A Comprehensive Review of the Current Status of the Cellular Neurobiology of Psychedelics
Abstract
:Simple Summary
Abstract
1. Introduction
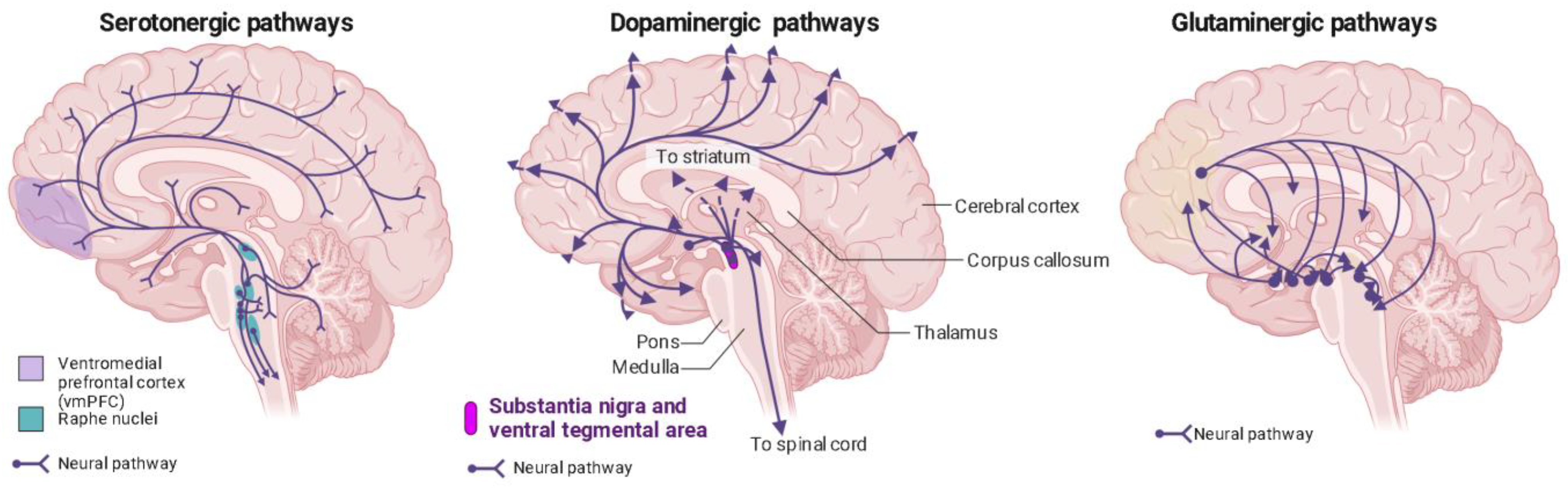
2. Psychedelics Exert Their Effects on the Brain at Multiple Levels, Engaging in Intricate and Multifaceted Mechanisms
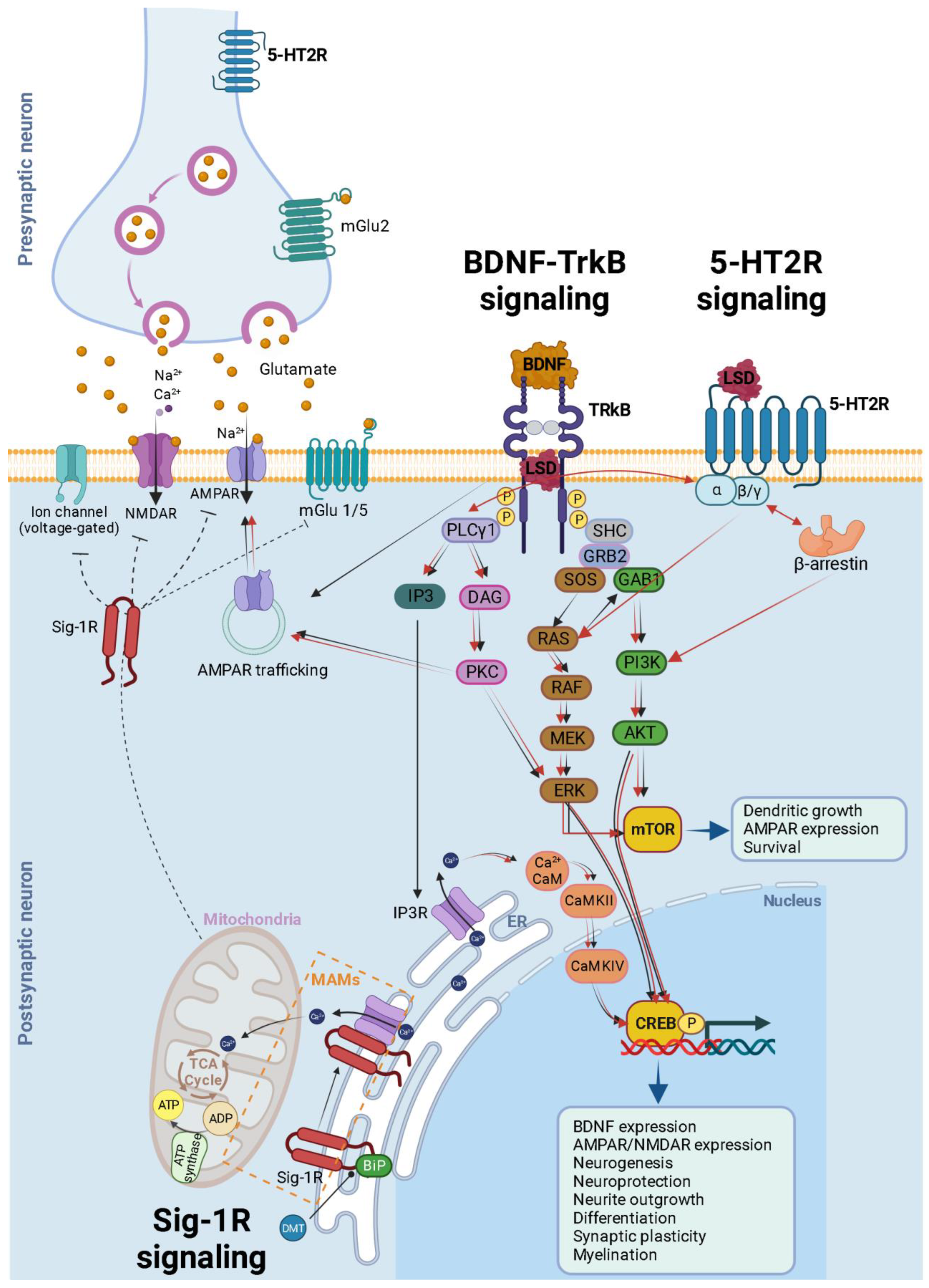
3. 5-HT2A Receptor Signaling
4. Classic Psychedelics Facilitate Plasticity via the TrkB-BDNF Signaling Pathway
5. What Do 5-HT2A and TrkB Pathways Reveal about the Role of Subjective Experience in Serotonergic Psychedelic Therapy?
6. Glutamate Signaling: A Shared Regulator of Neuroplasticity in Hallucinogens and Dissociative Anesthetics
7. Additional Receptors and Pathways Contributing to the Mechanisms of Action of Psychedelics
8. The Potential of Psychedelics as Anti-Inflammatory Agents
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Osmond, H. A Review of the Clinical Effects of Psychotomimetic Agents. Ann. N. Y. Acad. Sci. 1957, 66, 418–434. [Google Scholar] [CrossRef] [PubMed]
- Kelmendi, B.; Kaye, A.P.; Pittenger, C.; Kwan, A.C. Psychedelics. Curr. Biol. 2022, 32, R63–R67. [Google Scholar] [CrossRef]
- Nichols, D.E. Chemistry and Structure-Activity Relationships of Psychedelics. In Behavioral Neurobiology of Psychedelic Drugs; Current Topics in Behavioral Neurosciences; Springer: Berlin/Heidelberg, Germany, 2018; Volume 36, pp. 1–43. [Google Scholar] [CrossRef]
- Passie, T.; Halpern, J.H.; Stichtenoth, D.O.; Emrich, H.M.; Hintzen, A. The Pharmacology of Lysergic Acid Diethylamide: A Review. CNS Neurosci. Ther. 2008, 14, 295–314. [Google Scholar] [CrossRef]
- Carod-Artal, F.J. Hallucinogenic Drugs in Pre-Columbian Mesoamerican Cultures. Neurologia 2015, 30, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Guerra-Doce, E. Psychoactive Substances in Prehistoric Times: Examining the Archaeological Evidence. Time Mind 2015, 8, 91–112. [Google Scholar] [CrossRef]
- Nichols, D.E.; Walter, H. The History of Psychedelics in Psychiatry. Pharmacopsychiatry 2021, 54, 151–166. [Google Scholar] [CrossRef]
- Swanson, L.R. Unifying Theories of Psychedelic Drug Effects. Front. Pharmacol. 2018, 9, 172. [Google Scholar] [CrossRef]
- Hofmann, A. Chemical Constitution and Pharmacodynamic Actions; Dekker: New York, NY, USA, 1968. [Google Scholar]
- Pletscher, A.; Ladewig, D.; Symposium Schweizerische Akademie der Medizinischen Wissenschaften (Eds.) 50 Years of LSD: Current Status and Perspectives of Hallucinogens: A Symposium of the Swiss Academy of Medical Sciences, Lugano-Agno, Switzerland, 21–22 October 1993; Parthenon Pub. Group: New York, NY, USA, 1994. [Google Scholar]
- Vattano, A.J. Psychedelic Drugs Reconsidered. By Lester Grinspoon and James B. Bakalar. New York: Basic Books, 1979. 343 pp. $15.95 cloth. Child. Sch. 1981, 4, 71–72. [Google Scholar] [CrossRef]
- Malleson, N. Acute Adverse Reactions to LSD in Clinical and Experimental Use in the United Kingdom. Br. J. Psychiatry 1971, 118, 229–230. [Google Scholar] [CrossRef]
- Hoffer, A. The Uses and Implications of Hallucinogenic Drugs; Nature Publishing Group, a Division of Macmillan Publishers Limited: London, UK, 1970. [Google Scholar]
- Abramson, H.A. The Use of LSD in Psychotherapy and Alcoholism; Bobbs-Merrill: Indianapolis, IN, USA, 1967. [Google Scholar]
- Solomon, D. LSD: The Consciousness-Expanding Drug; Putnam: New York, NY, USA, 1964. [Google Scholar]
- Pahnke, W.N.; Kurland, A.A.; Goodman, L.E.; Richards, W.A. LSD-Assisted Psychotherapy with Terminal Cancer Patients. Curr. Psychiatr. Ther. 1969, 9, 144–152. [Google Scholar] [PubMed]
- Ladewig, D.; Pletscher, A. Fifty Years of LSD: Current Status and Perspectives of Hallucinogens; CRC Press: Boca Raton, FL, USA, 1994. [Google Scholar]
- Rucker, J.J.; Jelen, L.A.; Flynn, S.; Frowde, K.D.; Young, A.H. Psychedelics in the Treatment of Unipolar Mood Disorders: A Systematic Review. J. Psychopharmacol. 2016, 30, 1220–1229. [Google Scholar] [CrossRef] [PubMed]
- Pare, C.M.B. Neuro-Psychopharmacology. Proceedings of the Fifth International Congress of the Collegium Internationale Neuro-Psychopharmacologicum. Washington, D.C. March, 1966. Edited by H. Brill. Excerpta Medica Foundation. 1967. Pp. 1278. Price £21 15s. Br. J. Psychiatry 1968, 114, 1043. [Google Scholar] [CrossRef]
- Dyck, E. Flashback: Psychiatric Experimentation with LSD in Historical Perspective. Can. J. Psychiatry 2005, 50, 381–388. [Google Scholar] [CrossRef]
- Cowan, W.M.; Harter, D.H.; Kandel, E.R. The Emergence of Modern Neuroscience: Some Implications for Neurology and Psychiatry. Annu. Rev. Neurosci. 2000, 23, 343–391. [Google Scholar] [CrossRef] [PubMed]
- Vollenweider, F.X.; Kometer, M. The Neurobiology of Psychedelic Drugs: Implications for the Treatment of Mood Disorders. Nat. Rev. Neurosci. 2010, 11, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Hadar, A.; David, J.; Shalit, N.; Roseman, L.; Gross, R.; Sessa, B.; Lev-Ran, S. The Psychedelic Renaissance in Clinical Research: A Bibliometric Analysis of Three Decades of Human Studies with Psychedelics. J. Psychoact. Drugs 2023, 55, 1–10. [Google Scholar] [CrossRef]
- Sessa, B. The 21st Century Psychedelic Renaissance: Heroic Steps Forward on the Back of an Elephant. Psychopharmacology 2018, 235, 551–560. [Google Scholar] [CrossRef]
- Rivera-García, M.T.; Cruz, S.L. The Resurgence of Hallucinogen Drugs in Clinical Research. Rev. Investig. Clin. 2023, 75, 169–178. [Google Scholar] [CrossRef]
- Mithoefer, M.C.; Grob, C.S.; Brewerton, T.D. Novel Psychopharmacological Therapies for Psychiatric Disorders: Psilocybin and MDMA. Lancet Psychiatry 2016, 3, 481–488. [Google Scholar] [CrossRef]
- Nutt, D.; Erritzoe, D.; Carhart-Harris, R. Psychedelic Psychiatry’s Brave New World. Cell 2020, 181, 24–28. [Google Scholar] [CrossRef]
- Johnson, M.W.; Garcia-Romeu, A.; Griffiths, R.R. Long-Term Follow-up of Psilocybin-Facilitated Smoking Cessation. Am. J. Drug Alcohol Abus. 2017, 43, 55–60. [Google Scholar] [CrossRef]
- Bogenschutz, M.P.; Forcehimes, A.A.; Pommy, J.A.; Wilcox, C.E.; Barbosa, P.C.R.; Strassman, R.J. Psilocybin-Assisted Treatment for Alcohol Dependence: A Proof-of-Concept Study. J. Psychopharmacol. 2015, 29, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Andersen, K.A.A.; Carhart-Harris, R.; Nutt, D.J.; Erritzoe, D. Therapeutic Effects of Classic Serotonergic Psychedelics: A Systematic Review of Modern-Era Clinical Studies. Acta Psychiatr. Scand. 2021, 143, 101–118. [Google Scholar] [CrossRef]
- Bogenschutz, M.P.; Johnson, M.W. Classic Hallucinogens in the Treatment of Addictions. Progress. Neuro-Psychopharmacol. Biol. Psychiatry 2016, 64, 250–258. [Google Scholar] [CrossRef]
- Krebs, T.S.; Johansen, P.-Ø. Lysergic Acid Diethylamide (LSD) for Alcoholism: Meta-Analysis of Randomized Controlled Trials. J. Psychopharmacol. 2012, 26, 994–1002. [Google Scholar] [CrossRef]
- Moreno, F.A.; Wiegand, C.B.; Taitano, E.K.; Delgado, P.L. Safety, Tolerability, and Efficacy of Psilocybin in 9 Patients with Obsessive-Compulsive Disorder. J. Clin. Psychiatry 2006, 67, 1735–1740. [Google Scholar] [CrossRef] [PubMed]
- Ehrmann, K.; Allen, J.J.B.; Moreno, F.A. Psilocybin for the Treatment of Obsessive-Compulsive Disorders. Curr. Top. Behav. Neurosci. 2022, 56, 247–259. [Google Scholar] [CrossRef]
- Rucker, J.J.; Iliff, J.; Nutt, D.J. Psychiatry & the Psychedelic Drugs. Past, Present & Future. Neuropharmacology 2018, 142, 200–218. [Google Scholar] [PubMed]
- Kyzar, E.J.; Nichols, C.D.; Gainetdinov, R.R.; Nichols, D.E.; Kalueff, A.V. Psychedelic Drugs in Biomedicine. Trends Pharmacol. Sci. 2017, 38, 992–1005. [Google Scholar] [CrossRef] [PubMed]
- De Vos, C.M.; Mason, N.L.; Kuypers, K.P. Psychedelics and Neuroplasticity: A Systematic Review Unraveling the Biological Underpinnings of Psychedelics. Front. Psychiatry 2021, 12, 724606. [Google Scholar] [CrossRef]
- Elman, I.; Pustilnik, A.; Borsook, D. Beating Pain with Psychedelics: Matter over Mind? Neurosci. Biobehav. Rev. 2022, 134, 104482. [Google Scholar] [CrossRef] [PubMed]
- Lukasiewicz, K.; Baker, J.J.; Zuo, Y.; Lu, J. Serotonergic Psychedelics in Neural Plasticity. Front. Mol. Neurosci. 2021, 14, 748359. [Google Scholar] [CrossRef] [PubMed]
- Saeger, H.N.; Olson, D.E. Psychedelic-Inspired Approaches for Treating Neurodegenerative Disorders. J. Neurochem. 2022, 162, 109–127. [Google Scholar] [CrossRef]
- Leger, R.F.; Unterwald, E.M. Assessing the Effects of Methodological Differences on Outcomes in the Use of Psychedelics in the Treatment of Anxiety and Depressive Disorders: A Systematic Review and Meta-Analysis. J. Psychopharmacol. 2022, 36, 20–30. [Google Scholar] [CrossRef]
- Center for Drug Evaluation and Research. Psychedelic Drugs: Considerations for Clinical Investigations Guidance for Industry; Food and Drug Administration: Rockville, MD, USA, 2023.
- Davis, A.K.; Barrett, F.S.; May, D.G.; Cosimano, M.P.; Sepeda, N.D.; Johnson, M.W.; Finan, P.H.; Griffiths, R.R. Effects of Psilocybin-Assisted Therapy on Major Depressive Disorder: A Randomized Clinical Trial. JAMA Psychiatry 2021, 78, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Carhart-Harris, R.; Giribaldi, B.; Watts, R.; Baker-Jones, M.; Murphy-Beiner, A.; Murphy, R.; Martell, J.; Blemings, A.; Erritzoe, D.; Nutt, D.J. Trial of Psilocybin Versus Escitalopram for Depression. N. Engl. J. Med. 2021, 384, 1402–1411. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, R.R.; Johnson, M.W.; Carducci, M.A.; Umbricht, A.; Richards, W.A.; Richards, B.D.; Cosimano, M.P.; Klinedinst, M.A. Psilocybin Produces Substantial and Sustained Decreases in Depression and Anxiety in Patients with Life-Threatening Cancer: A Randomized Double-Blind Trial. J. Psychopharmacol. 2016, 30, 1181–1197. [Google Scholar] [CrossRef]
- Ross, S.; Bossis, A.; Guss, J.; Agin-Liebes, G.; Malone, T.; Cohen, B.; Mennenga, S.E.; Belser, A.; Kalliontzi, K.; Babb, J. Rapid and Sustained Symptom Reduction Following Psilocybin Treatment for Anxiety and Depression in Patients with Life-Threatening Cancer: A Randomized Controlled Trial. J. Psychopharmacol. 2016, 30, 1165–1180. [Google Scholar] [CrossRef]
- Carhart-Harris, R.L.; Bolstridge, M.; Rucker, J.; Day, C.M.; Erritzoe, D.; Kaelen, M.; Bloomfield, M.; Rickard, J.A.; Forbes, B.; Feilding, A. Psilocybin with Psychological Support for Treatment-Resistant Depression: An Open-Label Feasibility Study. Lancet Psychiatry 2016, 3, 619–627. [Google Scholar] [CrossRef]
- Mitchell, J.M.; Bogenschutz, M.; Lilienstein, A.; Harrison, C.; Kleiman, S.; Parker-Guilbert, K.; Ot’alora, G.M.; Garas, W.; Paleos, C.; Gorman, I. MDMA-Assisted Therapy for Severe PTSD: A Randomized, Double-Blind, Placebo-Controlled Phase 3 Study. Nat. Med. 2021, 27, 1025–1033. [Google Scholar] [CrossRef]
- Xiong, J.; Lipsitz, O.; Nasri, F.; Lui, L.M.; Gill, H.; Phan, L.; Chen-Li, D.; Iacobucci, M.; Ho, R.; Majeed, A. Impact of COVID-19 Pandemic on Mental Health in the General Population: A Systematic Review. J. Affect. Disord. 2020, 277, 55–64. [Google Scholar] [CrossRef]
- Halberstadt, A.L.; Geyer, M.A. Multiple Receptors Contribute to the Behavioral Effects of Indoleamine Hallucinogens. Neuropharmacology 2011, 61, 364–381. [Google Scholar] [CrossRef] [PubMed]
- Moliner, R.; Girych, M.; Brunello, C.A.; Kovaleva, V.; Biojone, C.; Enkavi, G.; Antenucci, L.; Kot, E.F.; Goncharuk, S.A.; Kaurinkoski, K.; et al. Psychedelics Promote Plasticity by Directly Binding to BDNF Receptor TrkB. Nat. Neurosci. 2023, 26, 1032–1041. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrova, L.R.; Phillips, A.G. Neuroplasticity as a Convergent Mechanism of Ketamine and Classical Psychedelics. Trends Pharmacol. Sci. 2021, 42, 929–942. [Google Scholar] [CrossRef] [PubMed]
- Mason, N.; Kuypers, K.; Müller, F.; Reckweg, J.; Tse, D.; Toennes, S.; Hutten, N.; Jansen, J.; Stiers, P.; Feilding, A. Me, myself, bye: Regional Alterations in Glutamate and the Experience of Ego Dissolution with Psilocybin. Neuropsychopharmacology 2020, 45, 2003–2011. [Google Scholar] [CrossRef]
- Nardou, R.; Lewis, E.M.; Rothhaas, R.; Xu, R.; Yang, A.; Boyden, E.; Dölen, G. Oxytocin-Dependent Reopening of a Social Reward Learning Critical Period with MDMA. Nature 2019, 569, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Holze, F.; Avedisian, I.; Varghese, N.; Eckert, A.; Liechti, M.E. Role of the 5-HT(2A) Receptor in Acute Effects of LSD on Empathy and Circulating Oxytocin. Front. Pharmacol. 2021, 12, 711255. [Google Scholar] [CrossRef]
- Hutten, N.R.; Mason, N.L.; Dolder, P.C.; Theunissen, E.L.; Holze, F.; Liechti, M.E.; Varghese, N.; Eckert, A.; Feilding, A.; Ramaekers, J.G. Low Doses of LSD Acutely Increase BDNF Blood Plasma Levels in Healthy Volunteers. ACS Pharmacol. Transl. Sci. 2020, 4, 461–466. [Google Scholar] [CrossRef]
- Holze, F.; Vizeli, P.; Ley, L.; Müller, F.; Dolder, P.; Stocker, M.; Duthaler, U.; Varghese, N.; Eckert, A.; Borgwardt, S. Acute Dose-Dependent Effects of Lysergic acid Diethylamide in a Double-Blind Placebo-Controlled Study in Healthy Subjects. Neuropsychopharmacology 2021, 46, 537–544. [Google Scholar] [CrossRef]
- Flanagan, T.W.; Nichols, C.D. Psychedelics as Anti-Inflammatory Agents. Int. Rev. Psychiatry 2018, 30, 363–375. [Google Scholar] [CrossRef]
- Nichols, C.D.; Sanders-Bush, E. A Single Dose of Lysergic Acid Diethylamide Influences Gene Expression Patterns within the Mammalian Brain. Neuropsychopharmacology 2002, 26, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Kanen, J.W.; Luo, Q.; Rostami Kandroodi, M.; Cardinal, R.N.; Robbins, T.W.; Nutt, D.J.; Carhart-Harris, R.L.; den Ouden, H.E.M. Effect of Lysergic acid Diethylamide (LSD) on Reinforcement Learning in Humans. Psychol. Med. 2022, 53, 6434–6445. [Google Scholar] [CrossRef]
- Knudsen, G.M. Sustained Effects of Single Doses of Classical Psychedelics in Humans. Neuropsychopharmacology 2023, 48, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Yu, J.; Wang, H.; Luo, Z.; Liu, X.; He, L.; Qi, J.; Fan, L.; Tang, L.; Chen, Z.; et al. Structure-Based Discovery of Nonhallucinogenic Psychedelic Analogs. Science 2022, 375, 403–411. [Google Scholar] [CrossRef]
- Vollenweider, F.X.; Geyer, M.A. A Systems Model of Altered Consciousness: Integrating Natural and Drug-Induced Psychoses. Brain Res. Bull. 2001, 56, 495–507. [Google Scholar] [CrossRef] [PubMed]
- Carhart-Harris, R.L.; Friston, K.J. REBUS and the Anarchic Brain: Toward a Unified Model of the Brain Action of Psychedelics. Pharmacol. Rev. 2019, 71, 316–344. [Google Scholar] [CrossRef] [PubMed]
- Doss, M.K.; Madden, M.B.; Gaddis, A.; Nebel, M.B.; Griffiths, R.R.; Mathur, B.N.; Barrett, F.S. Models of Psychedelic Drug Action: Modulation of Cortical-Subcortical Circuits. Brain 2022, 145, 441–456. [Google Scholar] [CrossRef]
- Dai, R.; Larkin, T.E.; Huang, Z.; Tarnal, V.; Picton, P.; Vlisides, P.E.; Janke, E.; McKinney, A.; Hudetz, A.G.; Harris, R.E. Classical and Non-Classical Psychedelic Drugs Induce Common Network Changes in Human Cortex. NeuroImage 2023, 273, 120097. [Google Scholar] [CrossRef]
- Ly, C.; Greb, A.C.; Cameron, L.P.; Wong, J.M.; Barragan, E.V.; Wilson, P.C.; Burbach, K.F.; Zarandi, S.S.; Sood, A.; Paddy, M.R. Psychedelics Promote Structural and Functional Neural Plasticity. Cell Rep. 2018, 23, 3170–3182. [Google Scholar] [CrossRef]
- Barrett, F.S.; Krimmel, S.R.; Griffiths, R.R.; Seminowicz, D.A.; Mathur, B.N. Psilocybin Acutely Alters the Functional Connectivity of the Claustrum with Brain Networks that Support Perception, Memory, and Attention. Neuroimage 2020, 218, 116980. [Google Scholar] [CrossRef]
- Carhart-Harris, R.L.; Muthukumaraswamy, S.; Roseman, L.; Kaelen, M.; Droog, W.; Murphy, K.; Tagliazucchi, E.; Schenberg, E.E.; Nest, T.; Orban, C. Neural Correlates of the LSD Experience Revealed by Multimodal Neuroimaging. Proc. Natl. Acad. Sci. USA 2016, 113, 4853–4858. [Google Scholar] [CrossRef]
- Preller, K.H.; Duerler, P.; Burt, J.B.; Ji, J.L.; Adkinson, B.; Stńmpfli, P.; Seifritz, E.; Repovš, G.; Krystal, J.H.; Murray, J.D. Psilocybin Induces Time-Dependent Changes in Global Functional Connectivity. Biol. Psychiatry 2020, 88, 197–207. [Google Scholar] [CrossRef]
- Kaelen, M.; Roseman, L.; Kahan, J.; Santos-Ribeiro, A.; Orban, C.; Lorenz, R.; Barrett, F.S.; Bolstridge, M.; Williams, T.; Williams, L. LSD Modulates Music-Induced Imagery via Changes in Parahippocampal Connectivity. Eur. Neuropsychopharmacol. 2016, 26, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Petri, G.; Expert, P.; Turkheimer, F.; Carhart-Harris, R.; Nutt, D.; Hellyer, P.J.; Vaccarino, F. Homological Scaffolds of Brain Functional Networks. J. R. Soc. Interface 2014, 11, 20140873. [Google Scholar] [CrossRef] [PubMed]
- Smigielski, L.; Scheidegger, M.; Kometer, M.; Vollenweider, F.X. Psilocybin-Assisted Mindfulness Training Modulates Self-Consciousness and Brain Default Mode Network Connectivity with Lasting Effects. NeuroImage 2019, 196, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Olson, D.E. Psychoplastogens: A Promising Class of Plasticity-Promoting Neurotherapeutics. J. Exp. Neurosci. 2018, 12, 1179069518800508. [Google Scholar] [CrossRef]
- van Elk, M.; Yaden, D.B. Pharmacological, Neural, and Psychological Mechanisms Underlying Psychedelics: A Critical Review. Neurosci. Biobehav. Rev. 2022, 140, 104793. [Google Scholar] [CrossRef] [PubMed]
- Kwan, A.C.; Olson, D.E.; Preller, K.H.; Roth, B.L. The Neural Basis of Psychedelic Action. Nat. Neurosci. 2022, 25, 1407–1419. [Google Scholar] [CrossRef]
- Vollenweider, F.X.; Csomor, P.A.; Knappe, B.; Geyer, M.A.; Quednow, B.B. The Effects of the Preferential 5-HT2A Agonist Psilocybin on Prepulse Inhibition of Startle in Healthy Human Volunteers Depend on Interstimulus Interval. Neuropsychopharmacology 2007, 32, 1876–1887. [Google Scholar] [CrossRef]
- Quednow, B.B.; Kometer, M.; Geyer, M.A.; Vollenweider, F.X. Psilocybin-Induced Deficits in Automatic and Controlled Inhibition Are Attenuated by Ketanserin in Healthy Human Volunteers. Neuropsychopharmacology 2012, 37, 630–640. [Google Scholar] [CrossRef]
- Schmid, Y.; Enzler, F.; Gasser, P.; Grouzmann, E.; Preller, K.H.; Vollenweider, F.X.; Brenneisen, R.; Müller, F.; Borgwardt, S.; Liechti, M.E. Acute Effects of Lysergic Acid Diethylamide in Healthy Subjects. Biol. Psychiatry 2015, 78, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Riba, J.; Rodríguez-Fornells, A.; Barbanoj, M.J. Effects of Ayahuasca on Sensory and Sensorimotor Gating in Humans as Measured by P50 Suppression and Prepulse Inhibition of the Startle Reflex, Respectively. Psychopharmacology 2002, 165, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Preller, K.H.; Burt, J.B.; Ji, J.L.; Schleifer, C.H.; Adkinson, B.D.; Stämpfli, P.; Seifritz, E.; Repovs, G.; Krystal, J.H.; Murray, J.D. Changes in Global and Thalamic Brain Connectivity in LSD-Induced Altered States of Consciousness Are Attributable to the 5-HT2A Receptor. Elife 2018, 7, e35082. [Google Scholar] [CrossRef]
- Müller, F.; Lenz, C.; Dolder, P.; Lang, U.; Schmidt, A.; Liechti, M.; Borgwardt, S. Increased Thalamic Resting-State Connectivity as a Core Driver of LSD-Induced Hallucinations. Acta Psychiatr. Scand. 2017, 136, 648–657. [Google Scholar] [CrossRef]
- Carhart-Harris, R.L. The Entropic Brain-Revisited. Neuropharmacology 2018, 142, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Schartner, M.M.; Carhart-Harris, R.L.; Barrett, A.B.; Seth, A.K.; Muthukumaraswamy, S.D. Increased Spontaneous MEG Signal Diversity for Psychoactive Doses of Ketamine, LSD and Psilocybin. Sci. Rep. 2017, 7, 46421. [Google Scholar] [CrossRef]
- Timmermann, C.; Roseman, L.; Schartner, M.; Milliere, R.; Williams, L.T.; Erritzoe, D.; Muthukumaraswamy, S.; Ashton, M.; Bendrioua, A.; Kaur, O. Neural Correlates of the DMT Experience Assessed with Multivariate EEG. Sci. Rep. 2019, 9, 16324. [Google Scholar] [CrossRef]
- Lebedev, A.V.; Kaelen, M.; Lövdén, M.; Nilsson, J.; Feilding, A.; Nutt, D.J.; Carhart-Harris, R.L. LSD-Induced Entropic Brain Activity Predicts Subsequent Personality Change. Human Brain Mapp. 2016, 37, 3203–3213. [Google Scholar] [CrossRef]
- Atasoy, S.; Roseman, L.; Kaelen, M.; Kringelbach, M.L.; Deco, G.; Carhart-Harris, R.L. Connectome-Harmonic Decomposition of Human Brain Activity Reveals Dynamical Repertoire Re-Organization under LSD. Sci. Rep. 2017, 7, 17661. [Google Scholar] [CrossRef]
- Viol, A.; Palhano-Fontes, F.; Onias, H.; de Araujo, D.B.; Viswanathan, G.M. Shannon Entropy of Brain Functional Complex Networks under the Influence of the Psychedelic Ayahuasca. Sci. Rep. 2017, 7, 7388. [Google Scholar] [CrossRef]
- Mathur, B.N. The Claustrum in Review. Front. Syst. Neurosci. 2014, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Krimmel, S.R.; White, M.G.; Panicker, M.H.; Barrett, F.S.; Mathur, B.N.; Seminowicz, D.A. Resting State Functional Connectivity and Cognitive Task-Related Activation of the Human Claustrum. Neuroimage 2019, 196, 59–67. [Google Scholar] [CrossRef] [PubMed]
- White, M.G.; Mathur, B.N. Claustrum Circuit Components for Top–Down Input Processing and Cortical Broadcast. Brain Struct. Funct. 2018, 223, 3945–3958. [Google Scholar] [CrossRef]
- Barrett, F.S.; Preller, K.H.; Kaelen, M. Psychedelics and Music: Neuroscience and Therapeutic Implications. Int. Rev. Psychiatry 2018, 30, 350–362. [Google Scholar] [CrossRef]
- Nichols, D.E. Psychedelics. Pharmacol. Rev. 2016, 68, 264–355. [Google Scholar] [CrossRef]
- Wacker, D.; Wang, S.; McCorvy, J.D.; Betz, R.M.; Venkatakrishnan, A.J.; Levit, A.; Lansu, K.; Schools, Z.L.; Che, T.; Nichols, D.E.; et al. Crystal Structure of an LSD-Bound Human Serotonin Receptor. Cell 2017, 168, 377–389.e12. [Google Scholar] [CrossRef]
- Halberstadt, A.L.; Chatha, M.; Klein, A.K.; Wallach, J.; Brandt, S.D. Correlation between the Potency of Hallucinogens in the Mouse Head-Twitch Response Assay and Their Behavioral and Subjective Effects in Other Species. Neuropharmacology 2020, 167, 107933. [Google Scholar] [CrossRef]
- Kim, K.; Che, T.; Panova, O.; DiBerto, J.F.; Lyu, J.; Krumm, B.E.; Wacker, D.; Robertson, M.J.; Seven, A.B.; Nichols, D.E. Structure of a Hallucinogen-Activated Gq-Coupled 5-HT2A Serotonin Receptor. Cell 2020, 182, 1574–1588.e19. [Google Scholar] [CrossRef]
- Preller, K.H.; Herdener, M.; Pokorny, T.; Planzer, A.; Kraehenmann, R.; Stämpfli, P.; Liechti, M.E.; Seifritz, E.; Vollenweider, F.X. The Fabric of Meaning and Subjective effects in LSD-Induced States Depend on Serotonin 2A Receptor Activation. Curr. Biol. 2017, 27, 451–457. [Google Scholar] [CrossRef]
- Kraehenmann, R.; Pokorny, D.; Aicher, H.; Preller, K.H.; Pokorny, T.; Bosch, O.G.; Seifritz, E.; Vollenweider, F.X. LSD Increases Primary Process Thinking via Serotonin 2A Receptor Activation. Front. Pharmacol. 2017, 8, 814. [Google Scholar] [CrossRef] [PubMed]
- Kraehenmann, R.; Pokorny, D.; Vollenweider, L.; Preller, K.H.; Pokorny, T.; Seifritz, E.; Vollenweider, F.X. Dreamlike Effects of LSD on Waking Imagery in Humans Depend on Serotonin 2A Receptor Activation. Psychopharmacology 2017, 234, 2031–2046. [Google Scholar] [CrossRef]
- Delli Pizzi, S.; Chiacchiaretta, P.; Sestieri, C.; Ferretti, A.; Onofrj, M.; Della Penna, S.; Roseman, L.; Timmermann, C.; Nutt, D.J.; Carhart-Harris, R.L.; et al. Spatial Correspondence of LSD-Induced Variations on Brain Functioning at Rest With Serotonin Receptor Expression. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2023, 8, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.M.; Klaiber, A.; Holze, F.; Istampoulouoglou, I.; Duthaler, U.; Varghese, N.; Eckert, A.; Liechti, M.E. Ketanserin Reverses the Acute Response to LSD in a Randomized, Double-Blind, Placebo-Controlled, Crossover Study in Healthy Participants. Int. J. Neuropsychopharmacol. 2023, 26, 97–106. [Google Scholar] [CrossRef]
- Vollenweider, F.X.; Vollenweider-Scherpenhuyzen, M.F.; Bäbler, A.; Vogel, H.; Hell, D. Psilocybin Induces Schizophrenia-Like Psychosis in Humans via a Serotonin-2 Agonist Action. Neuroreport 1998, 9, 3897–3902. [Google Scholar] [CrossRef]
- Singleton, S.P.; Timmermann, C.; Luppi, A.I.; Eckernas, E.; Roseman, L.; Carhart-Harris, R.L.; Kuceyeski, A. Time-Resolved Network Control Analysis Links Reduced Control Energy under DMT with the Serotonin 2a Receptor, Signal Diversity, and Subjective Experience. bioRxiv 2023. [Google Scholar] [CrossRef]
- Quednow, B.B.; Geyer, M.A.; Halberstadt, A.L. Serotonin and Schizophrenia; Elsevier: Amsterdam, The Netherlands, 2010; pp. 585–620. [Google Scholar] [CrossRef]
- Madsen, M.K.; Fisher, P.M.; Burmester, D.; Dyssegaard, A.; Stenbæk, D.S.; Kristiansen, S.; Johansen, S.S.; Lehel, S.; Linnet, K.; Svarer, C.; et al. Psychedelic Effects of Psilocybin Correlate with Serotonin 2A Receptor Occupancy and Plasma Psilocin Levels. Neuropsychopharmacology 2019, 44, 1328–1334. [Google Scholar] [CrossRef]
- Vollenweider, F.X.; Preller, K.H. Psychedelic Drugs: Neurobiology and Potential for Treatment of Psychiatric Disorders. Nat. Rev. Neurosci. 2020, 21, 611–624. [Google Scholar] [CrossRef]
- Beliveau, V.; Ganz, M.; Feng, L.; Ozenne, B.; Højgaard, L.; Fisher, P.M.; Svarer, C.; Greve, D.N.; Knudsen, G.M. A High-Resolution In Vivo Atlas of the Human Brain’s Serotonin System. J. Neurosci. 2017, 37, 120–128. [Google Scholar] [CrossRef]
- Weber, E.T.; Andrade, R. Htr2a Gene and 5-HT2A Receptor Expression in the Cerebral Cortex Studied Using Genetically Modified Mice. Front. Neurosci. 2010, 4, 36. [Google Scholar] [CrossRef]
- Celada, P.; Puig, M.V.; Artigas, F. Serotonin Modulation of Cortical Neurons and Networks. Front. Integr. Neurosci. 2013, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Jakab, R.L.; Goldman-Rakic, P.S. 5-Hydroxytryptamine2A Serotonin Receptors in the Primate Cerebral Cortex: Possible Site of Action of Hallucinogenic and Antipsychotic Drugs in Pyramidal Cell Apical Dendrites. Proc. Natl. Acad. Sci. USA 1998, 95, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Hall, H.; Farde, L.; Halldin, C.; Lundkvist, C.; Sedvall, G. Autoradiographic Localization of 5-HT2A Receptors in the Human Brain Using [3H] M100907 and [11C] M100907. Synapse 2000, 38, 421–431. [Google Scholar] [CrossRef]
- Saulin, A.; Savli, M.; Lanzenberger, R. Serotonin and Molecular Neuroimaging in Humans Using PET. Amino Acids 2012, 42, 2039–2057. [Google Scholar] [CrossRef]
- Willins, D.L.; Deutch, A.Y.; Roth, B.L. Serotonin 5-HT2A Receptors Are Expressed on Pyramidal Cells and Interneurons in the Rat Cortex. Synapse 1997, 27, 79–82. [Google Scholar] [CrossRef]
- Herzog, R.; Mediano, P.A.M.; Rosas, F.E.; Lodder, P.; Carhart-Harris, R.; Perl, Y.S.; Tagliazucchi, E.; Cofre, R. A Whole-Brain Model of the Neural Entropy Increase Elicited by Psychedelic Drugs. Sci. Rep. 2023, 13, 6244. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.; Kim, Y.; Moghaddam, B. Disruption of Prefrontal Cortex Large Scale Neuronal Activity by Different Classes of Psychotomimetic Drugs. J. Neurosci. 2012, 32, 3022–3031. [Google Scholar] [CrossRef]
- Miner, L.; Backstrom, J.; Sanders-Bush, E.; Sesack, S. Ultrastructural Localization of Serotonin2A Receptors in the Middle Layers of the Rat Prelimbic Prefrontal Cortex. Neuroscience 2003, 116, 107–117. [Google Scholar] [CrossRef]
- López-Giménez, J.F.; González-Maeso, J. Hallucinogens and Serotonin 5-HT 2A Receptor-Mediated Signaling Pathways. Behav. Neurobiol. Psychedelic Drugs 2018, 36, 45–73. [Google Scholar]
- Sleight, A.J.; Stam, N.J.; Mutel, V.; Vanderheyden, P.M. Radiolabelling of the Human 5-HT2A Receptor with an Agonist, a Partial Agonist and an Antagonist: Effects on Apparent Agonist Affinities. Biochem. Pharmacol. 1996, 51, 71–76. [Google Scholar] [CrossRef]
- Kenakin, T.P. Biased Signalling and Allosteric Machines: New Vistas and Challenges for Drug Discovery. Br. J. Pharmacol. 2012, 165, 1659–1669. [Google Scholar] [CrossRef]
- Urban, J.D.; Clarke, W.P.; Zastrow, M.v.; Nichols, D.E.; Kobilka, B.; Weinstein, H.; Javitch, J.A.; Roth, B.L.; Christopoulos, A.; Sexton, P.M.; et al. Functional Selectivity and Classical Concepts of Quantitative Pharmacology. J. Pharmacol. Exp. Ther. 2007, 320, 1–13. [Google Scholar] [CrossRef]
- Kenakin, T. Functional Selectivity and Biased Receptor Signaling. J. Pharmacol. Exp. Ther. 2011, 336, 296–302. [Google Scholar] [CrossRef]
- Pottie, E.; Poulie, C.B.M.; Simon, I.A.; Harpsøe, K.; D’Andrea, L.; Komarov, I.V.; Gloriam, D.E.; Jensen, A.A.; Kristensen, J.L.; Stove, C.P. Structure-Activity Assessment and In-Depth Analysis of Biased Agonism in a Set of Phenylalkylamine 5-HT(2A) Receptor Agonists. ACS Chem. Neurosci. 2023, 14, 2727–2742. [Google Scholar] [CrossRef] [PubMed]
- Popoli, M.; Yan, Z.; McEwen, B.S.; Sanacora, G. The Stressed Synapse: The Impact of Stress and Glucocorticoids on Glutamate Transmission. Nat. Rev. Neurosci. 2012, 13, 22–37. [Google Scholar] [CrossRef]
- Egan, C.; Grinde, E.; Dupre, A.; Roth, B.L.; Hake, M.; Teitler, M.; Herrick-Davis, K. Agonist High and Low Affinity State Ratios Predict Drug Intrinsic Activity and a Revised Ternary Complex Mechanism at Serotonin 5-HT(2A) and 5-HT(2C) Receptors. Synapse 2000, 35, 144–150. [Google Scholar] [CrossRef]
- Roth, B.L.; Nakaki, T.; Chuang, D.M.; Costa, E. Aortic Recognition Sites for Serotonin (5HT) Are Coupled to Phospholipase C and Modulate Phosphatidylinositol Turnover. Neuropharmacology 1984, 23, 1223–1225. [Google Scholar] [CrossRef] [PubMed]
- Roth, B.L.; Nakaki, T.; Chuang, D.M.; Costa, E. 5-Hydroxytryptamine2 Receptors Coupled to Phospholipase C in Rat Aorta: Modulation of Phosphoinositide Turnover by Phorbol Ester. J. Pharmacol. Exp. Ther. 1986, 238, 480–485. [Google Scholar] [PubMed]
- Banerjee, A.A.; Vaidya, V.A. Differential Signaling Signatures Evoked by DOI Versus Lisuride Stimulation of the 5-HT2A Receptor. Biochem. Biophys. Res. Commun. 2020, 531, 609–614. [Google Scholar] [CrossRef]
- Araneda, R.; Andrade, R. 5-Hydroxytryptamine2 and 5-Hydroxytryptamine1a Receptors Mediate Opposing Responses on Membrane Excitability in Rat Association Cortex. Neuroscience 1991, 40, 399–412. [Google Scholar] [CrossRef]
- Pilc, A.; Machaczka, A.; Kawalec, P.; Smith, J.L.; Witkin, J.M. Where Do We Go Next in Antidepressant Drug Discovery? A New Generation of Antidepressants: A Pivotal Role of AMPA Receptor Potentiation and mGlu2/3 Receptor Antagonism. Expert. Opin. Drug Discov. 2022, 17, 1131–1146. [Google Scholar] [CrossRef]
- Lisman, J.; Yasuda, R.; Raghavachari, S. Mechanisms of CaMKII Action in Long-Term Potentiation. Nat. Rev. Neurosci. 2012, 13, 169–182. [Google Scholar] [CrossRef]
- Pottie, E.; Dedecker, P.; Stove, C.P. Identification of Psychedelic New Psychoactive Substances (NPS) Showing Biased Agonism at the 5-HT(2A)R through Simultaneous Use of β-Arrestin 2 and miniGα(q) Bioassays. Biochem. Pharmacol. 2020, 182, 114251. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.A.; Bhatnagar, A.; Gurevich, V.V.; Roth, B.L. The Interaction of a Constitutively Active Arrestin with the Arrestin-Insensitive 5-HT(2A) Receptor Induces Agonist-Independent Internalization. Mol. Pharmacol. 2003, 63, 961–972. [Google Scholar] [CrossRef] [PubMed]
- Tedford, H.W.; Zamponi, G.W. Direct G Protein Modulation of Cav2 Calcium Channels. Pharmacol. Rev. 2006, 58, 837–862. [Google Scholar] [CrossRef] [PubMed]
- Betke, K.M.; Wells, C.A.; Hamm, H.E. GPCR Mediated Regulation of Synaptic Transmission. Progress. Neurobiol. 2012, 96, 304–321. [Google Scholar] [CrossRef]
- Benneyworth, M.A.; Smith, R.L.; Barrett, R.J.; Sanders-Bush, E. Complex Discriminative Stimulus Properties of (+) Lysergic Acid Diethylamide (LSD) in C57Bl/6J Mice. Psychopharmacology 2005, 179, 854–862. [Google Scholar] [CrossRef]
- Winter, J.; Rice, K.; Amorosi, D.; Rabin, R. Psilocybin-Induced Stimulus Control in the Rat. Pharmacol. Biochem. Behav. 2007, 87, 472–480. [Google Scholar] [CrossRef] [PubMed]
- González-Maeso, J.; Weisstaub, N.V.; Zhou, M.; Chan, P.; Ivic, L.; Ang, R.; Lira, A.; Bradley-Moore, M.; Ge, Y.; Zhou, Q.; et al. Hallucinogens Recruit Specific Cortical 5-HT(2A) Receptor-Mediated Signaling Pathways to Affect Behavior. Neuron 2007, 53, 439–452. [Google Scholar] [CrossRef] [PubMed]
- Béïque, J.C.; Imad, M.; Mladenovic, L.; Gingrich, J.A.; Andrade, R. Mechanism of the 5-Hydroxytryptamine 2A Receptor-Mediated Facilitation of Synaptic Activity in Prefrontal Cortex. Proc. Natl. Acad. Sci. USA 2007, 104, 9870–9875. [Google Scholar] [CrossRef]
- Krishnan, V.; Nestler, E.J. The Molecular Neurobiology of Depression. Nature 2008, 455, 894–902. [Google Scholar] [CrossRef]
- Zhang, G.; Cohen, S.J.; Munchow, A.H.; Barrera, M.P.; Stackman, R.W., Jr. Stimulation of Serotonin 2A Receptors Facilitates Consolidation and Extinction of Fear Memory in C57BL/6J Mice. Neuropharmacology 2013, 64, 403–413. [Google Scholar] [CrossRef]
- Ohira, K.; Takeuchi, R.; Shoji, H.; Miyakawa, T. Fluoxetine-Induced Cortical Adult Neurogenesis. Neuropsychopharmacology 2013, 38, 909–920. [Google Scholar] [CrossRef] [PubMed]
- Lima da Cruz, R.V.; Moulin, T.C.; Petiz, L.L.; Leão, R.N. A Single Dose of 5-MeO-DMT Stimulates Cell Proliferation, Neuronal Survivability, Morphological and Functional Changes in Adult Mice Ventral Dentate Gyrus. Front. Mol. Neurosci. 2018, 11, 312. [Google Scholar] [CrossRef]
- Catlow, B.J.; Song, S.; Paredes, D.A.; Kirstein, C.L.; Sanchez-Ramos, J. Effects of Psilocybin on Hippocampal Neurogenesis and Extinction of Trace Fear Conditioning. Exp. Brain Res. 2013, 228, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.A.; Srivastava, D.P.; Allen, J.A.; Strachan, R.T.; Roth, B.L.; Penzes, P. Rapid Modulation of Spine Morphology by the 5-HT2A Serotonin Receptor through Kalirin-7 Signaling. Proc. Natl. Acad. Sci. USA 2009, 106, 19575–19580. [Google Scholar] [CrossRef]
- Shao, L.-X.; Liao, C.; Gregg, I.; Davoudian, P.A.; Savalia, N.K.; Delagarza, K.; Kwan, A.C. Psilocybin Induces Rapid and Persistent Growth of Dendritic Spines in Frontal Cortex In Vivo. Neuron 2021, 109, 2535–2544.e4. [Google Scholar] [CrossRef] [PubMed]
- Kargieman, L.; Santana, N.; Mengod, G.; Celada, P.; Artigas, F. Antipsychotic Drugs Reverse the Disruption in Prefrontal Cortex Function Produced by NMDA Receptor Blockade with Phencyclidine. Proc. Natl. Acad. Sci. USA 2007, 104, 14843–14848. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Kanamaru, C.; Ohtani, A.; Li, F.; Senzaki, K.; Shiga, T. Subtype Specific Roles of Serotonin Receptors in the Spine Formation of Cortical Neurons In Vitro. Neurosci. Res. 2011, 71, 311–314. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Lee, H.-P.; Won, S.-D.; Park, E.-Y.; Lee, H.-Y.; Lee, B.-H.; Lee, S.-W.; Yoon, D.; Han, C.; Kim, D.-J. Low Plasma BDNF Is Associated with Suicidal Behavior in Major Depression. Progress. Neuro-Psychopharmacol. Biol. Psychiatry 2007, 31, 78–85. [Google Scholar] [CrossRef]
- Lee, B.-H.; Kim, H.; Park, S.-H.; Kim, Y.-K. Decreased Plasma BDNF Level in Depressive Patients. J. Affect. Disord. 2007, 101, 239–244. [Google Scholar] [CrossRef]
- Baumeister, D.; Barnes, G.; Giaroli, G.; Tracy, D. Classical Hallucinogens as Antidepressants? A Review of Pharmacodynamics and Putative Clinical Roles. Ther. Adv. Psychopharmacol. 2014, 4, 156–169. [Google Scholar] [CrossRef]
- Shimizu, E.; Hashimoto, K.; Okamura, N.; Koike, K.; Komatsu, N.; Kumakiri, C.; Nakazato, M.; Watanabe, H.; Shinoda, N.; Okada, S.-i. Alterations of Serum Levels of Brain-Derived Neurotrophic Factor (BDNF) in Depressed Patients with or without Antidepressants. Biol. Psychiatry 2003, 54, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Xu, Y.; Hu, W.; Gao, Y.; Ni, X.; Sheng, H.; Liu, Y. Exercise Ameliorates Depression-Like Behavior and Increases Hippocampal BDNF Level in Ovariectomized Rats. Neurosci. Lett. 2014, 573, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Wolkowitz, O.M.; Wolf, J.; Shelly, W.; Rosser, R.; Burke, H.M.; Lerner, G.K.; Reus, V.I.; Nelson, J.C.; Epel, E.S.; Mellon, S.H. Serum BDNF Levels before Treatment Predict SSRI Response in Depression. Progress. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 1623–1630. [Google Scholar] [CrossRef]
- Aydemir, O.; Deveci, A.; Taneli, F. The Effect of Chronic Antidepressant Treatment on Serum Brain-Derived Neurotrophic Factor Levels in Depressed Patients: A Preliminary Study. Progress. Neuro-Psychopharmacol. Biol. Psychiatry 2005, 29, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Colaço, C.S.; Alves, S.S.; Nolli, L.M.; Pinheiro, W.O.; de Oliveira, D.G.R.; Santos, B.W.L.; Pic-Taylor, A.; Mortari, M.R.; Caldas, E.D. Toxicity of Ayahuasca after 28 Days Daily Exposure and Effects on Monoamines and Brain-Derived Neurotrophic Factor (BDNF) in Brain of Wistar rats. Metab. Brain Dis. 2020, 35, 739–751. [Google Scholar] [CrossRef]
- Almeida, R.N.d.; Galvão, A.C.d.M.; Da Silva, F.S.; Silva, E.A.d.S.; Palhano-Fontes, F.; Maia-de-Oliveira, J.P.; de Araújo, L.-S.B.; Lobão-Soares, B.; Galvão-Coelho, N.L. Modulation of Serum Brain-Derived Neurotrophic Factor by a Single Dose of Ayahuasca: Observation from a Randomized Controlled Trial. Front. Psychol. 2019, 10, 1234. [Google Scholar] [CrossRef]
- Becker, A.M.; Holze, F.; Grandinetti, T.; Klaiber, A.; Toedtli, V.E.; Kolaczynska, K.E.; Duthaler, U.; Varghese, N.; Eckert, A.; Grünblatt, E. Acute Effects of Psilocybin after Escitalopram or Placebo Pretreatment in a Randomized, Double-Blind, Placebo-Controlled, Crossover Study in Healthy Subjects. Clin. Pharmacol. Ther. 2022, 111, 886–895. [Google Scholar] [CrossRef]
- Castrén, E.; Antila, H. Neuronal Plasticity and Neurotrophic Factors in Drug Responses. Mol. Psychiatry 2017, 22, 1085–1095. [Google Scholar] [CrossRef]
- Casarotto, P.C.; Girych, M.; Fred, S.M.; Kovaleva, V.; Moliner, R.; Enkavi, G.; Biojone, C.; Cannarozzo, C.; Sahu, M.P.; Kaurinkoski, K. Antidepressant Drugs Act by Directly Binding to TRKB Neurotrophin Receptors. Cell 2021, 184, 1299–1313.e19. [Google Scholar] [CrossRef]
- Hibicke, M.; Landry, A.N.; Kramer, H.M.; Talman, Z.K.; Nichols, C.D. Psychedelics, but Not Ketamine, Produce Persistent Antidepressant-Like Effects in a Rodent Experimental System for the Study of Depression. ACS Chem. Neurosci. 2020, 11, 864–871. [Google Scholar] [CrossRef]
- Park, H.; Poo, M.-m. Neurotrophin Regulation of Neural Circuit Development and Function. Nat. Rev. Neurosci. 2013, 14, 7–23. [Google Scholar] [CrossRef]
- Davoudian, P.A.; Shao, L.X.; Kwan, A.C. Shared and Distinct Brain Regions Targeted for Immediate Early Gene Expression by Ketamine and Psilocybin. ACS Chem. Neurosci. 2023, 14, 468–480. [Google Scholar] [CrossRef] [PubMed]
- Azogu, I.; Plamondon, H. Inhibition of TrkB at the Nucleus Accumbens, Using ANA-12, Regulates Basal and Stress-Induced Orexin a Expression within the Mesolimbic System and Affects Anxiety, Sociability and Motivation. Neuropharmacology 2017, 125, 129–145. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, V.; Han, M.-H.; Graham, D.L.; Berton, O.; Renthal, W.; Russo, S.J.; LaPlant, Q.; Graham, A.; Lutter, M.; Lagace, D.C.; et al. Molecular Adaptations Underlying Susceptibility and Resistance to Social Defeat in Brain Reward Regions. Cell 2007, 131, 391–404. [Google Scholar] [CrossRef]
- Lindvall, O.; Kokaia, Z.; Bengzon, J.; Elme’r, E.; Kokaia, M. Neurotrophins and Brain Insults. Trends Neurosci. 1994, 17, 490–496. [Google Scholar] [CrossRef]
- Berton, O.; McClung, C.A.; Dileone, R.J.; Krishnan, V.; Renthal, W.; Russo, S.J.; Graham, D.; Tsankova, N.M.; Bolanos, C.A.; Rios, M.; et al. Essential Role of BDNF in the Mesolimbic Dopamine Pathway in Social Defeat Stress. Science 2006, 311, 864–868. [Google Scholar] [CrossRef]
- Vargas-Perez, H.; Grieder, T.E.; van der Kooy, D. Neural Plasticity in the Ventral Tegmental Area, Aversive Motivation during Drug Withdrawal and Hallucinogenic Therapy. J. Psychoact. Drugs 2023, 55, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Azogu, I.; Plamondon, H. Blockade of TrkB Receptors in the Nucleus Accumbens Prior to Heterotypic Stress Alters Corticotropin-Releasing Hormone (CRH), Vesicular Glutamate Transporter 2 (vGluT2) and Glucocorticoid Receptor (GR) within the Mesolimbic Pathway. Horm. Behav. 2017, 90, 98–112. [Google Scholar] [CrossRef]
- Flores Mosri, D. Affective Features Underlying Depression in Addiction: Understanding What It Feels Like. Front. Psychol. 2019, 10, 2318. [Google Scholar] [CrossRef]
- Cai, S.; Huang, S.; Hao, W. New Hypothesis and Treatment Targets of Depression: An Integrated View of Key Findings. Neurosci. Bull. 2015, 31, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Binder, D.K.; Croll, S.D.; Gall, C.M.; Scharfman, H.E. BDNF and Epilepsy: Too Much of a Good Thing? Trends Neurosci. 2001, 24, 47–53. [Google Scholar] [CrossRef]
- Coull, J.A.; Beggs, S.; Boudreau, D.; Boivin, D.; Tsuda, M.; Inoue, K.; Gravel, C.; Salter, M.W.; De Koninck, Y. BDNF from Microglia Causes the Shift in Neuronal Anion Gradient Underlying Neuropathic Pain. Nature 2005, 438, 1017–1021. [Google Scholar] [CrossRef] [PubMed]
- Groves, J. Is It Time to Reassess the BDNF Hypothesis of Depression? Mol. Psychiatry 2007, 12, 1079–1088. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Perez, H.; Ting-A-Kee, R.; Van Der Kooy, D. Different Neural Systems Mediate Morphine Reward and Its Spontaneous Withdrawal Aversion. Eur. J. Neurosci. 2009, 29, 2029–2034. [Google Scholar] [CrossRef] [PubMed]
- Yuen, E.Y.; Jiang, Q.; Chen, P.; Feng, J.; Yan, Z. Activation of 5-HT2A/C Receptors Counteracts 5-HT1A Regulation of n-Methyl-D-Aspartate Receptor Channels in Pyramidal Neurons of Prefrontal Cortex. J. Biol. Chem. 2008, 283, 17194–17204. [Google Scholar] [CrossRef]
- Kang, H.; Welcher, A.A.; Shelton, D.; Schuman, E.M. Neurotrophins and Time: Different Roles for TrkB Signaling in Hippocampal Long-Term Potentiation. Neuron 1997, 19, 653–664. [Google Scholar] [CrossRef]
- Andreska, T.; Lüningschrör, P.; Sendtner, M. Regulation of TrkB Cell Surface Expression—A Mechanism for Modulation of Neuronal Responsiveness to Brain-Derived Neurotrophic Factor. Cell Tissue Res. 2020, 382, 5–14. [Google Scholar] [CrossRef]
- Muschamp, J.W.; Regina, M.J.; Hull, E.M.; Winter, J.C.; Rabin, R.A. Lysergic Acid Diethylamide and [−]-2, 5-Dimethoxy-4-Methylamphetamine Increase Extracellular Glutamate in Rat Prefrontal Cortex. Brain Res. 2004, 1023, 134–140. [Google Scholar] [CrossRef]
- Puig, M.V.; Celada, P.; Díaz-Mataix, L.; Artigas, F. In Vivo Modulation of the Activity of Pyramidal Neurons in the Rat Medial Prefrontal Cortex by 5-HT2A Receptors: Relationship to Thalamocortical Afferents. Cereb. Cortex 2003, 13, 870–882. [Google Scholar] [CrossRef]
- Lladó-Pelfort, L.; Celada, P.; Riga, M.; Troyano-Rodriguez, E.; Santana, N.; Artigas, F. Effects of Hallucinogens on Neuronal Activity. Behav. Neurobiol. Psychedelic Drugs 2018, 36, 75–105. [Google Scholar]
- Banks, M.I.; Zahid, Z.; Jones, N.T.; Sultan, Z.W.; Wenthur, C.J. Catalysts for Change: The Cellular Neurobiology of Psychedelics. Mol. Biol. Cell 2021, 32, 1135–1144. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Martinowich, K.; Lee, F. BDNF at the Synapse: Why Location Matters. Mol. Psychiatry 2017, 22, 1370–1375. [Google Scholar] [CrossRef] [PubMed]
- McNamee, S.; Devenot, N.; Buisson, M. Studying Harms Is Key to Improving Psychedelic-Assisted Therapy—Participants Call for Changes to Research Landscape. JAMA Psychiatry 2023, 80, 411–412. [Google Scholar] [CrossRef] [PubMed]
- Aday, J.S.; Carhart-Harris, R.L.; Woolley, J.D. Emerging Challenges for Psychedelic Therapy. JAMA Psychiatry 2023, 80, 533–534. [Google Scholar] [CrossRef]
- Vargas, M.V.; Meyer, R.; Avanes, A.A.; Rus, M.; Olson, D.E. Psychedelics and Other Psychoplastogens for Treating Mental Illness. Front. Psychiatry 2021, 12, 727117. [Google Scholar] [CrossRef]
- Schlag, A.K.; Aday, J.; Salam, I.; Neill, J.C.; Nutt, D.J. Adverse Effects of Psychedelics: From Anecdotes and Misinformation to Systematic Science. J. Psychopharmacol. 2022, 36, 258–272. [Google Scholar] [CrossRef]
- Cohen, S. Lysergic Acid Diethylamide: Side Effects and Complications. J. Nerv. Ment. Dis. 1960, 130, 30–40. [Google Scholar] [CrossRef]
- McGlothlin, W.H.; Arnold, D.O. LSD Revisited: A Ten-Year Follow-up of Medical LSD Use. Arch. Gen. Psychiatry 1971, 24, 35–49. [Google Scholar] [CrossRef]
- Carbonaro, T.M.; Bradstreet, M.P.; Barrett, F.S.; MacLean, K.A.; Jesse, R.; Johnson, M.W.; Griffiths, R.R. Survey Study of Challenging Experiences after Ingesting Psilocybin Mushrooms: Acute and Enduring Positive and Negative Consequences. J. Psychopharmacol. 2016, 30, 1268–1278. [Google Scholar] [CrossRef]
- Cerón Tapia, H.R.; González Guzmán, M.A.; Córdoba Ortiz, S.A. Ayahuasca-Induced Psychosis: A Case Report. Rev. Colomb. De Psiquiatr. 2022, 51, 236–239. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, R.G.; Bouso, J.C.; Hallak, J.E. Ayahuasca, Dimethyltryptamine, and Psychosis: A Systematic Review of Human Studies. Ther. Adv. Psychopharmacol. 2017, 7, 141–157. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.W.; Richards, W.A.; Griffiths, R.R. Human Hallucinogen Research: Guidelines for Safety. J. Psychopharmacol. 2008, 22, 603–620. [Google Scholar] [CrossRef] [PubMed]
- Zeifman, R.J.; Singhal, N.; Breslow, L.; Weissman, C.R. On the Relationship between Classic Psychedelics and Suicidality: A Systematic Review. ACS Pharmacol. Transl. Sci. 2021, 4, 436–451. [Google Scholar] [CrossRef]
- American Psychiatric Association; DSMTF; American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5; American Psychiatric Association: Washington, DC, USA, 2013; Volume 5. [Google Scholar]
- Grinspoon, L.; Bakalar, J.B. Psychedelic Drugs Reconsidered; Basic Books: New York, NY, USA, 1979; Volume 168. [Google Scholar]
- Müller, F.; Kraus, E.; Holze, F.; Becker, A.; Ley, L.; Schmid, Y.; Vizeli, P.; Liechti, M.E.; Borgwardt, S. Flashback Phenomena after Administration of LSD and Psilocybin in Controlled Studies with Healthy Participants. Psychopharmacology 2022, 239, 1933–1943. [Google Scholar] [CrossRef]
- Halpern, J.H.; Pope, H.G., Jr. Hallucinogen Persisting Perception Disorder: What Do We Know after 50 Years? Drug Alcohol Depend. 2003, 69, 109–119. [Google Scholar] [CrossRef]
- Halpern, J.; Lerner, A.; Passie, T. Behavioral Neurobiology of Psychedelic Drugs; Halberstadt, A.L., Vollenweider, F.X., Nichols, D.E., Eds.; Current Topics in Behavioral Neurosciences, Volume 36; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Rudin, D.; Liechti, M.E.; Luethi, D. Molecular and Clinical Aspects of Potential Neurotoxicity Induced by New Psychoactive Stimulants and Psychedelics. Exp. Neurol. 2021, 343, 113778. [Google Scholar] [CrossRef]
- Cameron, L.P.; Tombari, R.J.; Lu, J.; Pell, A.J.; Hurley, Z.Q.; Ehinger, Y.; Vargas, M.V.; McCarroll, M.N.; Taylor, J.C.; Myers-Turnbull, D. A Non-Hallucinogenic Psychedelic Analogue with Therapeutic Potential. Nature 2021, 589, 474–479. [Google Scholar] [CrossRef]
- Hesselgrave, N.; Troppoli, T.A.; Wulff, A.B.; Cole, A.B.; Thompson, S.M. Harnessing Psilocybin: Antidepressant-Like Behavioral and Synaptic Actions of Psilocybin Are Independent of 5-HT2R Activation in Mice. Proc. Natl. Acad. Sci. USA 2021, 118, e2022489118. [Google Scholar] [CrossRef]
- Kaplan, A.L.; Confair, D.N.; Kim, K.; Barros-Álvarez, X.; Rodriguiz, R.M.; Yang, Y.; Kweon, O.S.; Che, T.; McCorvy, J.D.; Kamber, D.N. Bespoke Library Docking for 5-HT2A Receptor Agonists with Antidepressant Activity. Nature 2022, 610, 582–591. [Google Scholar] [CrossRef]
- Dunlap, L.E.; Azinfar, A.; Ly, C.; Cameron, L.P.; Viswanathan, J.; Tombari, R.J.; Myers-Turnbull, D.; Taylor, J.C.; Grodzki, A.C.; Lein, P.J. Identification of Psychoplastogenic N, N-Dimethylaminoisotryptamine (isoDMT) Analogues through Structure–Activity Relationship Studies. J. Med. Chem. 2020, 63, 1142–1155. [Google Scholar] [CrossRef]
- Dong, C.; Ly, C.; Dunlap, L.E.; Vargas, M.V.; Sun, J.; Hwang, I.-W.; Azinfar, A.; Oh, W.C.; Wetsel, W.C.; Olson, D.E. Psychedelic-Inspired Drug Discovery Using an Engineered Biosensor. Cell 2021, 184, 2779–2792.e18. [Google Scholar] [CrossRef]
- Lewis, V.; Bonniwell, E.M.; Lanham, J.K.; Ghaffari, A.; Sheshbaradaran, H.; Cao, A.B.; Calkins, M.M.; Bautista-Carro, M.A.; Arsenault, E.; Telfer, A.; et al. A Non-Hallucinogenic LSD Analog with Therapeutic Potential for Mood Disorders. Cell Rep. 2023, 42, 112203. [Google Scholar] [CrossRef]
- Tomašević, N.; Vujović, M.; Kostić, E.; Ragavendran, V.; Arsić, B.; Matić, S.L.; Božović, M.; Fioravanti, R.; Proia, E.; Ragno, R.; et al. Molecular Docking Assessment of Cathinones as 5-HT(2A)R Ligands: Developing of Predictive Structure-Based Bioactive Conformations and Three-Dimensional Structure-Activity Relationships Models for Future Recognition of Abuse Drugs. Molecules 2023, 28, 6236. [Google Scholar] [CrossRef] [PubMed]
- O’Grady, C. No Trip Needed for Psychedelics to Lift Mood? Science 2023, 380, 999. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, R.S. Is the Psychedelic Experience an Essential Aspect of the Therapeutic Effect of Serotonergic Psychedelics? Conceptual, Discovery, Development and Implementation Implications for Psilocybin and Related Agents. Expert Opin. Drug Saf. 2023, 22, 881–883. [Google Scholar] [CrossRef]
- Olson, D.E. The Subjective Effects of Psychedelics May Not Be Necessary for Their Enduring Therapeutic Effects. ACS Pharmacol. Transl. Sci. 2021, 4, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Polito, V.; Liknaitzky, P. The Emerging Science of Microdosing: A Systematic Review of Research on Low Dose Psychedelics (1955–2021) and Recommendations for the Field. Neurosci. Biobehav. Rev. 2022, 139, 104706. [Google Scholar] [CrossRef]
- Yaden, D.B.; Griffiths, R.R. The Subjective Effects of Psychedelics Are Necessary for Their Enduring Therapeutic Effects. ACS Pharmacol. Transl. Sci. 2021, 4, 568–572. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, R.G.; Hallak, J.E.; Baker, G.; Dursun, S. Hallucinogenic/Psychedelic 5HT2A Receptor Agonists as Rapid Antidepressant Therapeutics: Evidence and Mechanisms of Action. J. Psychopharmacol. 2021, 35, 453–458. [Google Scholar] [CrossRef]
- Johnson, M.P.; Baez, M.; Kursar, J.D.; Nelson, D.L. Species Differences in 5-HT2A Receptors: Cloned Pig and Rhesus Monkey 5-HT2A Receptors Reveal Conserved Transmembrane Homology to the Human Rather Than Rat Sequence. Biochim. Biophys. Acta 1995, 1236, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Wise, T.; Taylor, M.J.; Herane-Vives, A.; Gammazza, A.M.; Cappello, F.; Lythgoe, D.J.; Williams, S.C.; Young, A.H.; Cleare, A.J.; Arnone, D. Glutamatergic Hypofunction in Medication-Free Major Depression: Secondary Effects of Affective Diagnosis and Relationship to Peripheral Glutaminase. J. Affect. Disord. 2018, 234, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Wojtas, A.; Bysiek, A.; Wawrzczak-Bargiela, A.; Szych, Z.; Majcher-Maślanka, I.; Herian, M.; Maćkowiak, M.; Gołembiowska, K. Effect of Psilocybin and Ketamine on Brain Neurotransmitters, Glutamate Receptors, DNA and Rat Behavior. Int. J. Mol. Sci. 2022, 23, 6713. [Google Scholar] [CrossRef] [PubMed]
- Scruggs, J.L.; Schmidt, D.; Deutch, A.Y. The Hallucinogen 1-[2, 5-Dimethoxy-4-Iodophenyl]-2-Aminopropane (DOI) Increases Cortical Extracellular Glutamate Levels in Rats. Neurosci. Lett. 2003, 346, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Manahan-Vaughan, D. Role of Metabotropic Glutamate Receptors in Persistent Forms of Hippocampal Plasticity and Learning. Neuropharmacology 2013, 66, 65–81. [Google Scholar] [CrossRef]
- González-Maeso, J.; Ang, R.L.; Yuen, T.; Chan, P.; Weisstaub, N.V.; López-Giménez, J.F.; Zhou, M.; Okawa, Y.; Callado, L.F.; Milligan, G.; et al. Identification of a Serotonin/Glutamate Receptor Complex Implicated in Psychosis. Nature 2008, 452, 93–97. [Google Scholar] [CrossRef]
- Moreno, J.L.; Miranda-Azpiazu, P.; García-Bea, A.; Younkin, J.; Cui, M.; Kozlenkov, A.; Ben-Ezra, A.; Voloudakis, G.; Fakira, A.K.; Baki, L.; et al. Allosteric Signaling through an mGlu2 and 5-HT2A Heteromeric Receptor Complex and Its Potential Contribution to Schizophrenia. Sci. Signal. 2016, 9, ra5. [Google Scholar] [CrossRef]
- Walker, A.G.; Wenthur, C.J.; Xiang, Z.; Rook, J.M.; Emmitte, K.A.; Niswender, C.M.; Lindsley, C.W.; Conn, P.J. Metabotropic Glutamate Receptor 3 Activation Is Required for Long-Term Depression in Medial Prefrontal Cortex and Fear Extinction. Proc. Natl. Acad. Sci. USA 2015, 112, 1196–1201. [Google Scholar] [CrossRef]
- Vaidya, V.A.; Marek, G.J.; Aghajanian, G.K.; Duman, R.S. 5-HT2A Receptor-Mediated Regulation of Brain-Derived Neurotrophic Factor mRNA in the Hippocampus and the Neocortex. J. Neurosci. 1997, 17, 2785–2795. [Google Scholar] [CrossRef]
- De Gregorio, D.; Popic, J.; Enns, J.P.; Inserra, A.; Skalecka, A.; Markopoulos, A.; Posa, L.; Lopez-Canul, M.; Qianzi, H.; Lafferty, C.K.; et al. Lysergic Acid Diethylamide (LSD) Promotes Social Behavior through mTORC1 in the Excitatory Neurotransmission. Proc. Natl. Acad. Sci. USA 2021, 118, e2020705118. [Google Scholar] [CrossRef]
- Zhang, C.; Marek, G.J. AMPA Receptor Involvement in 5-Hydroxytryptamine2a Receptor-Mediated Pre-Frontal Cortical Excitatory Synaptic Currents and DOI-Induced Head Shakes. Progress. Neuro-Psychopharmacol. Biol. Psychiatry 2008, 32, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Benneyworth, M.A.; Xiang, Z.; Smith, R.L.; Garcia, E.E.; Conn, P.J.; Sanders-Bush, E. A Selective Positive Allosteric Modulator of Metabotropic Glutamate Receptor Subtype 2 Blocks a Hallucinogenic Drug Model of Psychosis. Mol. Pharmacol. 2007, 72, 477–484. [Google Scholar] [CrossRef]
- Lambe, E.K.; Aghajanian, G.K. Hallucinogen-Induced UP States in the Brain Slice of Rat Prefrontal Cortex: Role of Glutamate Spillover and NR2B-NMDA Receptors. Neuropsychopharmacology 2006, 31, 1682–1689. [Google Scholar] [CrossRef] [PubMed]
- Barre, A.; Berthoux, C.; De Bundel, D.; Valjent, E.; Bockaert, J.; Marin, P.; Bécamel, C. Presynaptic Serotonin 2A Receptors Modulate Thalamocortical Plasticity and Associative Learning. Proc. Natl. Acad. Sci. USA 2016, 113, E1382–E1391. [Google Scholar] [CrossRef] [PubMed]
- Rex, C.S.; Lauterborn, J.C.; Lin, C.-Y.; Kramár, E.A.; Rogers, G.A.; Gall, C.M.; Lynch, G. Restoration of Long-Term Potentiation in Middle-Aged Hippocampus after Induction of Brain-Derived Neurotrophic Factor. J. Neurophysiol. 2006, 96, 677–685. [Google Scholar] [CrossRef]
- Goff, D.C.; Lamberti, J.S.; Leon, A.C.; Green, M.F.; Miller, A.L.; Patel, J.; Manschreck, T.; Freudenreich, O.; Johnson, S.A. A Placebo-Controlled Add-on Trial of the Ampakine, CX516, for Cognitive Deficits in Schizophrenia. Neuropsychopharmacology 2008, 33, 465–472. [Google Scholar] [CrossRef]
- Anis, N.A.; Berry, S.C.; Burton, N.R.; Lodge, D. The Dissociative Anaesthetics, Ketamine and Phencyclidine, Selectively Reduce Excitation of Central Mammalian Neurones by N-Methyl-Aspartate. Br. J. Pharmacol. 1983, 79, 565–575. [Google Scholar] [CrossRef]
- Moghaddam, B.; Adams, B.; Verma, A.; Daly, D. Activation of Glutamatergic Neurotransmission by Ketamine: A Novel Step in the Pathway from NMDA Receptor Blockade to Dopaminergic and Cognitive Disruptions Associated with the Prefrontal Cortex. J. Neurosci. 1997, 17, 2921–2927. [Google Scholar] [CrossRef]
- Quirk, M.C.; Sosulski, D.L.; Feierstein, C.E.; Uchida, N.; Mainen, Z.F. A Defined Network of Fast-Spiking Interneurons in Orbitofrontal Cortex: Responses to Behavioral Contingencies and Ketamine Administration. Front. Syst. Neurosci. 2009, 3, 13. [Google Scholar] [CrossRef]
- Jodo, E.; Suzuki, Y.; Katayama, T.; Hoshino, K.Y.; Takeuchi, S.; Niwa, S.; Kayama, Y. Activation of Medial Prefrontal Cortex by Phencyclidine Is Mediated via a Hippocampo-Prefrontal Pathway. Cereb. Cortex 2005, 15, 663–669. [Google Scholar] [CrossRef]
- Moghaddam, B.; Adams, B.W. Reversal of Phencyclidine Effects by a Group II Metabotropic Glutamate Receptor Agonist in Rats. Science 1998, 281, 1349–1352. [Google Scholar] [CrossRef] [PubMed]
- Ly, C.; Greb, A.C.; Vargas, M.V.; Duim, W.C.; Grodzki, A.C.G.; Lein, P.J.; Olson, D.E. Transient Stimulation with Psychoplastogens Is Sufficient to Initiate Neuronal Growth. ACS Pharmacol. Transl. Sci. 2021, 4, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Phoumthipphavong, V.; Barthas, F.; Hassett, S.; Kwan, A.C. Longitudinal Effects of Ketamine on Dendritic Architecture In Vivo in the Mouse Medial Frontal Cortex. eNeuro 2016, 3, ENEURO.0133-15.2016. [Google Scholar] [CrossRef]
- Fischer, R.; Marks, P.A.; Hill, R.M.; Rockey, M.A. Personality Structure as the Main Determinant of Drug Induced (Model) Psychoses. Nature 1968, 218, 296–298. [Google Scholar] [CrossRef] [PubMed]
- Maeng, S.; Zarate, C.A., Jr.; Du, J.; Schloesser, R.J.; McCammon, J.; Chen, G.; Manji, H.K. Cellular Mechanisms Underlying the Antidepressant Effects of Ketamine: Role of Alpha-Amino-3-Hydroxy-5-Methylisoxazole-4-Propionic Acid Receptors. Biol. Psychiatry 2008, 63, 349–352. [Google Scholar] [CrossRef]
- Cavus, I.; Duman, R.S. Influence of Estradiol, Stress, and 5-HT2A Agonist Treatment on Brain-Derived Neurotrophic Factor Expression in Female Rats. Biol. Psychiatry 2003, 54, 59–69. [Google Scholar] [CrossRef]
- Garcia, L.S.; Comim, C.M.; Valvassori, S.S.; Réus, G.Z.; Stertz, L.; Kapczinski, F.; Gavioli, E.C.; Quevedo, J. Ketamine Treatment Reverses Behavioral and Physiological Alterations Induced by Chronic Mild Stress in Rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 2009, 33, 450–455. [Google Scholar] [CrossRef]
- Wacker, D.; Wang, C.; Katritch, V.; Han, G.W.; Huang, X.P.; Vardy, E.; McCorvy, J.D.; Jiang, Y.; Chu, M.; Siu, F.Y.; et al. Structural Features for Functional Selectivity at Serotonin Receptors. Science 2013, 340, 615–619. [Google Scholar] [CrossRef]
- Nichols, D.E. Hallucinogens. Pharmacol. Ther. 2004, 101, 131–181. [Google Scholar] [CrossRef]
- Kroeze, W.K.; Sassano, M.F.; Huang, X.P.; Lansu, K.; McCorvy, J.D.; Giguère, P.M.; Sciaky, N.; Roth, B.L. PRESTO-Tango as an Open-Source Resource for Interrogation of the Druggable Human GPCRome. Nat. Struct. Mol. Biol. 2015, 22, 362–369. [Google Scholar] [CrossRef]
- Lawn, T.; Dipasquale, O.; Vamvakas, A.; Tsougos, I.; Mehta, M.A.; Howard, M.A. Differential Contributions of Serotonergic and Dopaminergic Functional Connectivity to the Phenomenology of LSD. Psychopharmacology 2022, 239, 1797–1808. [Google Scholar] [CrossRef]
- Marona-Lewicka, D.; Thisted, R.A.; Nichols, D.E. Distinct Temporal Phases in the Behavioral Pharmacology of LSD: Dopamine D2 Receptor-Mediated Effects in the Rat and Implications for Psychosis. Psychopharmacology 2005, 180, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Erkizia-Santamaría, I.; Alles-Pascual, R.; Horrillo, I.; Meana, J.J.; Ortega, J.E. Serotonin 5-HT(2A), 5-HT(2c) and 5-HT(1A) Receptor Involvement in the Acute Effects of Psilocybin in Mice. In Vitro Pharmacological Profile and Modulation of Thermoregulation and Head-Twich Response. Biomed. Pharmacother. 2022, 154, 113612. [Google Scholar] [CrossRef] [PubMed]
- Geiger, H.A.; Wurst, M.G.; Daniels, R.N. DARK Classics in Chemical Neuroscience: Psilocybin. ACS Chem. Neurosci. 2018, 9, 2438–2447. [Google Scholar] [CrossRef]
- Davies, M.F.; Deisz, R.A.; Prince, D.A.; Peroutka, S.J. Two Distinct Effects of 5-Hydroxytryptamine on Single Cortical Neurons. Brain Res. 1987, 423, 347–352. [Google Scholar] [CrossRef]
- Inserra, A.; De Gregorio, D.; Gobbi, G. Psychedelics in Psychiatry: Neuroplastic, Immunomodulatory, and Neurotransmitter Mechanisms. Pharmacol. Rev. 2021, 73, 202–277. [Google Scholar] [CrossRef]
- Savalia, N.K.; Shao, L.-X.; Kwan, A.C. A Dendrite-Focused Framework for Understanding the Actions of Ketamine and Psychedelics. Trends Neurosci. 2021, 44, 260–275. [Google Scholar] [CrossRef] [PubMed]
- Vollenweider, F.X.; Vontobel, P.; Hell, D.; Leenders, K.L. 5-HT Modulation of Dopamine Release in Basal Ganglia in Psilocybin-Induced Psychosis in Man—A PET Study with [11C] Raclopride. Neuropsychopharmacology 1999, 20, 424–433. [Google Scholar] [CrossRef]
- Ichikawa, J.; Meltzer, H.Y. The Effect of Serotonin1A Receptor Agonism on Antipsychotic Drug-Induced Dopamine Release in Rat Striatum and Nucleus Accumbens. Brain Res. 2000, 858, 252–263. [Google Scholar] [CrossRef]
- Marona-Lewicka, D.; Nichols, D.E. Further Evidence that the Delayed Temporal Dopaminergic Effects of LSD Are Mediated by a Mechanism Different than the First Temporal Phase of Action. Pharmacol. Biochem. Behav. 2007, 87, 453–461. [Google Scholar] [CrossRef]
- Ubhayarathna, M.; Langmead, C.J.; Diepenhorst, N.A.; Stewart, G.D. Molecular and Structural Insights into the 5-HT(2C) Receptor as a Therapeutic Target for Substance Use Disorders. Br. J. Pharmacol. 2023. early view. [Google Scholar] [CrossRef]
- De Gregorio, D.; Posa, L.; Ochoa-Sanchez, R.; McLaughlin, R.; Maione, S.; Comai, S.; Gobbi, G. The Hallucinogen D-Lysergic Diethylamide (LSD) Decreases Dopamine Firing Activity through 5-HT1A, D2 and TAAR1 Receptors. Pharmacol. Res. 2016, 113, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Shahar, O.; Botvinnik, A.; Esh-Zuntz, N.; Brownstien, M.; Wolf, R.; Lotan, A.; Wolf, G.; Lerer, B.; Lifschytz, T. Role of 5-HT2A, 5-HT2C, 5-HT1A and TAAR1 Receptors in the Head Twitch Response Induced by 5-Hydroxytryptophan and Psilocybin: Translational Implications. Int. J. Mol. Sci. 2022, 23, 14148. [Google Scholar] [CrossRef] [PubMed]
- Pabba, M. The Essential Roles of Protein–Protein Interaction in Sigma-1 Receptor Functions; Frontiers Media SA: Lausanne, Switzerland, 2013; Volume 7, p. 50. [Google Scholar]
- Weng, T.Y.; Tsai, S.A.; Su, T.P. Roles of Sigma-1 Receptors on Mitochondrial Functions Relevant to Neurodegenerative Diseases. J. Biomed. Sci. 2017, 24, 74. [Google Scholar] [CrossRef]
- Inserra, A. Hypothesis: The Psychedelic Ayahuasca Heals Traumatic Memories via a Sigma 1 Receptor-Mediated Epigenetic-Mnemonic Process. Front. Pharmacol. 2018, 9, 330. [Google Scholar] [CrossRef]
- Albayrak, Y.; Hashimoto, K. Sigma-1 Receptor Agonists and Their Clinical Implications in Neuropsychiatric Disorders. Sigma Recept. Their Role Dis. Ther. Targets 2017, 964, 153–161. [Google Scholar]
- Zhang, H.; Cuevas, J. Sigma Receptors Inhibit High-Voltage–Activated Calcium Channels in Rat Sympathetic and Parasympathetic Neurons. J. Neurophysiol. 2002, 87, 2867–2879. [Google Scholar] [CrossRef]
- Penke, B.; Fulop, L.; Szucs, M.; Frecska, E. The Role of Sigma-1 Receptor, an Intracellular Chaperone in Neurodegenerative Diseases. Curr. Neuropharmacol. 2018, 16, 97–116. [Google Scholar] [CrossRef]
- Frecska, E.; Szabo, A.; Winkelman, M.J.; Luna, L.E.; McKenna, D.J. A Possibly Sigma-1 Receptor Mediated Role of Dimethyltryptamine in Tissue Protection, Regeneration, and Immunity. J. Neural Transm. 2013, 120, 1295–1303. [Google Scholar] [CrossRef]
- Fontanilla, D.; Johannessen, M.; Hajipour, A.R.; Cozzi, N.V.; Jackson, M.B.; Ruoho, A.E. The Hallucinogen N, N-Dimethyltryptamine (DMT) Is an Endogenous Sigma-1 Receptor Regulator. Science 2009, 323, 934–937. [Google Scholar] [CrossRef]
- Su, T.-P.; Hayashi, T.; Vaupel, D.B. When the Endogenous Hallucinogenic Trace Amine N, N-Dimethyltryptamine Meets the Sigma-1 Receptor. Sci. Signal. 2009, 2, pe12. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Rizzuto, R.; Hajnoczky, G.; Su, T.-P. MAM: More Than Just a Housekeeper. Trends Cell Biol. 2009, 19, 81–88. [Google Scholar] [CrossRef]
- Aydar, E.; Palmer, C.P.; Klyachko, V.A.; Jackson, M.B. The Sigma Receptor as a Ligand-Regulated Auxiliary Potassium Channel Subunit. Neuron 2002, 34, 399–410. [Google Scholar] [CrossRef]
- Nardai, S.; László, M.; Szabó, A.; Alpár, A.; Hanics, J.; Zahola, P.; Merkely, B.; Frecska, E.; Nagy, Z. N, N-Dimethyltryptamine Reduces Infarct Size and Improves Functional Recovery Following Transient Focal Brain Ischemia in Rats. Exp. Neurol. 2020, 327, 113245. [Google Scholar] [CrossRef] [PubMed]
- Morales-Garcia, J.A.; Calleja-Conde, J.; Lopez-Moreno, J.A.; Alonso-Gil, S.; Sanz-SanCristobal, M.; Riba, J.; Perez-Castillo, A. N, N-Dimethyltryptamine Compound Found in the Hallucinogenic Tea Ayahuasca, Regulates Adult Neurogenesis In Vitro and In Vivo. Transl. Psychiatry 2020, 10, 331. [Google Scholar] [CrossRef]
- Rueda, C.B.; Llorente-Folch, I.; Traba, J.; Amigo, I.; Gonzalez-Sanchez, P.; Contreras, L.; Juaristi, I.; Martinez-Valero, P.; Pardo, B.; Del Arco, A. Glutamate Excitotoxicity and Ca2+-Regulation of Respiration: Role of the Ca2+ Activated Mitochondrial Transporters (CaMCs). Biochim. Biophys. Acta (BBA)-Bioenerg. 2016, 1857, 1158–1166. [Google Scholar] [CrossRef]
- Brimson, J.M.; Safrany, S.T.; Qassam, H.; Tencomnao, T. Dipentylammonium Binds to the Sigma-1 Receptor and Protects against Glutamate Toxicity, Attenuates Dopamine Toxicity and Potentiates Neurite Outgrowth in Various Cultured Cell Lines. Neurotox. Res. 2018, 34, 263–272. [Google Scholar] [CrossRef]
- Shen, Y.C.; Wang, Y.H.; Chou, Y.C.; Liou, K.T.; Yen, J.C.; Wang, W.Y.; Liao, J.F. Dimemorfan Protects Rats against Ischemic Stroke through Activation of Sigma-1 Receptor-Mediated Mechanisms by Decreasing Glutamate Accumulation. J. Neurochem. 2008, 104, 558–572. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sattler, R.; Yang, E.J.; Nunes, A.; Ayukawa, Y.; Akhtar, S.; Ji, G.; Zhang, P.-W.; Rothstein, J.D. Harmine, a Natural Beta-Carboline Alkaloid, Upregulates Astroglial Glutamate Transporter Expression. Neuropharmacology 2011, 60, 1168–1175. [Google Scholar] [CrossRef]
- Thompson, C.; Szabo, A. Psychedelics as a Novel Approach to Treating Autoimmune Conditions. Immunol. Lett. 2020, 228, 45–54. [Google Scholar] [CrossRef]
- McEwen, B.S. Protection and Damage from Acute and Chronic Stress: Allostasis and Allostatic Overload and Relevance to the Pathophysiology of Psychiatric Disorders. Ann. N. Y. Acad. Sci. 2004, 1032, 1–7. [Google Scholar] [CrossRef]
- Wichers, M.; Maes, M. The Psychoneuroimmuno-Pathophysiology of Cytokine-Induced Depression in Humans. Int. J. Neuropsychopharmacol. 2002, 5, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Vezzani, A.; Viviani, B. Neuromodulatory Properties of Inflammatory Cytokines and Their Impact on Neuronal Excitability. Neuropharmacology 2015, 96, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.S.; Zurek, A.A.; Lecker, I.; Yu, J.; Abramian, A.M.; Avramescu, S.; Davies, P.A.; Moss, S.J.; Lu, W.Y.; Orser, B.A. Memory Deficits Induced by Inflammation Are Regulated by α5-Subunit-Containing GABAA Receptors. Cell Rep. 2012, 2, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Szabo, A.; Kovacs, A.; Frecska, E.; Rajnavolgyi, E. Psychedelic N, N-Dimethyltryptamine and 5-Methoxy-N, N-Dimethyltryptamine Modulate Innate and Adaptive Inflammatory Responses through the Sigma-1 Receptor of Human Monocyte-Derived Dendritic Cells. PLoS ONE 2014, 9, e106533. [Google Scholar] [CrossRef] [PubMed]
- House, R.; Thomas, P.; Bhargava, H. Immunological Consequences of In Vitro Exposure to Lysergic Acid Diethylamide (LSD). Immunopharmacol. Immunotoxicol. 1994, 16, 23–40. [Google Scholar] [CrossRef] [PubMed]
- Nkadimeng, S.M.; Steinmann, C.M.; Eloff, J.N. Anti-Inflammatory Effects of Four Psilocybin-Containing Magic Mushroom Water Extracts In Vitro on 15-Lipoxygenase Activity and on Lipopolysaccharide-Induced Cyclooxygenase-2 and Inflammatory Cytokines in Human U937 Macrophage Cells. J. Inflamm. Res. 2021, 14, 3729. [Google Scholar] [CrossRef] [PubMed]
- Galvão-Coelho, N.L.; de Menezes Galvão, A.C.; de Almeida, R.N.; Palhano-Fontes, F.; Campos Braga, I.; Lobão Soares, B.; Maia-de-Oliveira, J.P.; Perkins, D.; Sarris, J.; de Araujo, D.B. Changes in Inflammatory Biomarkers Are Related to the Antidepressant Effects of Ayahuasca. J. Psychopharmacol. 2020, 34, 1125–1133. [Google Scholar] [CrossRef]
- Uthaug, M.V.; Lancelotta, R.; Szabo, A.; Davis, A.K.; Riba, J.; Ramaekers, J.G. Prospective Examination of Synthetic 5-Methoxy-N,N-Dimethyltryptamine Inhalation: Effects on Salivary IL-6, Cortisol Levels, Affect, and Non-Judgment. Psychopharmacology 2020, 237, 773–785. [Google Scholar] [CrossRef]
- Nau, F., Jr.; Yu, B.; Martin, D.; Nichols, C.D. Serotonin 5-HT2A Receptor Activation Blocks TNF-α Mediated Inflammation In Vivo. PLoS ONE 2013, 8, e75426. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Becnel, J.; Zerfaoui, M.; Rohatgi, R.; Boulares, A.H.; Nichols, C.D. Serotonin 5-Hydroxytryptamine(2A) Receptor Activation Suppresses Tumor Necrosis Factor-Alpha-Induced Inflammation with Extraordinary Potency. J. Pharmacol. Exp. Ther. 2008, 327, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Robinson, G.I.; Li, D.; Wang, B.; Zahoruiko, Y.; Gerasymchuk, M.; Hudson, D.; Kovalchuk, O.; Kovalchuk, I. Anti-Inflammatory Effects of Serotonin Receptor and Transient Receptor Potential Channel Ligands in Human Small Intestinal Epithelial Cells. Curr. Issues Mol. Biol. 2023, 45, 6743–6774. [Google Scholar] [CrossRef] [PubMed]
- Galvão, A.C.M.; de Almeida, R.N.; Silva, E.; Freire, F.A.M.; Palhano-Fontes, F.; Onias, H.; Arcoverde, E.; Maia-de-Oliveira, J.P.; de Araújo, D.B.; Lobão-Soares, B.; et al. Cortisol Modulation by Ayahuasca in Patients with Treatment Resistant Depression and Healthy Controls. Front. Psychiatry 2018, 9, 185. [Google Scholar] [CrossRef] [PubMed]
- Hasler, F.; Grimberg, U.; Benz, M.A.; Huber, T.; Vollenweider, F.X. Acute Psychological and Physiological Effects of Psilocybin in Healthy Humans: A Double-Blind, Placebo-Controlled Dose-Effect Study. Psychopharmacology 2004, 172, 145–156. [Google Scholar] [CrossRef]
- Brouwer, A.; Carhart-Harris, R.L. Pivotal Mental States. J. Psychopharmacol. 2021, 35, 319–352. [Google Scholar] [CrossRef]
- Jørgensen, H.; Knigge, U.; Kjaer, A.; Møller, M.; Warberg, J. Serotonergic Stimulation of Corticotropin-Releasing Hormone and Pro-Opiomelanocortin Gene Expression. J. Neuroendocrinol. 2002, 14, 788–795. [Google Scholar] [CrossRef]
- Van de Kar, L.D.; Javed, A.; Zhang, Y.; Serres, F.; Raap, D.K.; Gray, T.S. 5-HT2A Receptors Stimulate ACTH, Corticosterone, Oxytocin, Renin, and Prolactin Release and Activate Hypothalamic CRF and Oxytocin-Expressing Cells. J. Neurosci. 2001, 21, 3572–3579. [Google Scholar] [CrossRef]
- Krabbe, G.; Matyash, V.; Pannasch, U.; Mamer, L.; Boddeke, H.W.; Kettenmann, H. Activation of Serotonin Receptors Promotes Microglial Injury-Induced Motility but Attenuates Phagocytic Activity. Brain Behav. Immun. 2012, 26, 419–428. [Google Scholar] [CrossRef]
- Haroon, E.; Miller, A.H.; Sanacora, G. Inflammation, Glutamate, and Glia: A Trio of Trouble in Mood Disorders. Neuropsychopharmacology 2017, 42, 193–215. [Google Scholar] [CrossRef]
- Herr, N.; Bode, C.; Duerschmied, D. The Effects of Serotonin in Immune Cells. Front. Cardiovasc. Med. 2017, 4, 48. [Google Scholar] [CrossRef]
- Wolff, M.; Evens, R.; Mertens, L.J.; Koslowski, M.; Betzler, F.; Gründer, G.; Jungaberle, H. Learning to Let Go: A Cognitive-Behavioral Model of How Psychedelic Therapy Promotes Acceptance. Front. Psychiatry 2020, 11, 5. [Google Scholar] [CrossRef] [PubMed]
- Roseman, L.; Nutt, D.J.; Carhart-Harris, R.L. Quality of Acute Psychedelic Experience Predicts Therapeutic Efficacy of Psilocybin for Treatment-Resistant Depression. Front. Pharmacol. 2017, 8, 974. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, S.; Kulsakdinun, C.; Battaglia, G.; Jaffe, J.; De Souza, E. Initial Identification and Characterization of Sigma Receptors on Human Peripheral Blood Leukocytes. J. Pharmacol. Exp. Ther. 1988, 247, 1114–1119. [Google Scholar]
- Bourrie, B.; Bouaboula, M.; Benoit, J.M.; Derocq, J.M.; Esclangon, M.; Le Fur, G.; Casellas, P. Enhancement of Endotoxin-Induced Interleukin-10 Production by SR 31747A, a Sigma Ligand. Eur. J. Immunol. 1995, 25, 2882–2887. [Google Scholar] [CrossRef]
- Carayon, P.; Bouaboula, M.; Loubet, J.; Bourrie, B.; Petitpretre, G.; Le Fur, G.; Casellas, P. The Sigma Ligand SR 31747 Prevents the Development of Acute Graft-Versus-Host Disease in Mice by Blocking IFN-γ and GM-CSF mRNA Expression. Int. J. Immunopharmacol. 1995, 17, 753–761. [Google Scholar] [CrossRef]
- Szabo, A. Psychedelics and Immunomodulation: Novel Approaches and Therapeutic Opportunities. Front. Immunol. 2015, 6, 358. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banushi, B.; Polito, V. A Comprehensive Review of the Current Status of the Cellular Neurobiology of Psychedelics. Biology 2023, 12, 1380. https://doi.org/10.3390/biology12111380
Banushi B, Polito V. A Comprehensive Review of the Current Status of the Cellular Neurobiology of Psychedelics. Biology. 2023; 12(11):1380. https://doi.org/10.3390/biology12111380
Chicago/Turabian StyleBanushi, Blerida, and Vince Polito. 2023. "A Comprehensive Review of the Current Status of the Cellular Neurobiology of Psychedelics" Biology 12, no. 11: 1380. https://doi.org/10.3390/biology12111380




