AIF1: Function and Connection with Inflammatory Diseases
Abstract
:Simple Summary
Abstract
1. Introduction
2. Structure
3. Functions
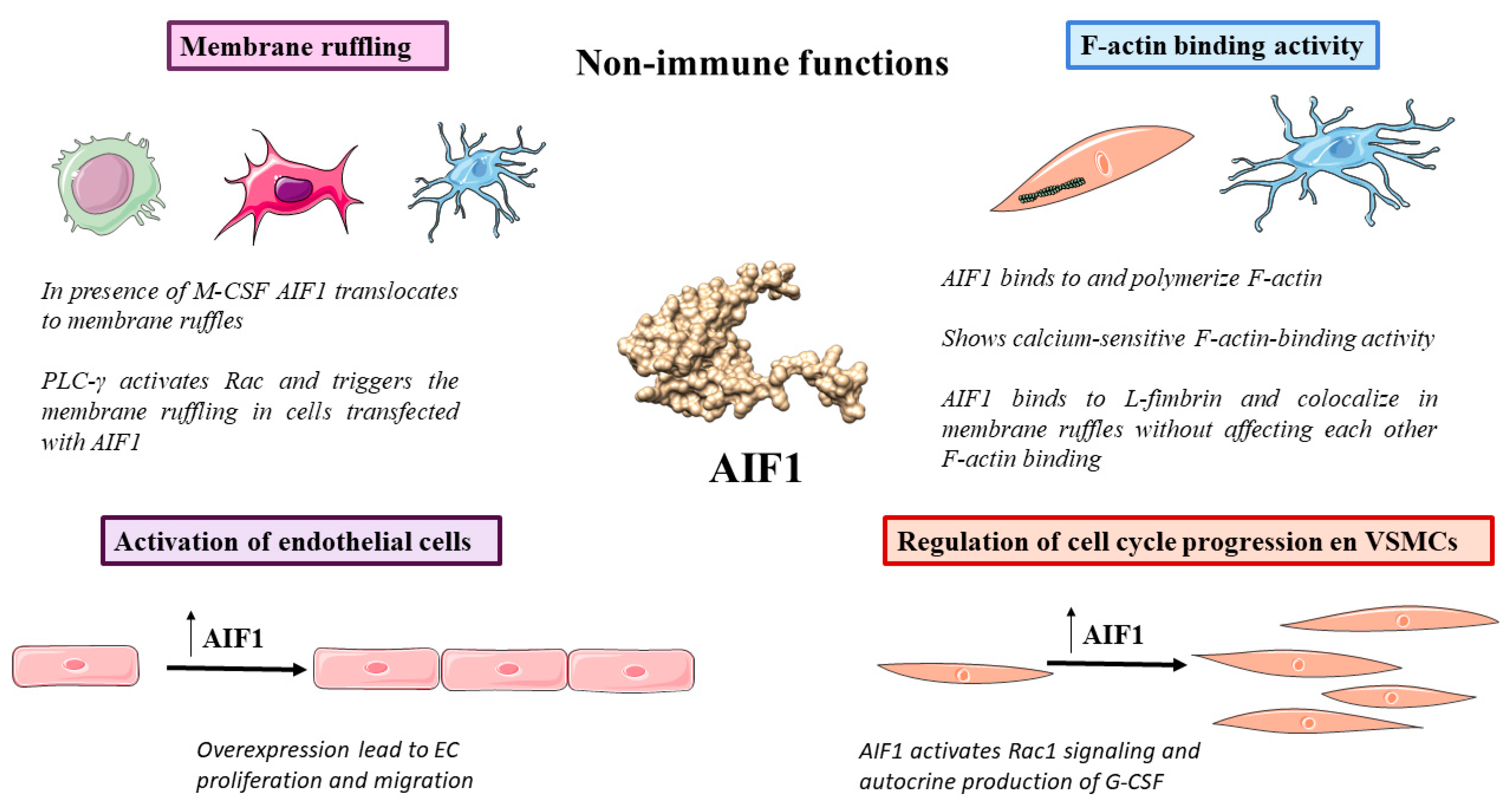
3.1. Membrane Ruffling
3.2. F-Actin Binding Activity
3.3. Regulation of Cell Cycle Progression
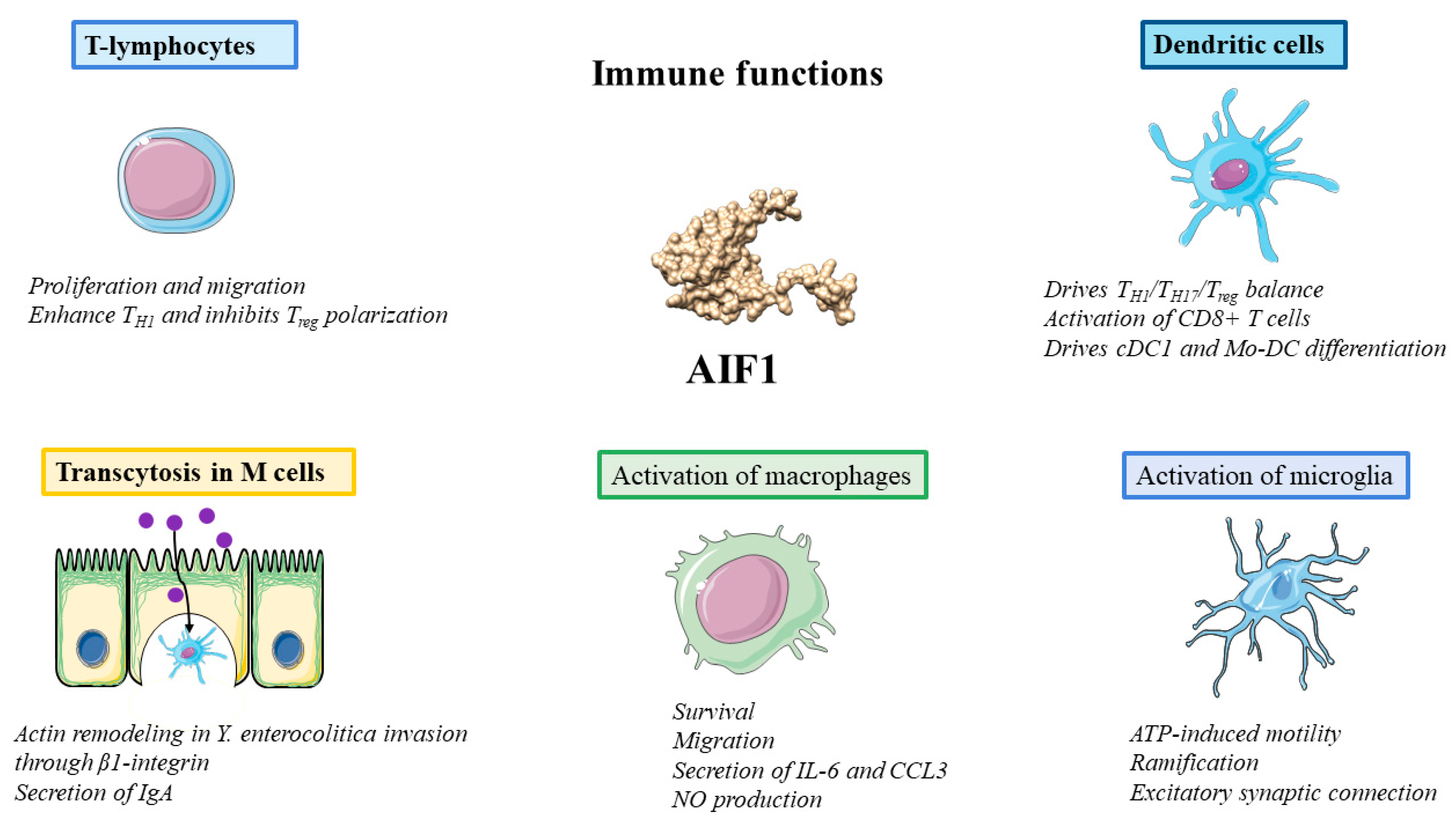
3.4. Functions in Immunity: Macrophages, T Lymphocytes and Dendritic Cells
3.5. Activation of Microglia
3.6. Transcytosis in M Cells
3.7. Activation of Endothelial Cells (ECs)
4. Clinical Relevance
4.1. Kidney Disease
4.2. Rheumatoid Arthritis
4.3. Cancer
4.4. CVDs
4.5. Metabolic Disease
4.6. Neurological Disorders
4.7. Transplants
| Pathology | AIF1 Expression Level | Expressing-AIF1 Cell Type | Target | Mechanism of Action/Correlation | References | |||
|---|---|---|---|---|---|---|---|---|
| Kidney disease | CKD | Upregulated | EC and VSMCs | Aldosterone upregulates AIF1, leading to calcium influx and calcification of VSMC | [32] | |||
| DKD | Upregulated | Glomerular EC | NF-κB signaling pathway | Inflammation and oxidative stress | [104] | |||
| Overexpression | HRGEC | miR-34a | miR-34a targets ATG4B Glucose upregulates AIF1 | [105] | ||||
| High levels | Serum | Correlated positively with albuminuria and negatively with eGFR | [108] | |||||
| RIF | Upregulated | Macrophages of renal interstitium | p38 or Akt/mTOR | Aldosterone upregulates AIF1, leading to formation of myofibroblasts via p38 in fibroblasts and activates macrophages via Akt/mTOR | [113,114] | |||
| Rheumatoid arthritis | Upregulated | Monocytes, infiltrating macrophages, synovial fibroblasts, blood and synovial fluid | Circulating monocytes correlated positively with DAS28 and Sharp erosion score AIF1 rs2259571 CC genotype associated with active form of RA and resistance to MTX | [120,121,122] | ||||
| Cancer | HCC | Upregulated | HCC | IGF/IGF1R axis | AIF1 activates IGF/IGF1R pathway AIF1 expression correlates with median tumor size, number of tumor deposits, the Barcelona Clinic Liver Cancer and portal vein tumor thrombus | [131,132] | ||
| Breast cancer | Upregulated | Epithelia of ductal carcinoma and MAMs | NF-κB Cyclin D1 p38 | Proliferation via NF-κB and upregulating cyclin D1 Migration via p38, which upregulates TNF-α Resistance to cisplatin | [133,134,135,136] | |||
| Lymphocytes and macrophages infiltrated | IL-6 | AIF1v1 expression was correlated with age, menopausal status, and CYP19A1 and IL-6 expression | [45] | |||||
| Glioma | Upregulated | Microglia and macrophages | Positively correlated with the level of immune infiltration and poor prognosis | [137,138] | ||||
| Esophageal cancer | Upregulated | TIGIT | AIF1 identified as a prognostic factor and associated with infiltration of macrophages, T cells, Treg cells and NK through TIGIT expression | [34,139] | ||||
| Haemangioma | Upregulated | EC | Trigger the recruitment of myeloid cells | [140] | ||||
| NSCLC | Upregulated | p38 and JAK/STAT | Associated with metastasis, higher TNM stage, and poorer survival via activation of p38-MAPK and JAK/STAT signaling | [141] | ||||
| Gallbladder cancer | Upregulated | TGF-β1/p38 pathway | Secretion of inflammatory factors, cell proliferation, inhibition of cell apoptosis and invasion and EMT, via TGF-β1/p38 pathway | [142] | ||||
| Gastric cancer | Downregulated | β-catenin | Proliferation and upregulation of β-catenin | [143] | ||||
| Atherosclerosis | Higher | Macrophages of atherosclerotic plaque in arterial wall | p65 of NF-κB pathway | ApoE -/- AIF1 mice showed increased area of atherosclerotic lesion Macrophages transfected with AIF1 incorporate higher levels of LDL, due to upregulation of SRA AIF1 activates macrophages through NF-κB pathway (phosphorylation of p65) | [35,37,154] | |||
| Higher | VSMCs | VSMCs express MMP2/9 and incorporate LDL | [155] | |||||
| Higher | Myeloid calcifying cells | Myeloid calcifying cells promote calcification of atherosclerotic plaque through paracrine activity | [156] | |||||
| Metabolic diseases | Obesity | Higher | Adipose-tissue macrophages | Macrophages secrete AIF1, increasing intracellular accumulation of lipids, production of ROS and release of TNF-α, IL-6 and resistin, and decreasing secretion of adiponectin; glucose uptake was suppressed and less consumed, NF-κB pathway was activated, with decreased GLUT4 on the plasma membrane and reduced Akt phosphorylation | [162] | |||
| Higher | Serum | Correlate positively with fasting plasma glucose, hemoglobin A1C, triglycerides, uric acid, waist circumference and body mass index | [159] | |||||
| Type 1 diabetes | Higher | Macrophages in pancreas | Accumulation in insulitis pancreatic islets | [42] | ||||
| DR | Serum | Correlated positively with HRS and IL-1β, IL-6 or TNF-α | [170] | |||||
| Neurological disorders | AD | Upregulated | Microglia | Microglia loss expression of TMEM119 and P2Y12. Correlated positively with age and CHI3L2 and CHID1 | [173,174,176] | |||
| Cerebral infarction | Upregulation | Microglia | Detected in first three days of glial reaction | [75] | ||||
| CJD | Upregulation | Microglia, macrophages and neurons | [180] | |||||
| Borna disease virus infection | Upregulated | Microglia | Activation of microglia and infiltration of macrophages | [181] | ||||
| CNS injury | Upregulated | Microglia and macrophages | Accumulation of microglia and macrophages in parenchymal pan-necrotic areas and perivascular Virchow–Robin spaces. Dexamethasone reduces accumulation of AIF1-expressing cell | [182,183] | ||||
| CIDP and VAS | Upregulated | Macrophages, T cells and VSMCs | Macrophages mainly in endoneurium, T cells near the blood vessels and some VSMCs in vessel walls | [184] | ||||
| EAN | Upregulation | Spleen and sciatic nerves | Upregulation of AIF1 at preclinical, and at height of clinical, EAN | [186] | ||||
| EAE | Upregulated | Macrophages and microglia | Macrophages in autoimmune lesions and microglia in the injured brain Inhibition of AIF1 led to a lower risk of developing EAE | [187] | ||||
| Transplants | Cardiac | Upregulated | Cardiomyocytes and mononuclear cells | Correlated with rejection and associated with CAV and Quilty B lesions | [36,190,191] | |||
| Kidney | Upregulated | Infiltrating macrophages and podocytes | Acute renal dysfunction associated with clinical rejection rs2269475 SNP associated with a lower risk of rejection | [192,193] | ||||
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marshall, J.S.; Warrington, R.; Watson, W.; Kim, H.L. An Introduction to Immunology and Immunopathology. Allergy Asthma. Clin. Immunol. 2018, 14, 49. [Google Scholar] [CrossRef]
- Turvey, S.E.; Broide, D.H. Innate Immunity. J. Allergy Clin. Immunol. 2010, 125, S24–S32. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, F.A.; Oettgen, H.C. Adaptive Immunity. J. Allergy Clin. Immunol. 2010, 125, S33–S40. [Google Scholar] [CrossRef] [PubMed]
- Nathan, C.; Ding, A. Nonresolving Inflammation. Cell 2010, 140, 871–882. [Google Scholar] [CrossRef]
- Medzhitov, R. Inflammation 2010: New Adventures of an Old Flame. Cell 2010, 140, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, O.; Akira, S. Pattern Recognition Receptors and Inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef]
- Granger, D.N.; Senchenkova, E. Inflammation and the Microcirculation. Inflamm. Microcirc. 2010, 2, 1–87. [Google Scholar] [CrossRef]
- Pahwa, R.; Goyal, A.; Jialal, I. Chronic Inflammation. Pathobiol. Hum. Dis. A Dyn. Encycl. Dis. Mech. 2022, 8, 300–314. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, M.; Ericsson, A.C. Function of Macrophages in Disease: Current Understanding on Molecular Mechanisms. Front. Immunol. 2021, 12, 635. [Google Scholar] [CrossRef]
- Wynn, T.A.; Chawla, A.; Pollard, J.W. Macrophage Biology in Development, Homeostasis and Disease. Nature 2013, 496, 445–455. [Google Scholar] [CrossRef]
- Murray, P.J.; Wynn, T.A. Protective and Pathogenic Functions of Macrophage Subsets. Nat. Rev. Immunol. 2011, 11, 723. [Google Scholar] [CrossRef] [PubMed]
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 Polarization. Eur. J. Pharmacol. 2020, 877, 173090. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J. Macrophage Polarization. Annu. Rev. Physiol. 2017, 79, 541–566. [Google Scholar] [CrossRef]
- Hirayama, D.; Iida, T.; Nakase, H. The Phagocytic Function of Macrophage-Enforcing Innate Immunity and Tissue Homeostasis. Int. J. Mol. Sci. 2018, 19, 92. [Google Scholar] [CrossRef]
- Duque, G.A.; Descoteaux, A. Macrophage Cytokines: Involvement in Immunity and Infectious Diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef]
- Ransohoff, R.M.; Perry, V.H. Microglial Physiology: Unique Stimuli, Specialized Responses. Annu. Rev. Immunol. 2009, 27, 119–145. [Google Scholar] [CrossRef]
- Colonna, M.; Butovsky, O. Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annu. Rev. Immunol. 2017, 35, 441. [Google Scholar] [CrossRef]
- Nayak, D.; Roth, T.L.; McGavern, D.B. Microglia Development and Function. Annu. Rev. Immunol. 2014, 32, 367. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Ma, Z.; Chen, X.; Shu, S. Microglia Activation in Central Nervous System Disorders: A Review of Recent Mechanistic Investigations and Development Efforts. Front. Neurol. 2023, 14, 1103416. [Google Scholar] [CrossRef]
- Woodburn, S.C.; Bollinger, J.L.; Wohleb, E.S. The Semantics of Microglia Activation: Neuroinflammation, Homeostasis, and Stress. J. Neuroinflamm. 2021, 18, 258. [Google Scholar] [CrossRef] [PubMed]
- Ginhoux, F.; Prinz, M. Origin of Microglia: Current Concepts and Past Controversies. Cold Spring Harb. Perspect. Biol. 2015, 7, a020537. [Google Scholar] [CrossRef]
- Vidal-Itriago, A.; Radford, R.A.W.; Aramideh, J.A.; Maurel, C.; Scherer, N.M.; Don, E.K.; Lee, A.; Chung, R.S.; Graeber, M.B.; Morsch, M. Microglia Morphophysiological Diversity and Its Implications for the CNS. Front. Immunol. 2022, 13, 5926. [Google Scholar] [CrossRef]
- Guo, S.; Wang, H.; Yin, Y. Microglia Polarization From M1 to M2 in Neurodegenerative Diseases. Front. Aging Neurosci. 2022, 14, 75. [Google Scholar] [CrossRef] [PubMed]
- Deininger, M.H.; Meyermann, R.; Schluesener, H.J. The Allograft Inflammatory Factor-1 Family of Proteins. FEBS Lett. 2002, 514, 115–121. [Google Scholar] [CrossRef]
- Utans, U.; Arceci, R.J.; Yamashita, Y.; Russell, M.E. Cloning and Characterization of Allograft Inflammatory Factor-1: A Novel Macrophage Factor Identified in Rat Cardiac Allografts with Chronic Rejection. J. Clin. Investig. 1995, 95, 2954–2962. [Google Scholar] [CrossRef]
- Iris, F.J.; Bougueleret, L.; Prieur, S.; Caterina, D.; Primas, G.; Perrot, V.; Jurka, J.; Rodriguez-Tome, P.; Claverie, J.M.; Dausset, J.; et al. Dense Alu Clustering and a Potential New Member of the NFκB Family within a 90 Kilobase HLA Class III Segment. Nat. Genet. 1993, 3, 137–145. [Google Scholar] [CrossRef]
- Autieri, M.V. CDNA Cloning of Human Allograft Inflammatory Factor-1: Tissue Distribution, Cytokine Induction, and MRNA Expression in Injured Rat Carotid Arteries. Biochem. Biophys. Res. Commun. 1996, 228, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Vizioli, J.; Verri, T.; Pagliara, P. Allograft Inflammatory Factor-1 in Metazoans: Focus on Invertebrates. Biology 2020, 9, 355. [Google Scholar] [CrossRef]
- Müller, W.E.; Krasko, A.; Skorokhod, A.; Bünz, C.; Grebenjuk, V.A.; Steffen, R.; Batel, R.; Schröder, H.C. Histocompatibility Reaction in Tissue and Cells of the Marine Sponge Suberites Domuncula in Vitro and in Vivo: Central Role of the Allograft Inflammatory Factor 1. Immunogenetics 2002, 54, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Xie, J.; Zhu, B.; Liu, X.; Wu, X. Allograft Inflammatory Factor 1 Functions as a Pro-Inflammatory Cytokine in the Oyster, Crassostrea Ariakensis. PLoS ONE 2014, 9, e95859. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Yan, D.J.; Chen, Z.W. Role of AIF-1 in the Regulation of Inflammatory Activation and Diverse Disease Processes. Cell Immunol. 2013, 284, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Hao, J.; Wang, X.; Liu, J.; Ni, J.; Hao, L. The Role of AIF-1 in the Aldosterone-Induced Vascular Calcification Related to Chronic Kidney Disease: Evidence From Mice Model and Cell Co-Culture Model. Front. Endocrinol. 2022, 1, 917356. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, K.; Słuczanowska-Głabowska, S.; Kurzawski, M.; Dziedziejko, V.; Kopytko, P.; Paczkowska, E.; Rogińska, D.; Safranow, K.; Machaliński, B.; Pawlik, A. Over-Expression of Allograft Inflammatory Factor-1 (AIF-1) in Patients with Rheumatoid Arthritis. Biomolecules 2020, 10, 1064. [Google Scholar] [CrossRef]
- Xu, X.; Wang, D.; Li, N.; Sheng, J.; Xie, M.; Zhou, Z.; Cheng, G.; Fan, Y. The Novel Tumor Microenvironment-Related Prognostic Gene AIF1 May Influence Immune Infiltrates and Is Correlated with TIGIT in Esophageal Cancer. Ann. Surg. Oncol. 2022, 29, 2930–2940. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, Y.; Wang, W.; Du, Z.; Yan, D.; Li, C.; Chen, Z. Daintain/AIF-1 Plays Roles in Coronary Heart Disease via Affecting the Blood Composition and Promoting Macrophage Uptake and Foam Cell Formation. Cell. Physiol. Biochem. 2013, 32, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; He, Z.; Henegar, J.; Allen, B.; Bigler, S. Expression of Allograft Inflammatory Factor-1 (AIF-1) in Acute Cellular Rejection of Cardiac Allografts. Cardiovasc. Pathol. 2011, 20, e177–e184. [Google Scholar] [CrossRef]
- Mishima, T.; Iwabuchi, K.; Fujii, S.; Tanaka, S.Y.; Ogura, H.; Watano-Miyata, K.; Ishimori, N.; Andoh, Y.; Nakai, Y.; Iwabuchi, C.; et al. Allograft Inflammatory Factor-1 Augments Macrophage Phagocytotic Activity and Accelerates the Progression of Atherosclerosis in ApoE-/- Mice. Int. J. Mol. Med. 2008, 21, 181–187. [Google Scholar] [CrossRef]
- Kelemen, S.E.; Autieri, M.V. Expression of Allograft Inflammatory Factor-1 in T Lymphocytes: A Role in T-Lymphocyte Activation and Proliferative Arteriopathies. Am. J. Pathol. 2005, 167, 619. [Google Scholar] [CrossRef]
- Kawasaki, H.; Kretsinger, R.H. Structural and Functional Diversity of EF-hand Proteins: Evolutionary Perspectives. Protein Sci. 2017, 26, 1898. [Google Scholar] [CrossRef]
- Lewit-Bentley, A.; Réty, S. EF-Hand Calcium-Binding Proteins. Curr. Opin. Struct. Biol. 2000, 10, 637–643. [Google Scholar] [CrossRef]
- Yamada, M.; Ohsawa, K.; Imai, Y.; Kohsaka, S.; Kamitori, S. X-Ray Structures of the Microglia/Macrophage-Specific Protein Iba1 from Human and Mouse Demonstrate Novel Molecular Conformation Change Induced by Calcium Binding. J. Mol. Biol. 2006, 364, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.W.; Ahren, B.; Östenson, C.G.; Cintra, A.; Bergman, T.; Möller, C.; Fuxe, K.; Mutt, V.; Jörnvall, H.; Efendic, S. Identification, Isolation, and Characterization of Daintain (Allograft Inflammatory Factor 1), a Macrophage Polypeptide with Effects on Insulin Secretion and Abundantly Present in the Pancreas of Prediabetic BB Rats. Proc. Natl. Acad. Sci. USA 1997, 94, 13879–13884. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Fuentes, E.J. Emerging Themes in PDZ Domain Signaling: Structure, Function, and Inhibition. Int. Rev. Cell Mol. Biol. 2019, 343, 129. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; DeVivo, M.; Dingus, J.; Harry, A.; Li, J.; Sui, J.; Carty, D.J.; Blank, J.L.; Exton, J.H.; Stoffel, R.H.; et al. A Region of Adenylyl Cyclase 2 Critical for Regulation by G Protein Beta Gamma Subunits. Science 1995, 268, 1166–1169. [Google Scholar] [CrossRef]
- Slim, F.A.; Ouellette, G.; Ennour-Idrissi, K.; Jacob, S.; Diorio, C.; Durocher, F. An Isoform of AIF1 Involved in Breast Cancer. Cancer Cell Int. 2018, 18, 167. [Google Scholar] [CrossRef]
- Borgo, C.; D’Amore, C.; Sarno, S.; Salvi, M.; Ruzzene, M. Protein Kinase CK2: A Potential Therapeutic Target for Diverse Human Diseases. Signal Transduct. Target. Ther. 2021, 6, 183. [Google Scholar] [CrossRef]
- Mahankali, M.; Peng, H.J.; Cox, D.; Gomez-Cambronero, J. The Mechanism of Cell Membrane Ruffling Relies on a Phospholipase D2 (PLD2), Grb2 and Rac2 Association. Cell Signal. 2011, 23, 1291–1298. [Google Scholar] [CrossRef]
- Ohsawa, K.; Imai, Y.; Kanazawa, H.; Sasaki, Y.; Kohsaka, S. Involvement of Iba1 in Membrane Ruffling and Phagocytosis of Macrophages/Microglia. J. Cell Sci. 2000, 113, 3073–3084. [Google Scholar] [CrossRef]
- Heasman, S.J.; Ridley, A.J. Mammalian Rho GTPases: New Insights into Their Functions from in Vivo Studies. Nat. Rev. Mol. Cell Biol. 2008, 9, 690–701. [Google Scholar] [CrossRef]
- Ridley, A.J.; Paterson, H.F.; Johnston, C.L.; Diekmann, D.; Hall, A. The Small GTP-Binding Protein Rac Regulates Growth Factor-Induced Membrane Ruffling. Cell 1992, 70, 401–410. [Google Scholar] [CrossRef]
- Kanazawa, H.; Ohsawa, K.; Sasaki, Y.; Kohsaka, S.; Imai, Y. Macrophage/Microglia-Specific Protein Iba1 Enhances Membrane Ruffling and Rac Activation via Phospholipase C-Gamma -Dependent Pathway. J. Biol. Chem. 2002, 277, 20026–20032. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.S.; Cantley, L.G.; Chen, C.S.; Kim, S.R.; Kwon, K.S.; Rhee, S.G. Activation of Phospholipase C-Gamma by Phosphatidylinositol 3,4,5-Trisphosphate. J. Biol. Chem. 1998, 273, 4465–4469. [Google Scholar] [CrossRef] [PubMed]
- Falasca, M.; Logan, S.K.; Lehto, V.P.; Baccante, G.; Lemmon, M.A.; Schlessinger, J. Activation of Phospholipase C Gamma by PI 3-Kinase-Induced PH Domain-Mediated Membrane Targeting. EMBO J. 1998, 17, 414–422. [Google Scholar] [CrossRef]
- Rönnstrand, L.; Siegbahn, A.; Rorsman, C.; Johnell, M.; Hansen, K.; Heldin, C.H. Overactivation of Phospholipase C-Gamma1 Renders Platelet-Derived Growth Factor Beta-Receptor-Expressing Cells Independent of the Phosphatidylinositol 3-Kinase Pathway for Chemotaxis. J. Biol. Chem. 1999, 274, 22089–22094. [Google Scholar] [CrossRef] [PubMed]
- Autieri, M.V.; Kelemen, S.E.; Wendt, K.W. AIF-1 Is an Actin-Polymerizing and Rac1-Activating Protein That Promotes Vascular Smooth Muscle Cell Migration. Circ. Res. 2003, 92, 1107–1114. [Google Scholar] [CrossRef]
- Sasaki, Y.; Ohsawa, K.; Kanazawa, H.; Kohsaka, S.; Imai, Y. Iba1 Is an Actin-Cross-Linking Protein in Macrophages/Microglia. Biochem. Biophys. Res. Commun. 2001, 286, 292–297. [Google Scholar] [CrossRef]
- Ohsawa, K.; Imai, Y.; Sasaki, Y.; Kohsaka, S. Microglia/Macrophage-Specific Protein Iba1 Binds to Fimbrin and Enhances Its Actin-Bundling Activity. J. Neurochem. 2004, 88, 844–856. [Google Scholar] [CrossRef]
- Gookin, S.; Min, M.; Phadke, H.; Chung, M.; Moser, J.; Miller, I.; Carter, D.; Spencer, S.L. A Map of Protein Dynamics during Cell-Cycle Progression and Cell-Cycle Exit. PLoS Biol. 2017, 15, e2003268. [Google Scholar] [CrossRef]
- Autieri, M.V.; Carbone, C.M. Overexpression of Allograft Inflammatory Factor-1 Promotes Proliferation of Vascular Smooth Muscle Cells by Cell Cycle Deregulation. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1421–1426. [Google Scholar] [CrossRef]
- Autieri, M.V.; Carbone, C.J.; Eisen, H. The Growth Enhancing Effects of Allograft Inflammatory Factor-1 (AIF-1) in VSMC Are Dose-Dependent and Mediated by Its Ability to Bind Calcium. J. Hear. Lung Transplant. 2001, 20, 198. [Google Scholar] [CrossRef]
- Chen, X.; Kelemen, S.E.; Autieri, M.V. AIF-1 Expression Modulates Proliferation of Human Vascular Smooth Muscle Cells by Autocrine Expression of G-CSF. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1217–1222. [Google Scholar] [CrossRef] [PubMed]
- Köhler, C. Allograft Inflammatory Factor-1/Ionized Calcium-Binding Adapter Molecule 1 Is Specifically Expressed by Most Subpopulations of Macrophages and Spermatids in Testis. Cell Tissue Res. 2007, 330, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.F.; Ho, D.W.; Lau, C.K.; Lam, C.T.; Lum, C.T.; Poon, R.T.P.; Fan, S.T. Allograft Inflammatory Factor-1 (AIF-1) Is Crucial for the Survival and pro-Inflammatory Activity of Macrophages. Int. Immunol. 2005, 17, 1391–1397. [Google Scholar] [CrossRef]
- Watano, K.; Iwabuchi, K.; Fujii, S.; Ishimori, N.; Mitsuhashi, S.; Ato, M.; Kitabatake, A.; Onoé, K. Allograft Inflammatory Factor-1 Augments Production of Interleukin-6, -10 and -12 by a Mouse Macrophage Line. Immunology 2001, 104, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Kadoya, M.; Yamamoto, A.; Hamaguchi, M.; Obayashi, H.; Mizushima, K.; Ohta, M.; Seno, T.; Oda, R.; Fujiwara, H.; Kohno, M.; et al. Allograft Inflammatory Factor-1 Stimulates Chemokine Production and Induces Chemotaxis in Human Peripheral Blood Mononuclear Cells. Biochem. Biophys. Res. Commun. 2014, 448, 287–291. [Google Scholar] [CrossRef]
- Tian, Y.; Kelemen, S.E.; Autieri, M.V. Inhibition of AIF-1 Expression by Constitutive SiRNA Expression Reduces Macrophage Migration, Proliferation, and Signal Transduction Initiated by Atherogenic Stimuli. Am. J. Physiol.—Cell Physiol. 2006, 290, C1083–C1091. [Google Scholar] [CrossRef]
- Cano-Martínez, D.; Monserrat, J.; Hernández-Breijo, B.; Sanmartín Salinas, P.; Álvarez-Mon, M.; Val Toledo-Lobo, M.; Guijarro, L.G. Extracellular Allograft Inflammatory Factor-1 (AIF-1) Potentiates Th1 Cell Differentiation and Inhibits Treg Response in Human Peripheral Blood Mononuclear Cells from Normal Subjects. Hum. Immunol. 2020, 81, 91–100. [Google Scholar] [CrossRef]
- Elizondo, D.M.; Andargie, T.E.; Yang, D.; Kacsinta, A.D.; Lipscomb, M.W. Inhibition of Allograft Inflammatory Factor-1 in Dendritic Cells Restrains CD4+ T Cell Effector Responses and Induces CD25+Foxp3+ T Regulatory Subsets. Front. Immunol. 2017, 8, 1502. [Google Scholar] [CrossRef]
- Elizondo, D.M.; Andargie, T.E.; Haddock, N.L.; da Silva, R.L.L.; de Moura, T.R.; Lipscomb, M.W. IL-10 Producing CD8+ CD122+ PD-1+ Regulatory T Cells Are Expanded by Dendritic Cells Silenced for Allograft Inflammatory Factor-1. J. Leukoc. Biol. 2019, 105, 123–130. [Google Scholar] [CrossRef]
- Huang, D.; Zhou, B.; Luo, Z.Z.; Yu, S.C.; Tang, B. Cigarette Smoke Extract Promotes DNA Methyltransferase 3a Expression in Dendritic Cells, Inducing Th-17/Treg Imbalance via the c-Jun/Allograft Inflammatory Factor 1 Axis. Kaohsiung J. Med. Sci. 2021, 37, 594–603. [Google Scholar] [CrossRef]
- Elizondo, D.M.; Brandy, N.Z.D.; Da Silva, R.L.L.; Haddock, N.L.; Kacsinta, A.D.; De Moura, T.R.; Lipscomb, M.W. Allograft Inflammatory Factor-1 Governs Hematopoietic Stem Cell Differentiation into CDC1 and Monocyte-Derived Dendritic Cells through IRF8 and RelB in Vitro. Front. Immunol. 2019, 10, 173. [Google Scholar] [CrossRef] [PubMed]
- Ito, D.; Imai, Y.; Ohsawa, K.; Nakajima, K.; Fukuuchi, Y.; Kohsaka, S. Microglia-Specific Localisation of a Novel Calcium Binding Protein, Iba1. Brain Res. Mol. Brain Res. 1998, 57, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mittelbronn, M.; Dietz, K.; Schluesener, H.J.; Meyermann, R. Local Distribution of Microglia in the Normal Adult Human Central Nervous System Differs by up to One Order of Magnitude. Acta Neuropathol. 2001, 101, 249–255. [Google Scholar] [CrossRef]
- Ahmed, Z.; Shaw, G.; Sharma, V.P.; Yang, C.; McGowan, E.; Dickson, D.W. Actin-Binding Proteins Coronin-1a and IBA-1 Are Effective Microglial Markers for Immunohistochemistry. J. Histochem. Cytochem. 2007, 55, 687–700. [Google Scholar] [CrossRef]
- Postler, E.; Rimner, A.; Beschorner, R.; Schluesener, H.J.; Meyermann, R. Allograft-Inflammatory-Factor-1 Is Upregulated in Microglial Cells in Human Cerebral Infarctions. J. Neuroimmunol. 2000, 104, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Norden, D.M.; Trojanowski, P.J.; Villanueva, E.; Navarro, E.; Godbout, J.P. Sequential Activation of Microglia and Astrocyte Cytokine Expression Precedes Increased Iba-1 or GFAP Immunoreactivity Following Systemic Immune Challenge. Glia 2016, 64, 300–316. [Google Scholar] [CrossRef] [PubMed]
- Lituma, P.J.; Woo, E.; O’Hara, B.F.; Castillo, P.E.; Sibinga, N.E.S.; Nandi, S. Altered Synaptic Connectivity and Brain Function in Mice Lacking Microglial Adapter Protein Iba1. Proc. Natl. Acad. Sci. USA 2021, 118, e2115539118. [Google Scholar] [CrossRef]
- Tanaka, S.; Suzuki, K.; Watanabe, M.; Matsuda, A.; Tone, S.; Koike, T. Upregulation of a New Microglial Gene, Mrf-1, in Response to Programmed Neuronal Cell Death and Degeneration. J. Neurosci. 1998, 18, 6358–6369. [Google Scholar] [CrossRef]
- Dillon, A.; Lo, D.D. M Cells: Intelligent Engineering of Mucosal Immune Surveillance. Front. Immunol. 2019, 10, 1499. [Google Scholar] [CrossRef]
- Kimura, S. Molecular Insights into the Mechanisms of M-Cell Differentiation and Transcytosis in the Mucosa-Associated Lymphoid Tissues. Anat. Sci. Int. 2018, 93, 23–34. [Google Scholar] [CrossRef]
- Kishikawa, S.; Sato, S.S.S.; Kaneto, S.; Uchino, S.; Kohsaka, S.; Nakamura, S.; Kiyono, H. Allograft Inflammatory Factor 1 Is a Regulator of Transcytosis in M Cells. Nat. Commun. 2017, 8, 14509. [Google Scholar] [CrossRef] [PubMed]
- Perrett, C.A.; Jepson, M.A. Regulation of Salmonella-Induced Membrane Ruffling by SipA Differs in Strains Lacking Other Effectors. Cell Microbiol. 2009, 11, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Rios, D.; Wood, M.B.; Li, J.; Chassaing, B.; Gewirtz, A.T.; Williams, I.R. Antigen Sampling by Intestinal M Cells Is the Principal Pathway Initiating Mucosal IgA Production to Commensal Enteric Bacteria. Mucosal Immunol. 2016, 9, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Tsubata, Y.; Sakatsume, M.; Ogawa, A.; Alchi, B.; Kaneko, Y.; Kuroda, T.; Kawachi, H.; Narita, I.; Yamamoto, T.; Gejyo, F. Expression of Allograft Inflammatory Factor-1 in Kidneys: A Novel Molecular Component of Podocyte. Kidney Int. 2006, 70, 1948–1954. [Google Scholar] [CrossRef]
- Jia, J.; Cai, Y.; Wang, R.; Fu, K.; Zhao, Y.F. Overexpression of Allograft Inflammatory Factor-1 Promotes the Proliferation and Migration of Human Endothelial Cells (HUV-EC-C) Probably by up-Regulation of Basic Fibroblast Growth Factor. Pediatr. Res. 2010, 67, 29–34. [Google Scholar] [CrossRef]
- Tian, Y.; Jain, S.; Kelemen, S.E.; Autieri, M.V. AIF-1 Expression Regulates Endothelial Cell Activation, Signal Transduction, and Vasculogenesis. Am. J. Physiol.—Cell Physiol. 2009, 296, 256–266. [Google Scholar] [CrossRef]
- Guijarro, L.G.; Cano-Martínez, D.; Toledo-Lobo, M.V.; Ruiz-Llorente, L.; Chaparro, M.; Guerra, I.; Iborra, M.; Cabriada, J.L.; Bujanda, L.; Taxonera, C.; et al. Evaluation of AIF-1 (Allograft Inflammatory Factor-1) as a Biomarker of Crohn’s Disease Severity. Biomedicines 2022, 10, 727. [Google Scholar] [CrossRef]
- Román, I.D.; Cano-Martínez, D.; Lobo, M.V.T.; Fernández-Moreno, M.D.; Hernández-Breijo, B.; Sacristán, S.; Sanmartín-Salinas, P.; Monserrat, J.; Gisbert, J.P.; Guijarro, L.G. Infliximab Therapy Reverses the Increase of Allograft Inflammatory Factor-1 in Serum and Colonic Mucosa of Rats with Inflammatory Bowel Disease. Biomarkers 2017, 22, 133–144. [Google Scholar] [CrossRef]
- Levey, A.S.; Coresh, J. Chronic Kidney Disease. Lancet 2012, 379, 165–180. [Google Scholar] [CrossRef]
- Marreiros, C.; Viegas, C.; Simes, D. Targeting a Silent Disease: Vascular Calcification in Chronic Kidney Disease. Int. J. Mol. Sci. 2022, 23, 16114. [Google Scholar] [CrossRef]
- Hostetter, T.H.; Ibrahim, H.N. Aldosterone in Chronic Kidney and Cardiac Disease. J. Am. Soc. Nephrol. 2003, 14, 2395–2401. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhou, X.; Huang, Z.; Fan, X.; Tan, X.; Lu, C.; Yang, J. Aldosterone Is a Possible New Stimulating Factor for Promoting Vascular Calcification. Front. Biosci. 2021, 26, 1052–1063. [Google Scholar] [CrossRef]
- Daniela, Q.; Federica, B.; Lofaro, F.D. The Biology of Vascular Calcification. Int. Rev. Cell Mol. Biol. 2020, 354, 261–353. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, I.K.; Jeon, J.H. Vascular Calcification—New Insights into Its Mechanism. Int. J. Mol. Sci. 2020, 21, 2685. [Google Scholar] [CrossRef]
- Zemaitis, M.R.; Foris, L.A.; Katta, S.; Bashir, K. Uremia. Urol. A Glance 2022, 7, 57–60. [Google Scholar] [CrossRef]
- Nigam, S.K.; Bush, K.T. Uremic Syndrome of Chronic Kidney Disease: Altered Remote Sensing and Signaling. Nat. Rev. Nephrol. 2019, 15, 301. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Tang, J.; Zhang, L.; Li, X.; Hao, L. The Crosstalk between Calcium Ions and Aldosterone Contributes to Inflammation, Apoptosis, and Calcification of VSMC via the AIF-1/NF- κ B Pathway in Uremia. Oxid. Med. Cell Longev. 2020, 2020, 3431597. [Google Scholar] [CrossRef]
- Reidy, K.; Kang, H.M.; Hostetter, T.; Susztak, K. Molecular Mechanisms of Diabetic Kidney Disease. J. Clin. Investig. 2014, 124, 2333. [Google Scholar] [CrossRef]
- Thomas, M.C.; Brownlee, M.; Susztak, K.; Sharma, K.; Jandeleit-Dahm, K.A.M.; Zoungas, S.; Rossing, P.; Groop, P.H.; Cooper, M.E. Diabetic Kidney Disease. Nat. Rev. Dis. Prim. 2015, 1, 15018. [Google Scholar] [CrossRef]
- Matoba, K.; Takeda, Y.; Nagai, Y.; Kawanami, D.; Utsunomiya, K.; Nishimura, R. Unraveling the Role of Inflammation in the Pathogenesis of Diabetic Kidney Disease. Int. J. Mol. Sci. 2019, 20, 3393. [Google Scholar] [CrossRef]
- Tanase, D.M.; Gosav, E.M.; Anton, M.I.; Floria, M.; Seritean Isac, P.N.; Hurjui, L.L.; Tarniceriu, C.C.; Costea, C.F.; Ciocoiu, M.; Rezus, C. Oxidative Stress and NRF2/KEAP1/ARE Pathway in Diabetic Kidney Disease (DKD): New Perspectives. Biomolecules 2022, 12, 1227. [Google Scholar] [CrossRef]
- Duni, A.; Liakopoulos, V.; Roumeliotis, S.; Peschos, D.; Dounousi, E. Oxidative Stress in the Pathogenesis and Evolution of Chronic Kidney Disease: Untangling Ariadne’s Thread. Int. J. Mol. Sci. 2019, 20, 3711. [Google Scholar] [CrossRef]
- Winiarska, A.; Knysak, M.; Nabrdalik, K.; Gumprecht, J.; Stompór, T. Inflammation and Oxidative Stress in Diabetic Kidney Disease: The Targets for SGLT2 Inhibitors and GLP-1 Receptor Agonists. Int. J. Mol. Sci. 2021, 22, 10822. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, X.; Zhang, L.; Ren, Y.; Hao, L. Allograft Inflammatory Factor-1 Enhances Inflammation and Oxidative Stress via the NF-ΚB Pathway in Diabetic Kidney Disease. Biochem. Biophys. Res. Commun. 2022, 614, 63–69. [Google Scholar] [CrossRef]
- Jianbing, H.; Xiaotian, L.; Jie, T.; Xueying, C.; Honge, J.; Bo, Z.; Lirong, H.; Lei, Z. The Effect of Allograft Inflammatory Factor-1 on Inflammation, Oxidative Stress, and Autophagy via MiR-34a/ATG4B Pathway in Diabetic Kidney Disease. Oxid. Med. Cell Longev. 2022, 2022, 1668000. [Google Scholar] [CrossRef]
- Wu, Y.; Dai, X.; Ni, Z.; Yan, X.; He, F.; Lian, J. The Downregulation of ATG4B Mediated by MicroRNA-34a/34c-5p Suppresses Rapamycin-Induced Autophagy. Iran. J. Basic Med. Sci. 2017, 20, 1125–1130. [Google Scholar] [CrossRef]
- Liu, X.J.; Hong, Q.; Wang, Z.; Yu, Y.Y.; Zou, X.; Xu, L.H. MicroRNA-34a Suppresses Autophagy in Tubular Epithelial Cells in Acute Kidney Injury. Am. J. Nephrol. 2015, 42, 168–175. [Google Scholar] [CrossRef]
- Fukui, M.; Tanaka, M.; Asano, M.; Yamazaki, M.; Hasegawa, G.; Imai, S.; Fujinami, A.; Ohta, M.; Obayashi, H.; Nakamura, N. Serum Allograft Inflammatory Factor-1 Is a Novel Marker for Diabetic Nephropathy. Diabetes Res. Clin. Pract. 2012, 97, 146–150. [Google Scholar] [CrossRef]
- Farris, A.B.; Colvin, R.B. Renal Interstitial Fibrosis: Mechanisms and Evaluation In: Current Opinion in Nephrology and Hypertension. Curr. Opin. Nephrol. Hypertens. 2012, 21, 289. [Google Scholar] [CrossRef]
- Wang, X.; Chen, J.; Xu, J.; Xie, J.; Harris, D.C.H.; Zheng, G. The Role of Macrophages in Kidney Fibrosis. Front. Physiol. 2021, 12, 987. [Google Scholar] [CrossRef]
- Wei, J.; Xu, Z.; Yan, X. The Role of the Macrophage-to-Myofibroblast Transition in Renal Fibrosis. Front. Immunol. 2022, 13, 934377. [Google Scholar] [CrossRef] [PubMed]
- Nikolic-Paterson, D.J.; Wang, S.; Lan, H.Y. Macrophages Promote Renal Fibrosis through Direct and Indirect Mechanisms. Kidney Int. Suppl. 2014, 4, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, X.; Zhang, L.; Yuan, X.; Hao, J.; Ni, J.; Hao, L. Upregulation of Allograft Inflammatory Factor-1 Expression and Secretion by Macrophages Stimulated with Aldosterone Promotes Renal Fibroblasts to a Profibrotic Phenotype. Int. J. Mol. Med. 2018, 42, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Wang, X.; Li, Y.; Li, X.; Zhang, S.; Hao, L. Aldosterone Promotes Renal Interstitial Fibrosis via the AIF-1/AKT/MTOR Signaling Pathway. Mol. Med. Rep. 2019, 20, 4033–4044. [Google Scholar] [CrossRef] [PubMed]
- Mueller, A.L.; Payandeh, Z.; Mohammadkhani, N.; Mubarak, S.M.H.; Zakeri, A.; Bahrami, A.A.; Brockmueller, A.; Shakibaei, M. Recent Advances in Understanding the Pathogenesis of Rheumatoid Arthritis: New Treatment Strategies. Cells 2021, 10, 3017. [Google Scholar] [CrossRef]
- Deane, K.D.; Holers, V.M. Rheumatoid Arthritis: Pathogenesis, Prediction and Prevention–An Emerging Paradigm Shift. Arthritis Rheumatol. 2021, 73, 181. [Google Scholar] [CrossRef]
- Smolen, J.S.; Aletaha, D.; Barton, A.; Burmester, G.R.; Emery, P.; Firestein, G.S.; Kavanaugh, A.; McInnes, I.B.; Solomon, D.H.; Strand, V.; et al. Rheumatoid Arthritis. Nat. Rev. Dis. Prim. 2018, 4, 18001. [Google Scholar] [CrossRef]
- Kimura, M.; Kawahito, Y.; Obayashi, H.; Ohta, M.; Hara, H.; Adachi, T.; Tokunaga, D.; Hojo, T.; Hamaguchi, M.; Omoto, A.; et al. A Critical Role for Allograft Inflammatory Factor-1 in the Pathogenesis of Rheumatoid Arthritis. J. Immunol. 2007, 178, 3316–3322. [Google Scholar] [CrossRef]
- Harney, S.M.J.; Vilariño-Güell, C.; Adamopoulos, I.E.; Sims, A.M.; Lawrence, R.W.; Cardon, L.R.; Newton, J.L.; Meisel, C.; Pointon, J.J.; Darke, C.; et al. Fine Mapping of the MHC Class III Region Demonstrates Association of AIF1 and Rheumatoid Arthritis. Rheumatology 2008, 47, 1761–1767. [Google Scholar] [CrossRef]
- Pawlik, A.; Kotrych, D.; Paczkowska, E.; Roginska, D.; Dziedziejko, V.; Safranow, K.; Machalinski, B. Expression of Allograft Inflammatory Factor-1 in Peripheral Blood Monocytes and Synovial Membranes in Patients with Rheumatoid Arthritis. Hum. Immunol. 2016, 77, 131–136. [Google Scholar] [CrossRef]
- Pawlik, A.; Kurzawski, M.; Dziedziejko, V.; Safranow, K.; Paczkowska, E.; Maslinski, W.; Drozdzik, M.; Gawronska-Szklarz, B. Allograft Inflammatory Factor-1 Gene Polymorphisms in Patients with Rheumatoid Arthritis. Genet. Test. Mol. Biomark. 2012, 16, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Pawlik, A.; Herczynska, M.M.; Dziedziejko, V.; Malinowski, D.; Kurzawski, M.; Drozdzik, M.; Gawronska-Szklarz, B. Effect of Allograft Inflammatory Factor-1 Gene Polymorphisms on Rheumatoid Arthritis Treatment with Methotrexate. Postepy Hig. Med. Dosw. 2013, 67, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. The Hallmarks of Cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and Tumor Progression: Signaling Pathways and Targeted Intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [CrossRef] [PubMed]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef]
- Qu, X.; Tang, Y.; Hua, S. Immunological Approaches towards Cancer and Inflammation: A Cross Talk. Front. Immunol. 2018, 9, 563. [Google Scholar] [CrossRef] [PubMed]
- Hiam-Galvez, K.J.; Allen, B.M.; Spitzer, M.H. Systemic Immunity in Cancer. Nat. Rev. Cancer 2021, 21, 345–359. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, H.; Hagerling, C.; Werb, Z. Roles of the Immune System in Cancer: From Tumor Initiation to Metastatic Progression. Genes Dev. 2018, 32, 1267. [Google Scholar] [CrossRef]
- Noy, R.; Pollard, J.W. Tumor-Associated Macrophages: From Mechanisms to Therapy. Immunity 2014, 41, 49–61. [Google Scholar] [CrossRef]
- Mantovani, A.; Marchesi, F.; Malesci, A.; Laghi, L.; Allavena, P. Tumor-Associated Macrophages as Treatment Targets In. Nat. Rev. Clin. Oncol. 2017, 14, 399. [Google Scholar] [CrossRef]
- Jia, S.; Du, Z.; Jiang, H.; Huang, X.; Chen, Z.; Chen, N. Daintain/AIF-1 Accelerates the Activation of Insulin-like Growth Factor-1 Receptor Signaling Pathway in HepG2 Cells. Oncol. Rep. 2015, 34, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Sun, S.; Zhu, C.; Xie, F.; Cai, Q.; Sun, H.; Chen, G.; Liang, X.; Xie, H.; Shi, J.; et al. Expression of Allograft Inflammatory Factor-1 (AIF-1) in Hepatocellular Carcinoma. Med. Sci. Monit. 2018, 24, 6218–6228. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Tan, W.Y.; Chen, Q.R.; Chen, X.P.; Fu, K.; Zhao, Y.Y.; Chen, Z.W. Daintain/AIF-1 Promotes Breast Cancer Proliferation via Activation of the NF-KappaB/Cyclin D1 Pathway and Facilitates Tumor Growth. Cancer Sci. 2008, 99, 952–957. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Feng, Z.; Jia, S.; Wang, W.; Du, Z.; Chen, N.; Chen, Z. Daintain/AIF-1 Promotes Breast Cancer Cell Migration by up-Regulated TNF-α via Activate P38 MAPK Signaling Pathway. Breast Cancer Res. Treat. 2012, 131, 891–898. [Google Scholar] [CrossRef]
- Zheng, W.; Zhao, D.; Zhang, H.; Chinnasamy, P.; Sibinga, N.; Pollard, J.W. Induction of Interferon Signaling and Allograft Inflammatory Factor 1 in Macrophages in a Mouse Model of Breast Cancer Metastases. Wellcome Open Res. 2021, 6, 52. [Google Scholar] [CrossRef]
- Jia, S.; Chaibou, M.A.; Chen, Z. Daintain/AIF-1 Reinforces the Resistance of Breast Cancer Cells to Cisplatin. Biosci. Biotechnol. Biochem. 2012, 76, 2338–2341. [Google Scholar] [CrossRef]
- Rao, M.; Yang, Z.; Huang, K.; Liu, W.; Chai, Y. Correlation of AIF-1 Expression with Immune and Clinical Features in 1270 Glioma Samples. J. Mol. Neurosci. 2022, 72, 420–432. [Google Scholar] [CrossRef]
- Deininger, M.H.; Seid, K.; Engel, S.; Meyermann, R.; Schluesener, H.J. Allograft Inflammatory Factor-1 Defines a Distinct Subset of Infiltrating Macrophages/Microglial Cells in Rat and Human Gliomas. Acta Neuropathol. 2000, 100, 673–680. [Google Scholar] [CrossRef]
- Harjunpää, H.; Guillerey, C. TIGIT as an Emerging Immune Checkpoint. Clin. Exp. Immunol. 2020, 200, 108–119. [Google Scholar] [CrossRef]
- Jia, J.; Bai, Y.; Fu, K.; Sun, Z.J.; Chen, X.M.; Zhao, Y.F. Expression of Allograft Inflammatory Factor-1 and CD68 in Haemangioma: Implication in the Progression of Haemangioma. Br. J. Dermatol. 2008, 159, 811–819. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, X.; Zheng, H.; Zhu, C.; Liu, Y. AIF-1, a Potential Biomarker of Aggressive Tumor Behavior in Patients with Non-Small Cell Lung Cancer. PLoS ONE 2022, 17, e0279211. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Fan, H.; Guo, J. Down-Regulation of AIF1 Inhibits Gallbladder Cancer Cell Proliferation, Invasion, and EMT by Regulating the TGF-Β1/P38 Pathway. Int. J. Clin. Exp. Med. 2019, 12, 9832–9838. [Google Scholar]
- Ye, Y.; Miao, S.; Lu, R.; Xia, X.; Chen, Y.; Zhang, J.; Wu, X.; He, S.; Qiang, F.; Zhou, J. Allograft Inflammatory Factor-1 Is an Independent Prognostic Indicator That Regulates β-Catenin in Gastric Cancer. Oncol. Rep. 2014, 31, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Shi, Y.; Ma, Y.; Pan, L.; Wang, Y.; Yuan, L.; Dong, J.; Ying, J. Chebulagic Acid Suppresses Gastric Cancer by Inhibiting the AURKA/β-Catenin/Wnt Pathway. Front. Pharmacol. 2023, 14, 1143427. [Google Scholar] [CrossRef]
- Lusis, A.J. Atherosclerosis. Nature 2000, 407, 233. [Google Scholar] [CrossRef]
- Henein, M.Y.; Vancheri, S.; Longo, G.; Vancheri, F. The Role of Inflammation in Cardiovascular Disease. Int. J. Mol. Sci. 2022, 23, 12906. [Google Scholar] [CrossRef]
- Herrington, W.; Lacey, B.; Sherliker, P.; Armitage, J.; Lewington, S. Epidemiology of Atherosclerosis and the Potential to Reduce the Global Burden of Atherothrombotic Disease. Circ. Res. 2016, 118, 535–546. [Google Scholar] [CrossRef]
- Moore, K.J.; Sheedy, F.J.; Fisher, E.A. Macrophages in Atherosclerosis: A Dynamic Balance. Nat. Rev. Immunol. 2013, 13, 709. [Google Scholar] [CrossRef]
- Farahi, L.; Sinha, S.K.; Lusis, A.J. Roles of Macrophages in Atherogenesis. Front. Pharmacol. 2021, 12, 3336. [Google Scholar] [CrossRef]
- Blagov, A.V.; Markin, A.M.; Bogatyreva, A.I.; Tolstik, T.V.; Sukhorukov, V.N.; Orekhov, A.N. The Role of Macrophages in the Pathogenesis of Atherosclerosis. Cells 2023, 12, 522. [Google Scholar] [CrossRef]
- Wilson, H.M. The Intracellular Signaling Pathways Governing Macrophage Activation and Function in Human Atherosclerosis. Biochem. Soc. Trans. 2022, 50, 1673–1682. [Google Scholar] [CrossRef] [PubMed]
- Lo Sasso, G.; Schlage, W.K.; Boué, S.; Veljkovic, E.; Peitsch, M.C.; Hoeng, J. The Apoe−/− Mouse Model: A Suitable Model to Study Cardiovascular and Respiratory Diseases in the Context of Cigarette Smoke Exposure and Harm Reduction. J. Transl. Med. 2016, 14, 146. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Suresh, M.V.; Voleti, B.; Agrawal, A. The Connection between C-Reactive Protein and Atherosclerosis. Ann. Med. 2008, 40, 110. [Google Scholar] [CrossRef] [PubMed]
- Egaña-Gorroño, L.; Chinnasamy, P.; Casimiro, I.; Almonte, V.M.; Parikh, D.; Oliveira-Paula, G.H.; Jayakumar, S.; Law, C.; Riascos-Bernal, D.F.; Sibinga, N.E.S. Allograft Inflammatory Factor-1 Supports Macrophage Survival and Efferocytosis and Limits Necrosis in Atherosclerotic Plaques. Atherosclerosis 2019, 289, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Sommerville, L.J.; Kelemen, S.E.; Ellison, S.P.; England, R.N.; Autieri, M.V. Increased Atherosclerosis and Vascular Smooth Muscle Cell Activation in AIF-1 Transgenic Mice Fed a High-Fat Diet. Atherosclerosis 2012, 220, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Albiero, M.; Rattazzi, M.; Menegazzo, L.; Boscaro, E.; Cappellari, R.; Pagnin, E.; Bertacco, E.; Poncina, N.; Dyar, K.; Ciciliot, S.; et al. Myeloid Calcifying Cells Promote Atherosclerotic Calcification via Paracrine Activity and Allograft Inflammatory Factor-1 Overexpression. Basic Res. Cardiol. 2013, 108, 368. [Google Scholar] [CrossRef] [PubMed]
- Ellulu, M.S.; Patimah, I.; Khaza’ai, H.; Rahmat, A.; Abed, Y. Obesity and Inflammation: The Linking Mechanism and the Complications. Arch. Med. Sci. 2017, 13, 851. [Google Scholar] [CrossRef]
- Cai, Z.; Huang, Y.; He, B. New Insights into Adipose Tissue Macrophages in Obesity and Insulin Resistance. Cells 2022, 11, 1424. [Google Scholar] [CrossRef]
- Fukui, M.; Tanaka, M.; Toda, H.; Asano, M.; Yamazaki, M.; Hasegawa, G.; Imai, S.; Fujinami, A.; Ohta, M.; Nakamura, N. The Serum Concentration of Allograft Inflammatory Factor-1 Is Correlated with Metabolic Parameters in Healthy Subjects. Metabolism 2012, 61, 1021–1025. [Google Scholar] [CrossRef]
- Rouskas, K.; Kouvatsi, A.; Paletas, K.; Papazoglou, D.; Tsapas, A.; Lobbens, S.; Vatin, V.; Durand, E.; Labrune, Y.; Delplanque, J.; et al. Common Variants in FTO, MC4R, TMEM18, PRL, AIF1, and PCSK1 Show Evidence of Association with Adult Obesity in the Greek Population. Obesity 2012, 20, 389–395. [Google Scholar] [CrossRef]
- Lorente-Cebrián, S.; Decaunes, P.; Dungner, E.; Bouloumié, A.; Arner, P.; Dahlman, I. Allograft Inflammatory Factor 1 (AIF-1) Is a New Human Adipokine Involved in Adipose Inflammation in Obese Women. BMC Endocr. Disord. 2013, 13, 54. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Lin, Y.; Tang, J.; Yue, H.; Zhao, Y. Allograft Inflammatory Factor-1 Mediates Macrophage-Induced Impairment of Insulin Signaling in Adipocytes. Cell Physiol. Biochem. 2018, 47, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Chinnasamy, P.; Casimiro, I.; Riascos-Bernal, D.F.; Venkatesh, S.; Parikh, D.; Maira, A.; Srinivasan, A.; Zheng, W.; Tarabra, E.; Zong, H.; et al. Increased Adipose Catecholamine Levels and Protection from Obesity with Loss of Allograft Inflammatory Factor-1. Nat. Commun. 2023, 14, 38. [Google Scholar] [CrossRef] [PubMed]
- Lier, J.; Winter, K.; Bleher, J.; Grammig, J.; Müller, W.; Streit, W.; Bechmann, I. Loss of IBA1-Expression in Brains from Individuals with Obesity and Hepatic Dysfunction. Brain Res. 2019, 1710, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhao, Y.; Jia, S.; Yan, D.; Chen, Z. Effects of Daintain/AIF-1 on Βcells Dysfunction in INS-1 Cells. Biosci. Biotechnol. Biochem. 2011, 75, 1842–1844. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.Y.; Huang, X.Y.; Chen, Z.W. Daintain/AIF-1 (Allograft Inflammatory Factor-1) Accelerates Type 1 Diabetes in NOD Mice. Biochem. Biophys. Res. Commun. 2012, 427, 513–517. [Google Scholar] [CrossRef]
- Elizondo, D.M.; Brandy, N.Z.D.; Da Silva, R.L.; De Moura, T.R.; Lipscomb, M.W. Allograft Inflammatory Factor-1 in Myeloid Cells Drives Autoimmunity in Type 1 Diabetes. JCI Insight 2020, 5, e136092. [Google Scholar] [CrossRef]
- Le, H.G.; Shakoor, A. Diabetic and Retinal Vascular Eye Disease. Med. Clin. N. Am. 2021, 105, 455–472. [Google Scholar] [CrossRef]
- Kinuthia, U.M.; Wolf, A.; Langmann, T. Microglia and Inflammatory Responses in Diabetic Retinopathy. Front. Immunol. 2020, 11, 564077. [Google Scholar] [CrossRef]
- Trotta, M.C.; Gesualdo, C.; Petrillo, F.; Cavasso, G.; Della Corte, A.; D’amico, G.; Hermenean, A.; Simonelli, F.; Rossi, S. Serum Iba-1, GLUT5, and TSPO in Patients With Diabetic Retinopathy: New Biomarkers for Early Retinal Neurovascular Alterations? A Pilot Study. Transl. Vis. Sci. Technol. 2022, 11, 16. [Google Scholar] [CrossRef]
- Gu, C.; Lhamo, T.; Zou, C.; Zhou, C.; Su, T.; Draga, D.; Luo, D.; Zheng, Z. Comprehensive Analysis of Angiogenesis- Related Genes and Pathways in Early Diabetic Retinopathy. BMC Med. Genom. 2020, 13, 142. [Google Scholar] [CrossRef]
- Li, Q.; Barres, B.A. Microglia and Macrophages in Brain Homeostasis and Disease. Nat. Rev. Immunol. 2017, 18, 225–242. [Google Scholar] [CrossRef] [PubMed]
- Kenkhuis, B.; Somarakis, A.; Kleindouwel, L.R.T.; van Roon-Mom, W.M.C.; Höllt, T.; van der Weerd, L. Co-Expression Patterns of Microglia Markers Iba1, TMEM119 and P2RY12 in Alzheimer’s Disease. Neurobiol. Dis. 2022, 167, 105684. [Google Scholar] [CrossRef] [PubMed]
- Sanfilippo, C.; Castrogiovanni, P.; Imbesi, R.; Di Rosa, M. CHI3L2 Expression Levels Are Correlated with AIF1, PECAM1, and CALB1 in the Brains of Alzheimer’s Disease Patients. J. Mol. Neurosci. 2020, 70, 1598–1610. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yang, Y.; Duan, H.; He, J.; Sun, L.; Hu, W.; Zeng, J. CHI3L2 Is a Novel Prognostic Biomarker and Correlated With Immune Infiltrates in Gliomas. Front. Oncol. 2021, 11, 611038. [Google Scholar] [CrossRef]
- Castrogiovanni, P.; Sanfilippo, C.; Imbesi, R.; Maugeri, G.; Furno, D.L.; Tibullo, D.; Castorina, A.; Musumeci, G.; Di Rosa, M. Brain CHID1 Expression Correlates with NRGN and CALB1 in Healthy Subjects and AD Patients. Cells 2021, 10, 882. [Google Scholar] [CrossRef]
- Castrogiovanni, P.; Barbagallo, I.; Imbesi, R.; Musumeci, G.; Sanfilippo, C.; Broggi, G.; Caltabiano, R.; Tibullo, D.; Giallongo, C.; Forte, S.; et al. Chitinase Domain Containing 1 Increase Is Associated with Low Survival Rate and M0 Macrophages Infiltrates in Colorectal Cancer Patients. Pathol.-Res. Pract. 2022, 237, 154038. [Google Scholar] [CrossRef]
- Kovaleva, O.V.; Rashidova, M.A.; Samoilova, D.V.; Podlesnaya, P.A.; Tabiev, R.M.; Mochalnikova, V.V.; Gratchev, A. CHID1 Is a Novel Prognostic Marker of Non-Small Cell Lung Cancer. Int. J. Mol. Sci. 2021, 22, 450. [Google Scholar] [CrossRef]
- Hopperton, K.E.; Mohammad, D.; Trépanier, M.O.; Giuliano, V.; Bazinet, R.P. Markers of Microglia in Post-Mortem Brain Samples from Patients with Alzheimer’s Disease: A Systematic Review. Mol. Psychiatry 2018, 23, 177–198. [Google Scholar] [CrossRef]
- Deininger, M.H.; Weinschenk, T.; Meyermann, R.; Schluesener, H.J. The Allograft Inflammatory Factor-1 in Creutzfeldt–Jakob Disease Brains. Neuropathol. Appl. Neurobiol. 2003, 29, 389–399. [Google Scholar] [CrossRef]
- Herden, C.; Schluesener, H.J.; Richt, J.A. Expression of Allograft Inflammatory Factor-1 and Haeme Oxygenase-1 in Brains of Rats Infected with the Neurotropic Borna Disease Virus. Neuropathol. Appl. Neurobiol. 2005, 31, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Schwab, J.M.; Frei, E.; Klusman, I.; Schnell, L.; Schwab, M.E.; Schluesener, H.J. AIF-1 Expression Defines a Proliferating and Alert Microglial r Macrophage Phenotype Following Spinal Cord Injury in Rats. J. Neuroimmunol. 2001, 119, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, Z.; Artelt, M.; Burnet, M.; Schluesener, H.J. Dexamethasone Attenuates Early Expression of Three Molecules Associated with Microglia/Macrophages Activation Following Rat Traumatic Brain Injury. Acta Neuropathol. 2007, 113, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Steck, A.J.; Kinter, J.; Renaud, S. Differential Gene Expression in Nerve Biopsies of Inflammatory Neuropathies. J. Peripher. Nerv. Syst. 2011, 16, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Shahrizaila, N.; Lehmann, H.C.; Kuwabara, S. Guillain-Barré Syndrome. Lancet 2021, 397, 1214–1228. [Google Scholar] [CrossRef]
- Pashenkov, M.; Efendic, S.; Zhu, J.; Zou, L.P.; Ostenson, C.G.; Mustafa, M. Augmented Expression of Daintain/Allograft Inflammatory Factor-1 Is Associated with Clinical Disease: Dynamics of Daintain/Allograft Inflammatory Factor-1 Expression in Spleen, Peripheral Nerves and Sera during Experimental Autoimmune Neuritis. Scand. J. Immunol. 2000, 52, 117–122. [Google Scholar] [CrossRef]
- Schluesener, H.J.; Seid, K.; Kretzschmar, J.; Meyermann, R. Allograft-Inflammatory Factor-1 in Rat Experimental Autoimmune Encephalomyelitis, Neuritis, and Uveitis: Expression by Activated Macrophages and Microglial Cells. Glia 1998, 24, 244–251. [Google Scholar] [CrossRef]
- Chinnasamy, P.; Lutz, S.E.; Riascos Bernal, D.F.; Jeganathan, V.; Casimiro, I.; Brosnan, C.F.; Sibinga, N.E. Loss of Allograft Inflammatory Factor-1 Ameliorates Experimental Autoimmune Encephalomyelitis by Limiting Encephalitogenic CD4 T-Cell Expansion. Mol. Med. 2015, 21, 233–241. [Google Scholar] [CrossRef]
- Utans, U.; Quist, W.C.; McManus, B.M.; Wilson, J.E.; Arceci, R.J.; Wallace, A.F.; Russell, M.E. Allograft Inflammatory Factory-1. A Cytokine-Responsive Macrophage Molecule Expressed in Transplanted Human Hearts. Transplantation 1996, 61, 1387–1392. [Google Scholar] [CrossRef]
- Autieri, M.V.; Kelemen, S.; Thomas, B.A.; Feller, E.D.; Goldman, B.I.; Eisen, H.J. Allograft Inflammatory Factor-1 Expression Correlates with Cardiac Rejection and Development of Cardiac Allograft Vasculopathy. Circulation 2002, 106, 2218–2223. [Google Scholar] [CrossRef]
- Pober, J.S.; Chih, S.; Kobashigawa, J.; Madsen, J.C.; Tellides, G. Cardiac Allograft Vasculopathy: Current Review and Future Research Directions. Cardiovasc. Res. 2021, 117, 2624–2638. [Google Scholar] [CrossRef] [PubMed]
- Grimm, P.C.; McKenna, R.; Nickerson, P.; Russell, M.E.; Gough, J.; Gospodarek, E.; Liu, B.; Jeffery, J.; Rush, D.N. Clinical Rejection Is Distinguished from Subclinical Rejection by Increased Infiltration by a Population of Activated Macrophages. J. Am. Soc. Nephrol. 1999, 10, 1582–1589. [Google Scholar] [CrossRef] [PubMed]
- Vu, D.; Tellez-Corrales, E.; Shah, T.; Hutchinson, I.; Min, D.I. Influence of Cyclooxygenase-2 (COX-2) Gene Promoter-1195 and Allograft Inflammatory Factor-1 (AIF-1) Polymorphisms on Allograft Outcome in Hispanic Kidney Transplant Recipients. Hum. Immunol. 2013, 74, 1386–1391. [Google Scholar] [CrossRef] [PubMed]
- Romanowski, M.; Kłoda, K.; Milczarek, S.; Pawlik, A.; Domański, L.; Safranow, K.; Ciechanowski, K. Influence of AIF1 Gene Polymorphisms on Long-Term Kidney Allograft Function. Ann. Transplant. 2015, 20, 506–511. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Leon-Oliva, D.; Garcia-Montero, C.; Fraile-Martinez, O.; Boaru, D.L.; García-Puente, L.; Rios-Parra, A.; Garrido-Gil, M.J.; Casanova-Martín, C.; García-Honduvilla, N.; Bujan, J.; et al. AIF1: Function and Connection with Inflammatory Diseases. Biology 2023, 12, 694. https://doi.org/10.3390/biology12050694
De Leon-Oliva D, Garcia-Montero C, Fraile-Martinez O, Boaru DL, García-Puente L, Rios-Parra A, Garrido-Gil MJ, Casanova-Martín C, García-Honduvilla N, Bujan J, et al. AIF1: Function and Connection with Inflammatory Diseases. Biology. 2023; 12(5):694. https://doi.org/10.3390/biology12050694
Chicago/Turabian StyleDe Leon-Oliva, Diego, Cielo Garcia-Montero, Oscar Fraile-Martinez, Diego Liviu Boaru, Luis García-Puente, Antonio Rios-Parra, Maria J. Garrido-Gil, Carlos Casanova-Martín, Natalio García-Honduvilla, Julia Bujan, and et al. 2023. "AIF1: Function and Connection with Inflammatory Diseases" Biology 12, no. 5: 694. https://doi.org/10.3390/biology12050694






