 2021, 43, 593–606. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Gao, F.; Wang, Q.; Lv, X.; Cheng, Z.; Wu, Y.; Chai, X.; Zetterberg, H.; Blennow, K.; Levey, A.I.; et al. Molecules of senescent glial cells differentiate Alzheimer’s disease from ageing. J. Neurol. Neurosurg. Psychiatry 2023, 94, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Justice, J.N.; Nambiar, A.M.; Tchkonia, T.; LeBrasseur, N.K.; Pascual, R.; Hashmi, S.K.; Prata, L.; Masternak, M.M.; Kritchevsky, S.B.; Musi, N.; et al. Senolytics in idiopathic pulmonary fibrosis: Results from a first-in-human, open-label, pilot study. EBioMedicine 2019, 40, 554–563. [Google Scholar] [CrossRef]
- Nambiar, A.; Kellogg, D., 3rd; Justice, J.; Goros, M.; Gelfond, J.; Pascual, R.; Hashmi, S.; Masternak, M.; Prata, L.; LeBrasseur, N.; et al. Senolytics dasatinib and quercetin in idiopathic pulmonary fibrosis: Results of a phase I, single-blind, single-center, randomized, placebo-controlled pilot trial on feasibility and tolerability. EBioMedicine 2023, 90, 104481. [Google Scholar] [CrossRef]
- Zhu, Y.; Prata, L.; Gerdes, E.O.W.; Netto, J.M.E.; Pirtskhalava, T.; Giorgadze, N.; Tripathi, U.; Inman, C.L.; Johnson, K.O.; Xue, A.; et al. Orally-active, clinically-translatable senolytics restore alpha-Klotho in mice and humans. EBioMedicine 2022, 77, 103912. [Google Scholar] [CrossRef]
- Gonzales, M.M.; Garbarino, V.R.; Marques Zilli, E.; Petersen, R.C.; Kirkland, J.L.; Tchkonia, T.; Musi, N.; Seshadri, S.; Craft, S.; Orr, M.E. Senolytic Therapy to Modulate the Progression of Alzheimer’s Disease (SToMP-AD): A Pilot Clinical Trial. J. Prev. Alzheimers Dis. 2022, 9, 22–29. [Google Scholar] [CrossRef]
- Orr, M.; Gonzales, M.; Garbarino, V.; Kautz, T.; Palavicini, J.; Lopez-Cruzan, M.; Dehkordi, S.K.; Mathews, J.; Zare, H.; Xu, P.; et al. Senolytic therapy to modulate the progression of Alzheimer’s Disease (SToMP-AD)—Outcomes from the first clinical trial of senolytic therapy for Alzheimer’s disease. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Bhisitkul, R. UBX1325, A Novel Senolytic Treatment for Patients with Advanced DME or Wet AMD: 24 Week Results of a Phase 1 Study ARVO 2022: AMD and Retinal Physiology. 2022. Available online: https://ir.unitybiotechnology.com/static-files/c8a69714-516b-47af-9339-ec7a5aa44156 (accessed on 1 July 2023).
- Bhisitkul, R.; Klier, S.; Tsuruda, P.; Xie, B.; Masaki, L.; Bautista, J.; Khan, A.; Dananberg, J. UBX1325, A Novel Senolytic Treatment for Patients with Advanced DME or wet AMD: 24-Week Results of a Phase 1 Study. Investig. Ophthalmol. Vis. Sci. 2022, 63, 4287. [Google Scholar]
- Safety, Tolerability and Evidence of Activity Study of UBX1325 in Patients with Diabetic Macular Edema (BEHOLD). Available online: https://clinicaltrials.gov/show/NCT04857996 (accessed on 1 July 2023).
- Unity Biotechnology Announces Positive 24-Week Data from Phase 2 Behold Study of UBX1325 in Patients with Diabetic Macular Edema. 2022. Available online: https://ir.unitybiotechnology.com/news-releases/news-release-details/unity-biotechnology-announces-positive-24-week-data-phase-2 (accessed on 1 July 2023).
- Senolytic Therapy to Modulate the Progression of Alzheimer’s Disease (SToMP-AD) Study. Available online: https://clinicaltrials.gov/show/NCT04685590 (accessed on 1 July 2023).
- Hematopoietic Stem Cell Transplant Survivors Study. Available online: https://clinicaltrials.gov/show/NCT02652052 (accessed on 1 July 2023).
- An Open-Label Intervention Trial to Reduce Senescence and Improve Frailty in Adult Survivors of Childhood Cancer. Available online: https://clinicaltrials.gov/show/NCT04733534 (accessed on 1 July 2023).
- Targeting Cellular Senescence with Senolytics to Improve Skeletal Health in Older Humans. Available online: https://clinicaltrials.gov/show/NCT04313634 (accessed on 1 July 2023).
- Senolytic Drugs Attenuate Osteoarthritis-Related Articular Cartilage Degeneration: A Clinical Trial. Available online: https://clinicaltrials.gov/show/NCT04210986 (accessed on 1 July 2023).
- Alleviation by Fisetin of Frailty, Inflammation, and Related Measures in Older Women. Available online: https://clinicaltrials.gov/show/NCT03430037 (accessed on 1 July 2023).
- Verdoorn, B.P.; Evans, T.K.; Hanson, G.J.; Zhu, Y.; Langhi Prata, L.G.P.; Pignolo, R.J.; Atkinson, E.J.; Wissler-Gerdes, E.O.; Kuchel, G.A.; Mannick, J.B.; et al. Fisetin for COVID-19 in skilled nursing facilities: Senolytic trials in the COVID era. J. Am. Geriatr. Soc. 2021, 69, 3023–3033. [Google Scholar] [CrossRef]
- COVID-FISETIN: Pilot in SARS-CoV-2 of Fisetin to Alleviate Dysfunction and Inflammation. Available online: https://clinicaltrials.gov/show/NCT04476953 (accessed on 1 July 2023).
- COVFIS-HOME: COVID-19 Pilot Study of Fisetin to Alleviate Dysfunction and Decrease Complications. Available online: https://clinicaltrials.gov/show/NCT04771611 (accessed on 1 July 2023).
- Gasek, N.S.; Kuchel, G.A.; Kirkland, J.L.; Xu, M. Strategies for Targeting Senescent Cells in Human Disease. Nat. Aging 2021, 1, 870–879. [Google Scholar] [CrossRef] [PubMed]
- Barzilai, N.; Crandall, J.P.; Kritchevsky, S.B.; Espeland, M.A. Metformin as a Tool to Target Aging. Cell Metab. 2016, 23, 1060–1065. [Google Scholar] [CrossRef]
- The TAME Trial: Targeting the Biology of Aging. Ushering a New Era of Interventions. American Federation for Aging Research. 2023. Available online: https://www.afar.org/tame-trial?token=mRxFYujTQkC61C0SDVNu5oFHe_vxrV1bscientific-awardsiagg-biological-sciences-program (accessed on 1 July 2023).
- Justice, J.N.; Ferrucci, L.; Newman, A.B.; Aroda, V.R.; Bahnson, J.L.; Divers, J.; Espeland, M.A.; Marcovina, S.; Pollak, M.N.; Kritchevsky, S.B.; et al. A framework for selection of blood-based biomarkers for geroscience-guided clinical trials: Report from the TAME Biomarkers Workgroup. Geroscience 2018, 40, 419–436. [Google Scholar] [CrossRef]
- Gillet, L.C.; Navarro, P.; Tate, S.; Rost, H.; Selevsek, N.; Reiter, L.; Bonner, R.; Aebersold, R. Targeted data extraction of the MS/MS spectra generated by data-independent acquisition: A new concept for consistent and accurate proteome analysis. Mol. Cell. Proteom. 2012, 11, O111.016717. [Google Scholar] [CrossRef]
- Collins, B.C.; Hunter, C.L.; Liu, Y.; Schilling, B.; Rosenberger, G.; Bader, S.L.; Chan, D.W.; Gibson, B.W.; Gingras, A.C.; Held, J.M.; et al. Multi-laboratory assessment of reproducibility, qualitative and quantitative performance of SWATH-mass spectrometry. Nat. Commun. 2017, 8, 291. [Google Scholar] [CrossRef]
- Sajic, T.; Liu, Y.; Aebersold, R. Using data-independent, high-resolution mass spectrometry in protein biomarker research: Perspectives and clinical applications. Proteom. Clin. Appl. 2015, 9, 307–321. [Google Scholar] [CrossRef]
- Dey, A.K.; Kumar, B.; Singh, A.K.; Ranjan, P.; Thiruvengadam, R.; Desiraju, B.K.; Kshetrapal, P.; Wadhwa, N.; Bhatnagar, S.; Rashid, F.; et al. Salivary proteome signatures in the early and middle stages of human pregnancy with term birth outcome. Sci. Rep. 2020, 10, 8022. [Google Scholar] [CrossRef]
- Kumar, B.; Dey, A.K.; Saha, S.; Singh, A.K.; Kshetrapal, P.; Wadhwa, N.; Thiruvengadam, R.; Desiraju, B.K.; Bhatnagar, S.; Salunke, D.M.; et al. Dynamic Alteration in the Vaginal Secretory Proteome across the Early and Mid-Trimesters of Pregnancy. J. Proteome Res. 2021, 20, 1190–1205. [Google Scholar] [CrossRef]
- Xie, X.; Shah, S.; Holtz, A.; Rose, J.; Basisty, N.; Schilling, B. Simultaneous Affinity Enrichment of Two Post-Translational Modifications for Quantification and Site Localization. J. Vis. Exp. 2020, 156, e60780. [Google Scholar] [CrossRef]
- Basisty, N.; Meyer, J.G.; Wei, L.; Gibson, B.W.; Schilling, B. Simultaneous Quantification of the Acetylome and Succinylome by ‘One-Pot’ Affinity Enrichment. Proteomics 2018, 18, e1800123. [Google Scholar] [CrossRef]
- Zhang, Y.; Bilbao, A.; Bruderer, T.; Luban, J.; Strambio-De-Castillia, C.; Lisacek, F.; Hopfgartner, G.; Varesio, E. The Use of Variable Q1 Isolation Windows Improves Selectivity in LC-SWATH-MS Acquisition. J. Proteome Res. 2015, 14, 4359–4371. [Google Scholar] [CrossRef]
- Amodei, D.; Egertson, J.; MacLean, B.X.; Johnson, R.; Merrihew, G.E.; Keller, A.; Marsh, D.; Vitek, O.; Mallick, P.; MacCoss, M.J. Improving Precursor Selectivity in Data-Independent Acquisition Using Overlapping Windows. J. Am. Soc. Mass Spectrom. 2019, 30, 669–684. [Google Scholar] [CrossRef] [PubMed]
- Pino, L.K.; Just, S.C.; MacCoss, M.J.; Searle, B.C. Acquiring and Analyzing Data Independent Acquisition Proteomics Experiments without Spectrum Libraries. Mol. Cell. Proteom. 2020, 19, 1088–1103. [Google Scholar] [CrossRef] [PubMed]
- Meier, F.; Brunner, A.D.; Koch, S.; Koch, H.; Lubeck, M.; Krause, M.; Goedecke, N.; Decker, J.; Kosinski, T.; Park, M.A.; et al. Online Parallel Accumulation-Serial Fragmentation (PASEF) with a Novel Trapped Ion Mobility Mass Spectrometer. Mol. Cell. Proteom. 2018, 17, 2534–2545. [Google Scholar] [CrossRef] [PubMed]
- Meier, F.; Brunner, A.D.; Frank, M.; Ha, A.; Bludau, I.; Voytik, E.; Kaspar-Schoenefeld, S.; Lubeck, M.; Raether, O.; Bache, N.; et al. diaPASEF: Parallel accumulation-serial fragmentation combined with data-independent acquisition. Nat. Methods 2020, 17, 1229–1236. [Google Scholar] [CrossRef] [PubMed]
- Rifai, N.; Gillette, M.A.; Carr, S.A. Protein biomarker discovery and validation: The long and uncertain path to clinical utility. Nat. Biotechnol. 2006, 24, 971–983. [Google Scholar] [CrossRef] [PubMed]
- Doll, S.; Burlingame, A.L. Mass spectrometry-based detection and assignment of protein posttranslational modifications. ACS Chem. Biol. 2015, 10, 63–71. [Google Scholar] [CrossRef]
- Olzscha, H. Posttranslational modifications and proteinopathies: How guardians of the proteome are defeated. Biol. Chem. 2019, 400, 895–915. [Google Scholar] [CrossRef]
- Retanal, C.; Ball, B.; Geddes-McAlister, J. Post-Translational Modifications Drive Success and Failure of Fungal-Host Interactions. J. Fungi 2021, 7, 124. [Google Scholar] [CrossRef]
- Ramazi, S.; Zahiri, J. Posttranslational modifications in proteins: Resources, tools and prediction methods. Database 2021, 2021, baab012. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Kashina, A. Post-translational Modifications of the Protein Termini. Front. Cell Dev. Biol. 2021, 9, 719590. [Google Scholar] [CrossRef]
- Smith, L.M.; Kelleher, N.L.; Linial, M.; Goodlett, D.; Langridge-Smith, P.; Ah Goo, Y.; Safford, G.; Bonilla, L.; Kruppa, G.; Zubarev, R.; et al. Proteoform: A single term describing protein complexity. Nat. Methods 2013, 10, 186–187. [Google Scholar] [CrossRef]
- Holtz, A.; Basisty, N.; Schilling, B. Quantification and Identification of Post-Translational Modifications Using Modern Proteomics Approaches. Methods Mol. Biol. 2021, 2228, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.M.; Kelleher, N.L. Proteoforms as the next proteomics currency. Science 2018, 359, 1106–1107. [Google Scholar] [CrossRef] [PubMed]
- Brodbelt, J.S. Deciphering combinatorial post-translational modifications by top-down mass spectrometry. Curr. Opin. Chem. Biol. 2022, 70, 102180. [Google Scholar] [CrossRef]
- Melby, J.A.; Roberts, D.S.; Larson, E.J.; Brown, K.A.; Bayne, E.F.; Jin, S.; Ge, Y. Novel Strategies to Address the Challenges in Top-Down Proteomics. J. Am. Soc. Mass Spectrom. 2021, 32, 1278–1294. [Google Scholar] [CrossRef] [PubMed]
- Toby, T.K.; Fornelli, L.; Kelleher, N.L. Progress in Top-Down Proteomics and the Analysis of Proteoforms. Annu. Rev. Anal. Chem. 2016, 9, 499–519. [Google Scholar] [CrossRef]
- Zhong, Q.; Xiao, X.; Qiu, Y.; Xu, Z.; Chen, C.; Chong, B.; Zhao, X.; Hai, S.; Li, S.; An, Z.; et al. Protein posttranslational modifications in health and diseases: Functions, regulatory mechanisms, and therapeutic implications. MedComm 2023, 4, e261. [Google Scholar] [CrossRef]
- Tong, Y.; Sun, M.; Chen, L.; Wang, Y.; Li, Y.; Li, L.; Zhang, X.; Cai, Y.; Qie, J.; Pang, Y.; et al. Proteogenomic insights into the biology and treatment of pancreatic ductal adenocarcinoma. J. Hematol. Oncol. 2022, 15, 168. [Google Scholar] [CrossRef]
- Krug, K.; Jaehnig, E.J.; Satpathy, S.; Blumenberg, L.; Karpova, A.; Anurag, M.; Miles, G.; Mertins, P.; Geffen, Y.; Tang, L.C.; et al. Proteogenomic Landscape of Breast Cancer Tumorigenesis and Targeted Therapy. Cell 2020, 183, 1436–1456.e31. [Google Scholar] [CrossRef] [PubMed]
- Satpathy, S.; Krug, K.; Jean Beltran, P.M.; Savage, S.R.; Petralia, F.; Kumar-Sinha, C.; Dou, Y.; Reva, B.; Kane, M.H.; Avanessian, S.C.; et al. A proteogenomic portrait of lung squamous cell carcinoma. Cell 2021, 184, 4348–4371.e40. [Google Scholar] [CrossRef] [PubMed]
- Melani, R.D.; Gerbasi, V.R.; Anderson, L.C.; Sikora, J.W.; Toby, T.K.; Hutton, J.E.; Butcher, D.S.; Negrão, F.; Seckler, H.S.; Srzentić, K.; et al. The Blood Proteoform Atlas: A reference map of proteoforms in human hematopoietic cells. Science 2022, 375, 411–418. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, D.; Cupp-Sutton, K.A.; Liu, X.; Smith, K.; Wu, S. Development of an Online 2D Ultrahigh-Pressure Nano-LC System for High-pH and Low-pH Reversed Phase Separation in Top-Down Proteomics. Anal. Chem. 2020, 92, 12774–12777. [Google Scholar] [CrossRef]
- McCool, E.N.; Lubeckyj, R.A.; Shen, X.; Chen, D.; Kou, Q.; Liu, X.; Sun, L. Deep Top-Down Proteomics Using Capillary Zone Electrophoresis-Tandem Mass Spectrometry: Identification of 5700 Proteoforms from the Escherichia coli Proteome. Anal. Chem. 2018, 90, 5529–5533. [Google Scholar] [CrossRef] [PubMed]
- Takemori, A.; Butcher, D.S.; Harman, V.M.; Brownridge, P.; Shima, K.; Higo, D.; Ishizaki, J.; Hasegawa, H.; Suzuki, J.; Yamashita, M.; et al. PEPPI-MS: Polyacrylamide-Gel-Based Prefractionation for Analysis of Intact Proteoforms and Protein Complexes by Mass Spectrometry. J. Proteome Res. 2020, 19, 3779–3791. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Rockwood, A.L.; Agarwal, A.M.; Anderson, L.C.; Weisbrod, C.R.; Hendrickson, C.L.; Marshall, A.G. Top-down proteomics-a near-future technique for clinical diagnosis? Ann. Transl. Med. 2020, 8, 136. [Google Scholar] [CrossRef]
- Moaddel, R.; Ubaida-Mohien, C.; Tanaka, T.; Lyashkov, A.; Basisty, N.; Schilling, B.; Semba, R.D.; Franceschi, C.; Gorospe, M.; Ferrucci, L. Proteomics in aging research: A roadmap to clinical, translational research. Aging Cell 2021, 20, e13325. [Google Scholar] [CrossRef]
- Hortin, G.L.; Sviridov, D. The dynamic range problem in the analysis of the plasma proteome. J. Proteom. 2010, 73, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yi, E.C.; Li, X.J.; Mallick, P.; Kelly-Spratt, K.S.; Masselon, C.D.; Camp, D.G., 2nd; Smith, R.D.; Kemp, C.J.; Aebersold, R. High throughput quantitative analysis of serum proteins using glycopeptide capture and liquid chromatography mass spectrometry. Mol. Cell. Proteom. 2005, 4, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Neverova, I.; Van Eyk, J.E. Role of chromatographic techniques in proteomic analysis. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2005, 815, 51–63. [Google Scholar] [CrossRef]
- Matt, P.; Fu, Z.; Fu, Q.; Van Eyk, J.E. Biomarker discovery: Proteome fractionation and separation in biological samples. Physiol. Genom. 2008, 33, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.Y.; Osman, J.; Low, T.Y.; Jamal, R. Plasma/serum proteomics: Depletion strategies for reducing high-abundance proteins for biomarker discovery. Bioanalysis 2019, 11, 1799–1812. [Google Scholar] [CrossRef] [PubMed]
- Tu, C.; Rudnick, P.A.; Martinez, M.Y.; Cheek, K.L.; Stein, S.E.; Slebos, R.J.; Liebler, D.C. Depletion of abundant plasma proteins and limitations of plasma proteomics. J. Proteome Res. 2010, 9, 4982–4991. [Google Scholar] [CrossRef]
- Guerrier, L.; Righetti, P.G.; Boschetti, E. Reduction of dynamic protein concentration range of biological extracts for the discovery of low-abundance proteins by means of hexapeptide ligand library. Nat. Protoc. 2008, 3, 883–890. [Google Scholar] [CrossRef]
- Leger, T.; Lavigne, D.; Le Caer, J.P.; Guerrier, L.; Boschetti, E.; Fareh, J.; Feldman, L.; Laprevote, O.; Meilhac, O. Solid-phase hexapeptide ligand libraries open up new perspectives in the discovery of biomarkers in human plasma. Clin. Chim. Acta 2011, 412, 740–747. [Google Scholar] [CrossRef]
- Pisanu, S.; Biosa, G.; Carcangiu, L.; Uzzau, S.; Pagnozzi, D. Comparative evaluation of seven commercial products for human serum enrichment/depletion by shotgun proteomics. Talanta 2018, 185, 213–220. [Google Scholar] [CrossRef]
- Boschetti, E.; Righetti, P.G. Low-Abundance Protein Enrichment for Medical Applications: The Involvement of Combinatorial Peptide Library Technique. Int. J. Mol. Sci. 2023, 24, 329. [Google Scholar] [CrossRef]
- Meng, R.; Gormley, M.; Bhat, V.B.; Rosenberg, A.; Quong, A.A. Low abundance protein enrichment for discovery of candidate plasma protein biomarkers for early detection of breast cancer. J. Proteom. 2011, 75, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Chen, Y.; Sun, X.; Li, Y.; Huang, C.; Deng, H.; Li, Z. Identification of potential serum biomarkers for rheumatoid arthritis by high-resolution quantitative proteomic analysis. Inflammation 2014, 37, 1459–1467. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Tang, H.Y.; Wang, H.; Liu, Q.; Speicher, D.W. Systematic comparison of fractionation methods for in-depth analysis of plasma proteomes. J. Proteome Res. 2012, 11, 3090–3100. [Google Scholar] [CrossRef] [PubMed]
- Mauri, P.; Scigelova, M. Multidimensional protein identification technology for clinical proteomic analysis. Clin. Chem. Lab. Med. 2009, 47, 636–646. [Google Scholar] [CrossRef]
- Geyer, P.E.; Holdt, L.M.; Teupser, D.; Mann, M. Revisiting biomarker discovery by plasma proteomics. Mol. Syst. Biol. 2017, 13, 942. [Google Scholar] [CrossRef]
- Kaur, G.; Poljak, A.; Ali, S.A.; Zhong, L.; Raftery, M.J.; Sachdev, P. Extending the Depth of Human Plasma Proteome Coverage Using Simple Fractionation Techniques. J. Proteome Res. 2021, 20, 1261–1279. [Google Scholar] [CrossRef]
- Laffoon, S.B.; Doecke, J.D.; Roberts, A.M.; Vance, J.A.; Reeves, B.D.; Pertile, K.K.; Rumble, R.L.; Fowler, C.J.; Trounson, B.; Ames, D.; et al. Analysis of plasma proteins using 2D gels and novel fluorescent probes: In search of blood based biomarkers for Alzheimer’s disease. Proteome Sci. 2022, 20, 2. [Google Scholar] [CrossRef]
- Moulder, R.; Bhosale, S.D.; Goodlett, D.R.; Lahesmaa, R. Analysis of the plasma proteome using iTRAQ and TMT-based Isobaric labeling. Mass Spectrom. Rev. 2018, 37, 583–606. [Google Scholar] [CrossRef]
- Keshishian, H.; Burgess, M.W.; Specht, H.; Wallace, L.; Clauser, K.R.; Gillette, M.A.; Carr, S.A. Quantitative, multiplexed workflow for deep analysis of human blood plasma and biomarker discovery by mass spectrometry. Nat. Protoc. 2017, 12, 1683–1701. [Google Scholar] [CrossRef]
- Ferdosi, S.; Tangeysh, B.; Brown, T.R.; Everley, P.A.; Figa, M.; McLean, M.; Elgierari, E.M.; Zhao, X.; Garcia, V.J.; Wang, T.; et al. Engineered nanoparticles enable deep proteomics studies at scale by leveraging tunable nano–bio interactions. Proc. Natl. Acad. Sci. USA 2022, 119, e2106053119. [Google Scholar] [CrossRef]
- Meng, Y.; Chen, J.; Liu, Y.; Zhu, Y.; Wong, Y.K.; Lyu, H.; Shi, Q.; Xia, F.; Gu, L.; Zhang, X.; et al. A highly efficient protein corona-based proteomic analysis strategy for the discovery of pharmacodynamic biomarkers. J. Pharm. Anal. 2022, 12, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Tenzer, S.; Docter, D.; Rosfa, S.; Wlodarski, A.; Kuharev, J.; Rekik, A.; Knauer, S.K.; Bantz, C.; Nawroth, T.; Bier, C.; et al. Nanoparticle size is a critical physicochemical determinant of the human blood plasma corona: A comprehensive quantitative proteomic analysis. ACS Nano 2011, 5, 7155–7167. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, N.; Grenier, P.; Mahmoudi, M.; Lima, E.M.; Appel, E.A.; Dormont, F.; Lim, J.M.; Karnik, R.; Langer, R.; Farokhzad, O.C. Mechanistic understanding of in vivo protein corona formation on polymeric nanoparticles and impact on pharmacokinetics. Nat. Commun. 2017, 8, 777. [Google Scholar] [CrossRef] [PubMed]
- Blume, J.E.; Manning, W.C.; Troiano, G.; Hornburg, D.; Figa, M.; Hesterberg, L.; Platt, T.L.; Zhao, X.; Cuaresma, R.A.; Everley, P.A.; et al. Rapid, deep and precise profiling of the plasma proteome with multi-nanoparticle protein corona. Nat. Commun. 2020, 11, 3662. [Google Scholar] [CrossRef] [PubMed]
- Ashkarran, A.A.; Gharibi, H.; Voke, E.; Landry, M.P.; Saei, A.A.; Mahmoudi, M. Measurements of heterogeneity in proteomics analysis of the nanoparticle protein corona across core facilities. Nat. Commun. 2022, 13, 6610. [Google Scholar] [CrossRef]
- Schopper, S.; Kahraman, A.; Leuenberger, P.; Feng, Y.; Piazza, I.; Muller, O.; Boersema, P.J.; Picotti, P. Measuring protein structural changes on a proteome-wide scale using limited proteolysis-coupled mass spectrometry. Nat. Protoc. 2017, 12, 2391–2410. [Google Scholar] [CrossRef]
- Pepelnjak, M.; de Souza, N.; Picotti, P. Detecting Protein-Small Molecule Interactions Using Limited Proteolysis-Mass Spectrometry (LiP-MS). Trends Biochem. Sci. 2020, 45, 919–920. [Google Scholar] [CrossRef]
- Park, C.; Marqusee, S. Pulse proteolysis: A simple method for quantitative determination of protein stability and ligand binding. Nat. Methods 2005, 2, 207–212. [Google Scholar] [CrossRef]
- Adhikari, J.; Fitzgerald, M.C. SILAC-pulse proteolysis: A mass spectrometry-based method for discovery and cross-validation in proteome-wide studies of ligand binding. J. Am. Soc. Mass Spectrom. 2014, 25, 2073–2083. [Google Scholar] [CrossRef]
- West, G.M.; Tang, L.; Fitzgerald, M.C. Thermodynamic analysis of protein stability and ligand binding using a chemical modification- and mass spectrometry-based strategy. Anal. Chem. 2008, 80, 4175–4185. [Google Scholar] [CrossRef]
- Strickland, E.C.; Geer, M.A.; Tran, D.T.; Adhikari, J.; West, G.M.; DeArmond, P.D.; Xu, Y.; Fitzgerald, M.C. Thermodynamic analysis of protein-ligand binding interactions in complex biological mixtures using the stability of proteins from rates of oxidation. Nat. Protoc. 2013, 8, 148–161. [Google Scholar] [CrossRef] [PubMed]
- Martinez Molina, D.; Jafari, R.; Ignatushchenko, M.; Seki, T.; Larsson, E.A.; Dan, C.; Sreekumar, L.; Cao, Y.; Nordlund, P. Monitoring drug target engagement in cells and tissues using the cellular thermal shift assay. Science 2013, 341, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Savitski, M.M.; Reinhard, F.B.; Franken, H.; Werner, T.; Savitski, M.F.; Eberhard, D.; Martinez Molina, D.; Jafari, R.; Dovega, R.B.; Klaeger, S.; et al. Tracking cancer drugs in living cells by thermal profiling of the proteome. Science 2014, 346, 1255784. [Google Scholar] [CrossRef] [PubMed]
- Gaetani, M.; Sabatier, P.; Saei, A.A.; Beusch, C.M.; Yang, Z.; Lundstrom, S.L.; Zubarev, R.A. Proteome Integral Solubility Alteration: A High-Throughput Proteomics Assay for Target Deconvolution. J. Proteome Res. 2019, 18, 4027–4037. [Google Scholar] [CrossRef]
- Kaur, S.; McGuire, L.; Tang, D.; Dollinger, G.; Huebner, V. Affinity selection and mass spectrometry-based strategies to identify lead compounds in combinatorial libraries. J. Protein Chem. 1997, 16, 505–511. [Google Scholar] [CrossRef]
- Jonker, N.; Kool, J.; Irth, H.; Niessen, W.M. Recent developments in protein-ligand affinity mass spectrometry. Anal. Bioanal. Chem. 2011, 399, 2669–2681. [Google Scholar] [CrossRef]
- Maecker, H.T.; McCoy, J.P.; Nussenblatt, R. Standardizing immunophenotyping for the Human Immunology Project. Nat. Rev. Immunol. 2012, 12, 191–200. [Google Scholar] [CrossRef]
- Gratama, J.W.; Sutherland, D.R.; Keeney, M.; Papa, S. Flow cytometric enumeration and immunophenotyping of hematopoietic stem and progenitor cells. J. Biol. Regul. Homeost. Agents 2001, 15, 14–22. [Google Scholar]
- Kim, K.M.; Noh, J.H.; Bodogai, M.; Martindale, J.L.; Yang, X.; Indig, F.E.; Basu, S.K.; Ohnuma, K.; Morimoto, C.; Johnson, P.F.; et al. Identification of senescent cell surface targetable protein DPP4. Genes Dev. 2017, 31, 1529–1534. [Google Scholar] [CrossRef]
- Herman, A.B.; Tsitsipatis, D.; Anerillas, C.; Mazan-Mamczarz, K.; Carr, A.E.; Gregg, J.M.; Wang, M.; Zhang, J.; Michel, M.; Henry-Smith, C.; et al. DPP4 inhibition impairs senohemostasis to improve plaque stability in atherosclerotic mice. J. Clin. Investig. 2023, 133, e165933. [Google Scholar] [CrossRef]
- Amor, C.; Feucht, J.; Leibold, J.; Ho, Y.J.; Zhu, C.; Alonso-Curbelo, D.; Mansilla-Soto, J.; Boyer, J.A.; Li, X.; Giavridis, T.; et al. Senolytic CAR T cells reverse senescence-associated pathologies. Nature 2020, 583, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Ekpenyong-Akiba, A.E.; Poblocka, M.; Macip, S. Targeted Senolytic Strategies Based on the Senescent Surfaceome. In Senolytics in Disease, Ageing and Longevity; Muñoz-Espin, D., Demaria, M., Eds.; Springer International Publishing: Cham, Swizterland, 2020; pp. 103–130. [Google Scholar] [CrossRef]
- Althubiti, M.; Lezina, L.; Carrera, S.; Jukes-Jones, R.; Giblett, S.M.; Antonov, A.; Barlev, N.; Saldanha, G.S.; Pritchard, C.A.; Cain, K.; et al. Characterization of novel markers of senescence and their prognostic potential in cancer. Cell Death Dis. 2014, 5, e1528. [Google Scholar] [CrossRef] [PubMed]
- Qu, A.; Wu, X.; Li, S.; Sun, M.; Xu, L.; Kuang, H.; Xu, C. An NIR-Responsive DNA-Mediated Nanotetrahedron Enhances the Clearance of Senescent Cells. Adv. Mater. 2020, 32, 2000184. [Google Scholar] [CrossRef] [PubMed]
- Hoare, M.; Ito, Y.; Kang, T.W.; Weekes, M.P.; Matheson, N.J.; Patten, D.A.; Shetty, S.; Parry, A.J.; Menon, S.; Salama, R.; et al. NOTCH1 mediates a switch between two distinct secretomes during senescence. Nat. Cell Biol. 2016, 18, 979–992. [Google Scholar] [CrossRef]
- Kim, K.M.; Noh, J.H.; Bodogai, M.; Martindale, J.L.; Pandey, P.R.; Yang, X.; Biragyn, A.; Abdelmohsen, K.; Gorospe, M. SCAMP4 enhances the senescent cell secretome. Genes Dev. 2018, 32, 909–914. [Google Scholar] [CrossRef]
- Doolittle, M.L.; Saul, D.; Kaur, J.; Rowsey, J.L.; Vos, S.J.; Pavelko, K.D.; Farr, J.N.; Monroe, D.G.; Khosla, S. Multiparametric senescent cell phenotyping reveals targets of senolytic therapy in the aged murine skeleton. Nat. Commun. 2023, 14, 4587. [Google Scholar] [CrossRef]
- Pauwels, J.; Fijalkowska, D.; Eyckerman, S.; Gevaert, K. Mass spectrometry and the cellular surfaceome. Mass Spectrom. Rev. 2022, 41, 804–841. [Google Scholar] [CrossRef]
- Meyfour, A.; Pahlavan, S.; Mirzaei, M.; Krijgsveld, J.; Baharvand, H.; Salekdeh, G.H. The quest of cell surface markers for stem cell therapy. Cell. Mol. Life Sci. 2021, 78, 469–495. [Google Scholar] [CrossRef]
- Wollscheid, B.; Bausch-Fluck, D.; Henderson, C.; O’Brien, R.; Bibel, M.; Schiess, R.; Aebersold, R.; Watts, J.D. Mass-spectrometric identification and relative quantification of N-linked cell surface glycoproteins. Nat. Biotechnol. 2009, 27, 378–386. [Google Scholar] [CrossRef]
- Bausch-Fluck, D.; Hofmann, A.; Wollscheid, B. Cell surface capturing technologies for the surfaceome discovery of hepatocytes. Methods Mol. Biol. 2012, 909, 1–16. [Google Scholar] [CrossRef]
- Berg Luecke, L.; Gundry, R.L. Assessment of Streptavidin Bead Binding Capacity to Improve Quality of Streptavidin-based Enrichment Studies. J. Proteome Res. 2021, 20, 1153–1164. [Google Scholar] [CrossRef] [PubMed]
- van Oostrum, M.; Muller, M.; Klein, F.; Bruderer, R.; Zhang, H.; Pedrioli, P.G.A.; Reiter, L.; Tsapogas, P.; Rolink, A.; Wollscheid, B. Classification of mouse B cell types using surfaceome proteotype maps. Nat. Commun. 2019, 10, 5734. [Google Scholar] [CrossRef] [PubMed]
- van Oostrum, M.; Campbell, B.; Seng, C.; Muller, M.; Tom Dieck, S.; Hammer, J.; Pedrioli, P.G.A.; Foldy, C.; Tyagarajan, S.K.; Wollscheid, B. Surfaceome dynamics reveal proteostasis-independent reorganization of neuronal surface proteins during development and synaptic plasticity. Nat. Commun. 2020, 11, 4990. [Google Scholar] [CrossRef]
- Luecke, L.B.; Waas, M.; Littrell, J.; Wojtkiewicz, M.; Castro, C.; Burkovetskaya, M.; Schuette, E.N.; Buchberger, A.R.; Churko, J.M.; Chalise, U.; et al. Surfaceome mapping of primary human heart cells with CellSurfer uncovers cardiomyocyte surface protein LSMEM2 and proteome dynamics in failing hearts. Nat. Cardiovasc. Res. 2023, 2, 76–95. [Google Scholar] [CrossRef] [PubMed]
- Muller, M.; Grabnitz, F.; Barandun, N.; Shen, Y.; Wendt, F.; Steiner, S.N.; Severin, Y.; Vetterli, S.U.; Mondal, M.; Prudent, J.R.; et al. Light-mediated discovery of surfaceome nanoscale organization and intercellular receptor interaction networks. Nat. Commun. 2021, 12, 7036. [Google Scholar] [CrossRef]
- Waas, M.; Littrell, J.; Gundry, R.L. CIRFESS: An Interactive Resource for Querying the Set of Theoretically Detectable Peptides for Cell Surface and Extracellular Enrichment Proteomic Studies. J. Am. Soc. Mass Spectrom. 2020, 31, 1389–1397. [Google Scholar] [CrossRef]
- Waas, M.; Snarrenberg, S.T.; Littrell, J.; Jones Lipinski, R.A.; Hansen, P.A.; Corbett, J.A.; Gundry, R.L. SurfaceGenie: A web-based application for prioritizing cell-type-specific marker candidates. Bioinformatics 2020, 36, 1367–4811. [Google Scholar] [CrossRef]
- Omasits, U.; Ahrens, C.H.; Müller, S.; Wollscheid, B. Protter: Interactive protein feature visualization and integration with experimental proteomic data. Bioinformatics 2014, 30, 884–886. [Google Scholar] [CrossRef]
- Bausch-Fluck, D.; Hofmann, A.; Bock, T.; Frei, A.P.; Cerciello, F.; Jacobs, A.; Moest, H.; Omasits, U.; Gundry, R.L.; Yoon, C.; et al. A Mass Spectrometric-Derived Cell Surface Protein Atlas. PLoS ONE 2015, 10, e0121314. [Google Scholar] [CrossRef]
- Bausch-Fluck, D.; Goldmann, U.; Müller, S.; van Oostrum, M.; Müller, M.; Schubert Olga, T.; Wollscheid, B. The in silico human surfaceome. Proc. Natl. Acad. Sci. USA 2018, 115, E10988–E10997. [Google Scholar] [CrossRef]

| Surface Protein | Cell/Tissue Type | Senescence Inducer | Strategy | Senolytic Validation | Reference |
|---|---|---|---|---|---|
| DEP1/PTPRJ/CD148 | Bladder cancer (EJp21 and EJp16); human lung fibroblast (IMR-90); human fibrosarcoma (HT1080p21); mouse lung adenomas (V600EBRAF); human melanocytes | p21/p16 overexpression; RS, RAS OIS - - - | PM isolation, in-gel digestion, and LC-MS/MS | [195] | |
| B2MG | Bladder cancer (EJp21 and EJp16); mouse lung adenomas (V600EBRAF) | p21/p16 overexpression - | PM isolation, in-gel digestion, and LC-MS/MS | Gold nanoparticles, ADCs | [13,195,196] |
| NOTCH1 | Human lung fibroblast (IMR-90); mouse pancreatic neoplasm (p48-cre) | HRASG12V, OIS, Etoposide KrasG12D OIS | SILAC | [197] | |
| DPP4/CD26 | Human lung fibroblast (WI-38, IMR-90); human aortic endothelial cells (HAEC); human umbilical vein endothelial cells (HUVEC); mouse embryonic fibroblasts (MEF) | RS, IR, Doxorubicin IR IR HRASG12V OIS | PM isolation, in-gel digestion, and LC-MS/MS | ADCC | [191] |
| SCAMP4 | Human lung fibroblast (WI-38, IMR-90); human aortic endothelial cells (HAEC); human umbilical vein endothelial cells (HUVEC) | RS, IR, Doxorubicin, HRASG12V OIS IR IR | PM isolation, in-gel digestion, and LC-MS/MS | [198] | |
| CD24 | Bone marrow mesenchymal cells from INK-ATTAC mice | - | CyTOF | [199] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dey, A.K.; Banarjee, R.; Boroumand, M.; Rutherford, D.V.; Strassheim, Q.; Nyunt, T.; Olinger, B.; Basisty, N. Translating Senotherapeutic Interventions into the Clinic with Emerging Proteomic Technologies. Biology 2023, 12, 1301. https://doi.org/10.3390/biology12101301
Dey AK, Banarjee R, Boroumand M, Rutherford DV, Strassheim Q, Nyunt T, Olinger B, Basisty N. Translating Senotherapeutic Interventions into the Clinic with Emerging Proteomic Technologies. Biology. 2023; 12(10):1301. https://doi.org/10.3390/biology12101301
Chicago/Turabian StyleDey, Amit K., Reema Banarjee, Mozhgan Boroumand, Delaney V. Rutherford, Quinn Strassheim, Thedoe Nyunt, Bradley Olinger, and Nathan Basisty. 2023. "Translating Senotherapeutic Interventions into the Clinic with Emerging Proteomic Technologies" Biology 12, no. 10: 1301. https://doi.org/10.3390/biology12101301





