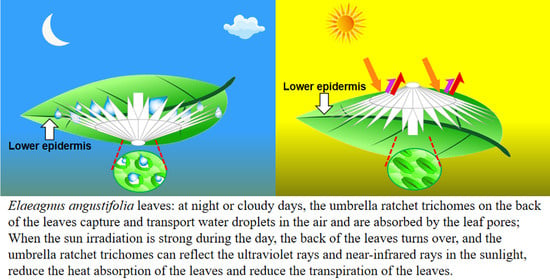
The Mechanism by Which Umbrella-Shaped Ratchet Trichomes on the Elaeagnus angustifolia Leaf Surface Collect Water and Reflect Light
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Characterization
2.2. Determination of Leaf Surface Wettability
2.3. Water-Collection Experiment
2.4. Photosynthesis Determination
2.5. Spectral Measurement and Statistical Analysis
3. Results
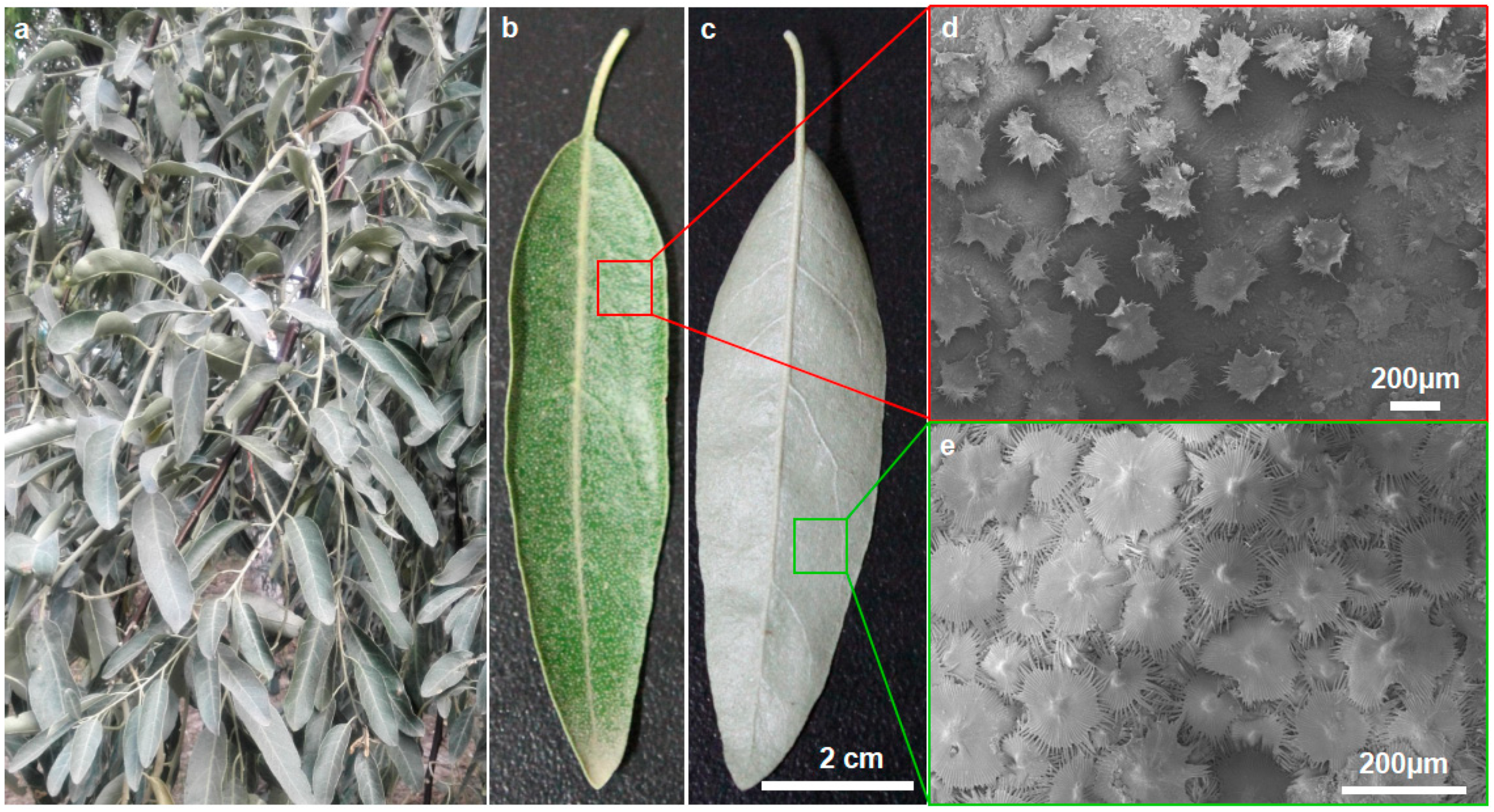
3.1. Leaf Surface Characteristics
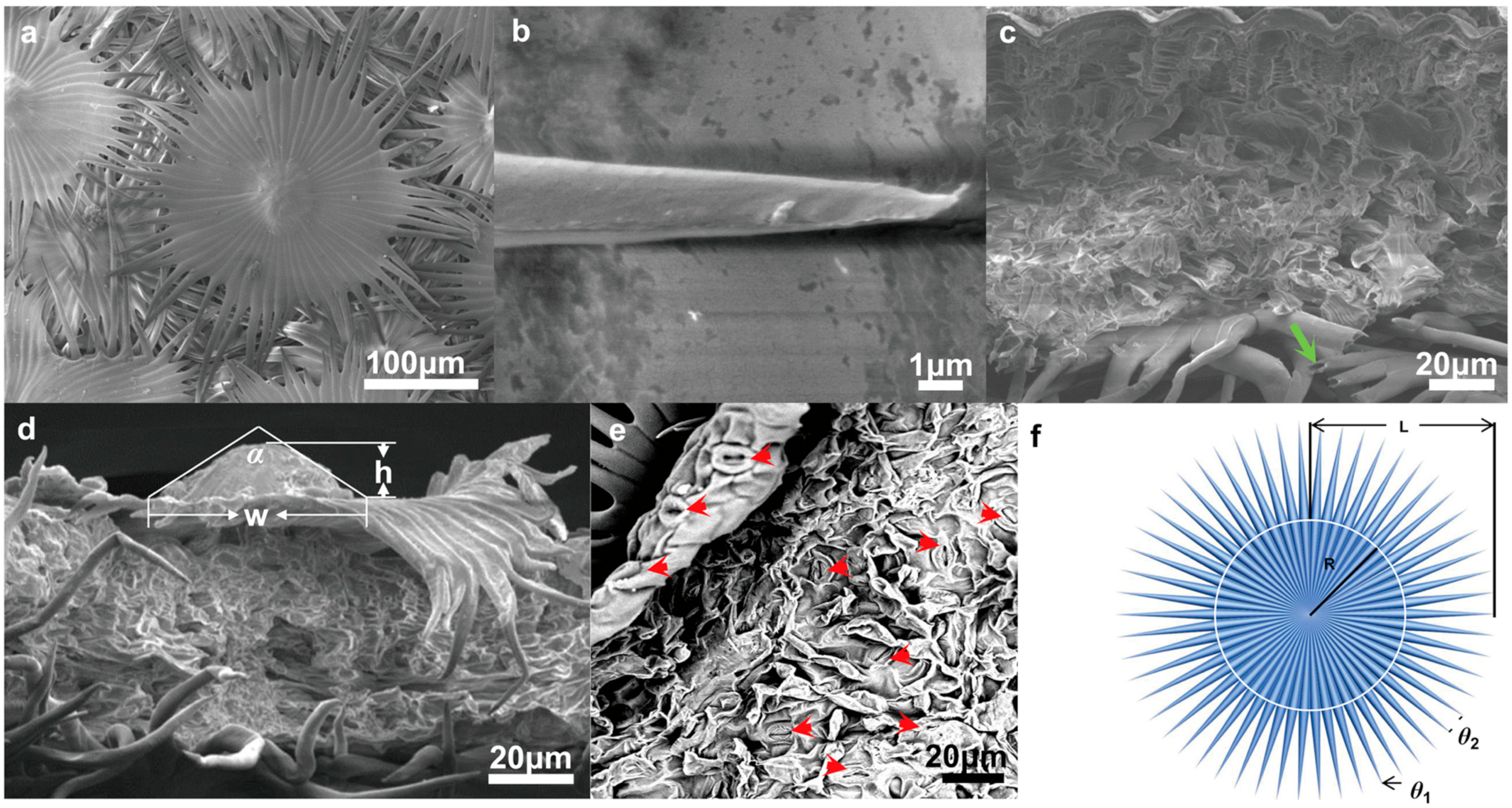
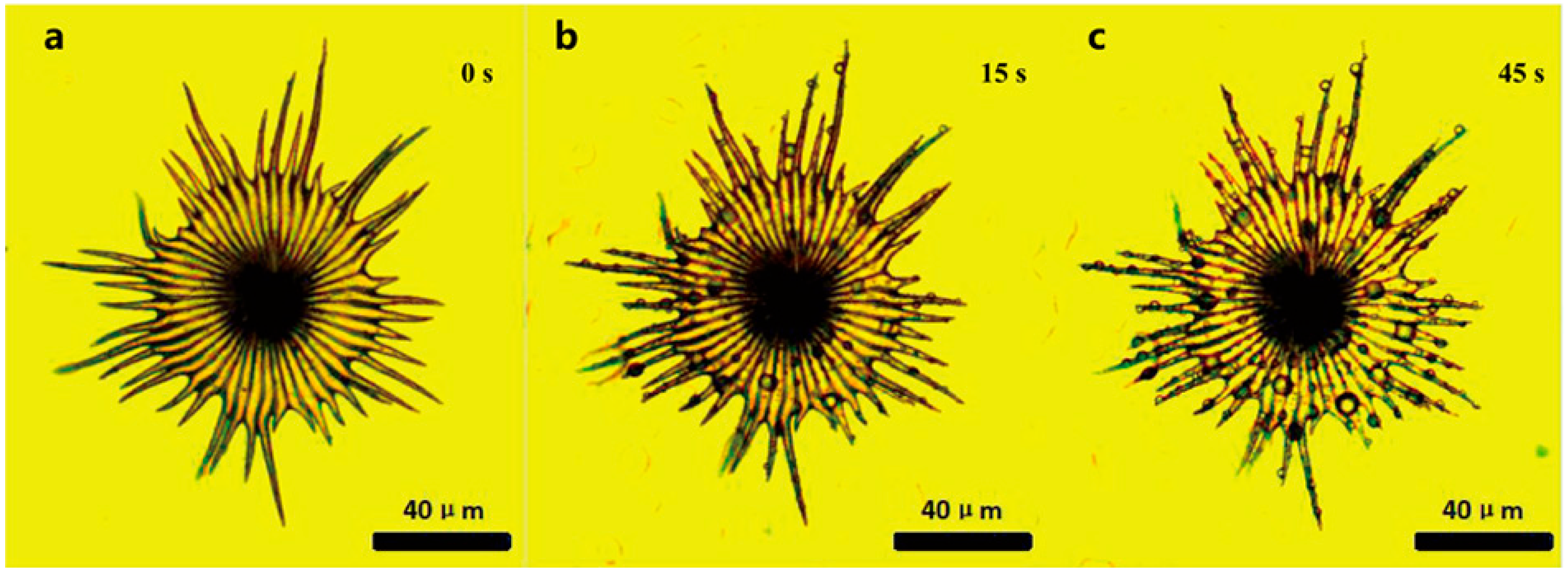
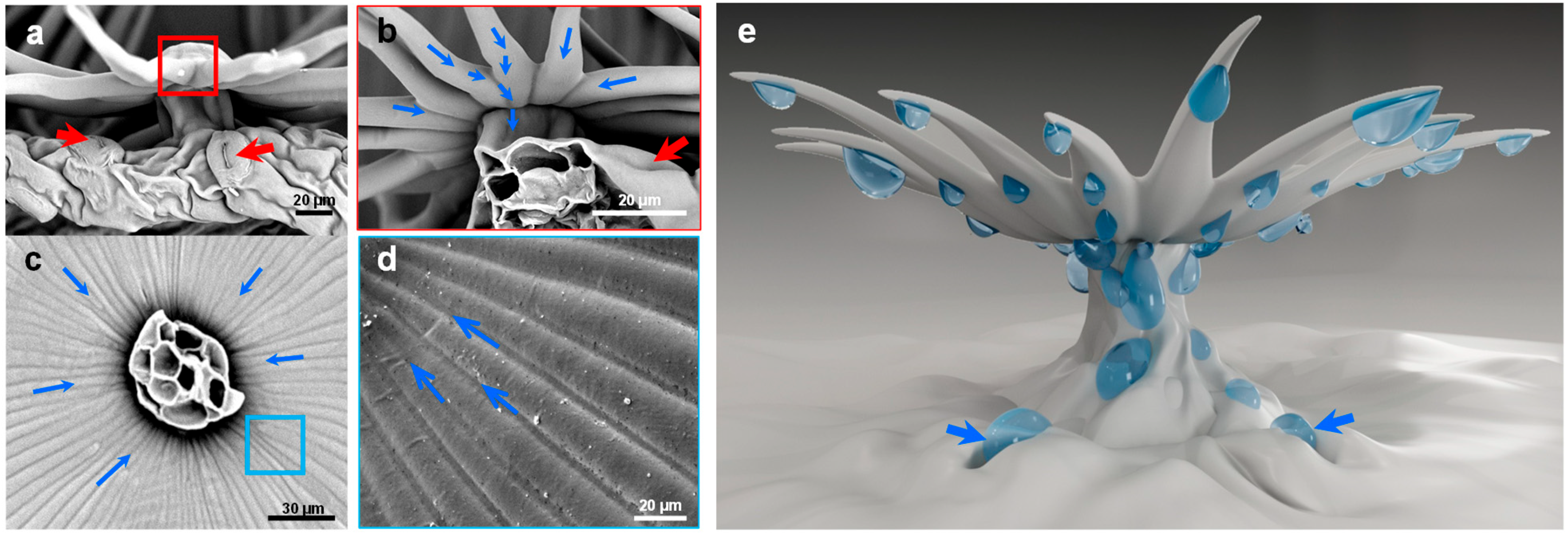
3.2. Structure and Characteristics of Trichomes
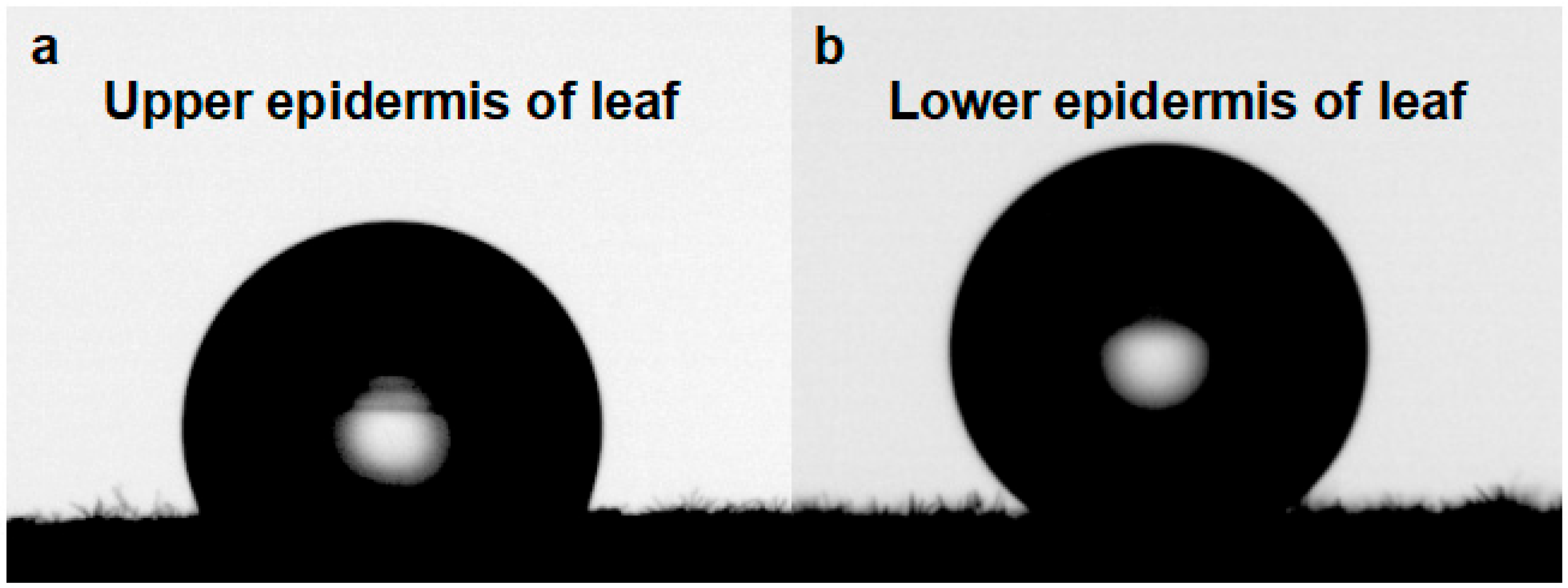
3.3. Leaf Wettability
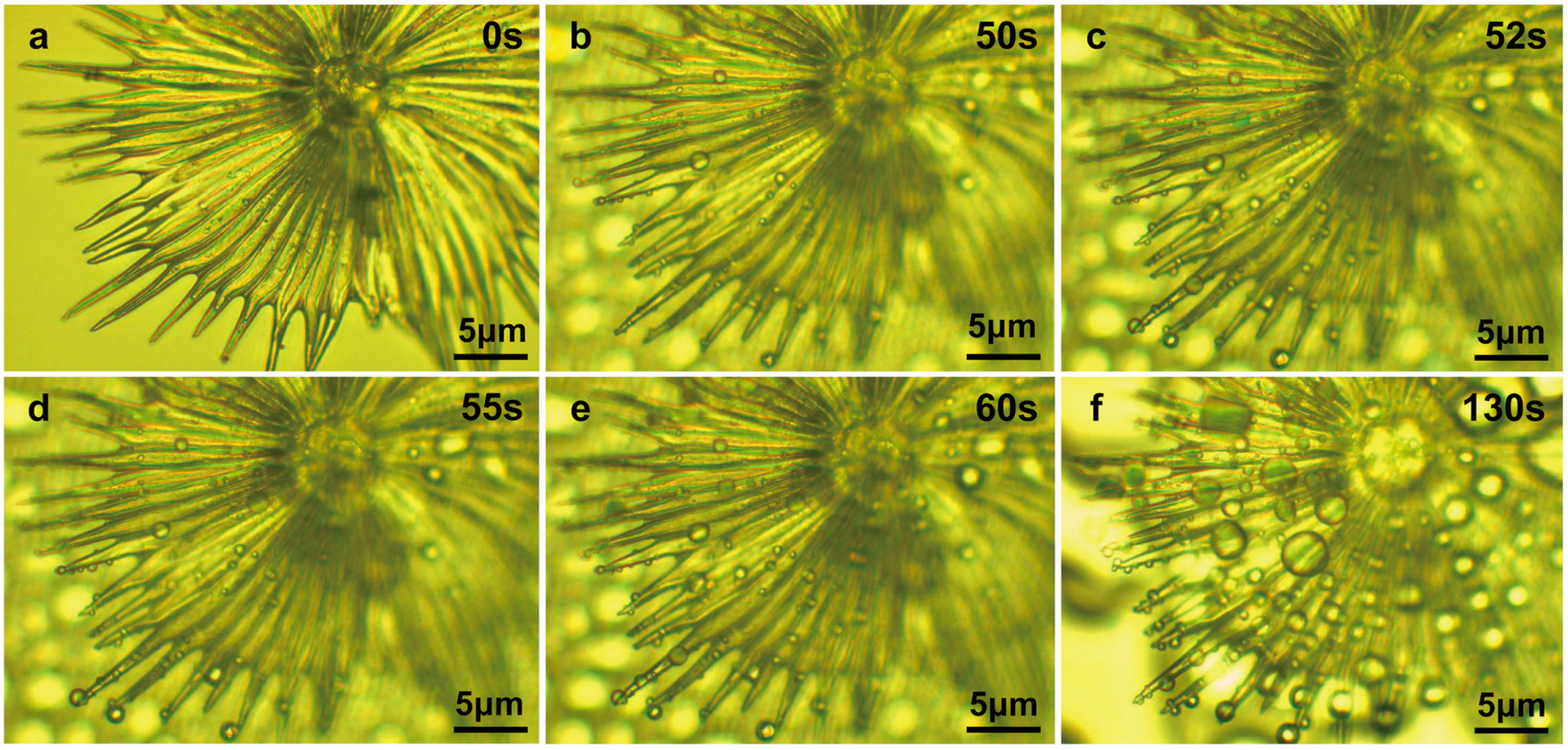
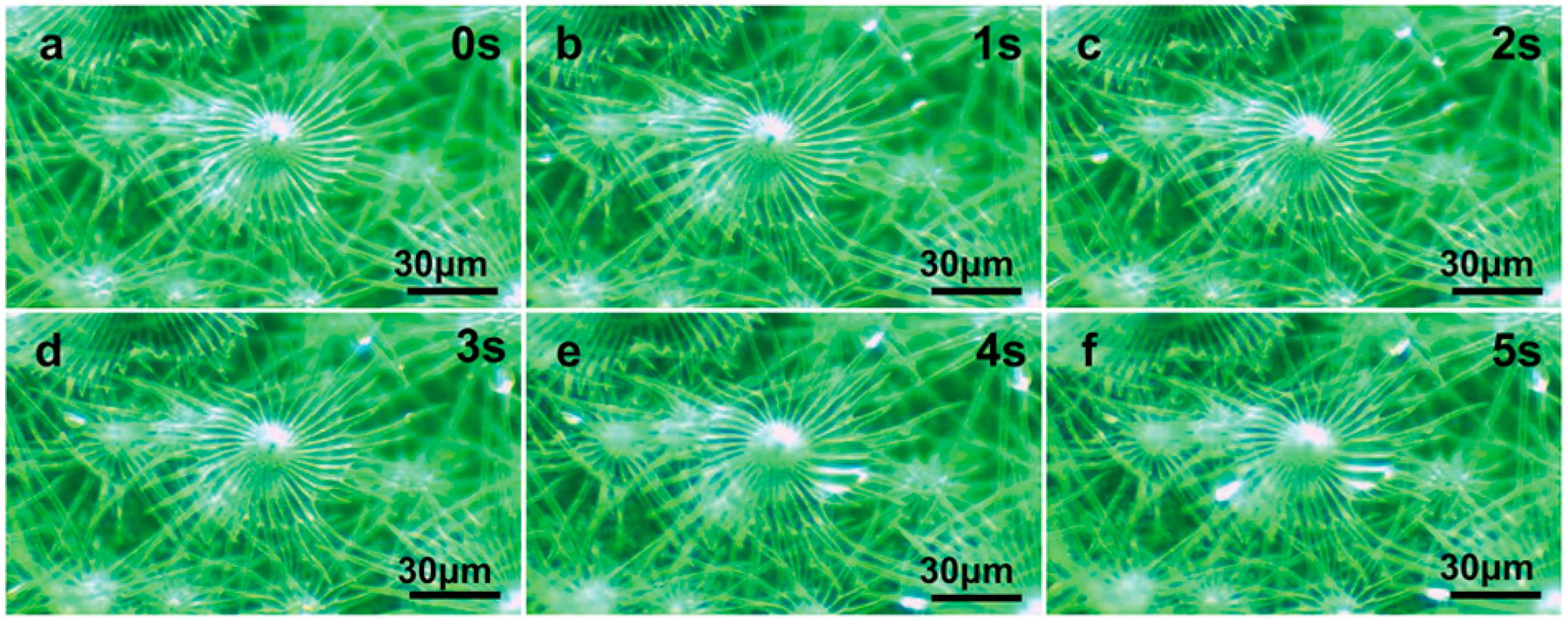
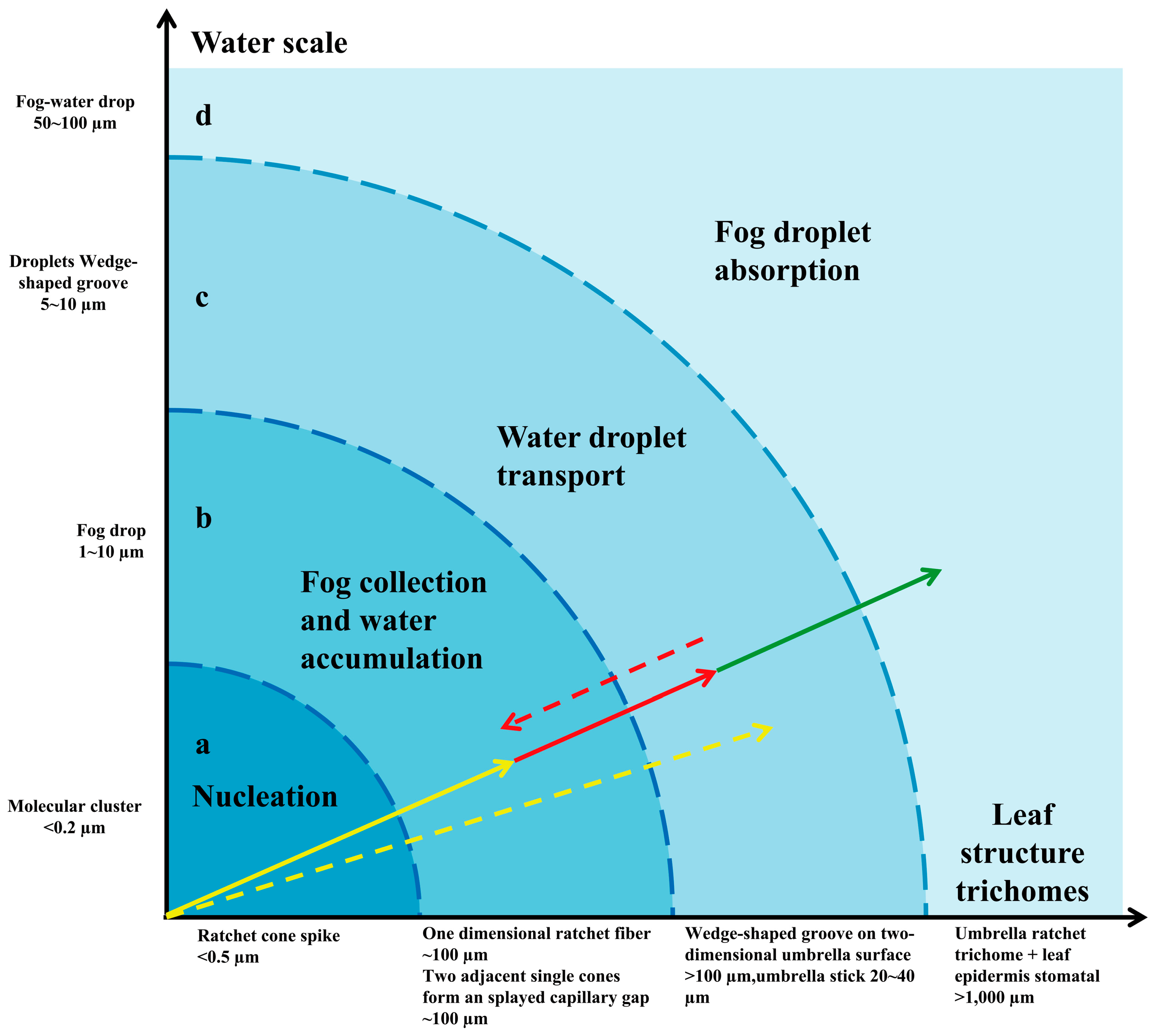
3.4. Multifunctional Hierarchical Water-Collection Mechanism of Trichomes
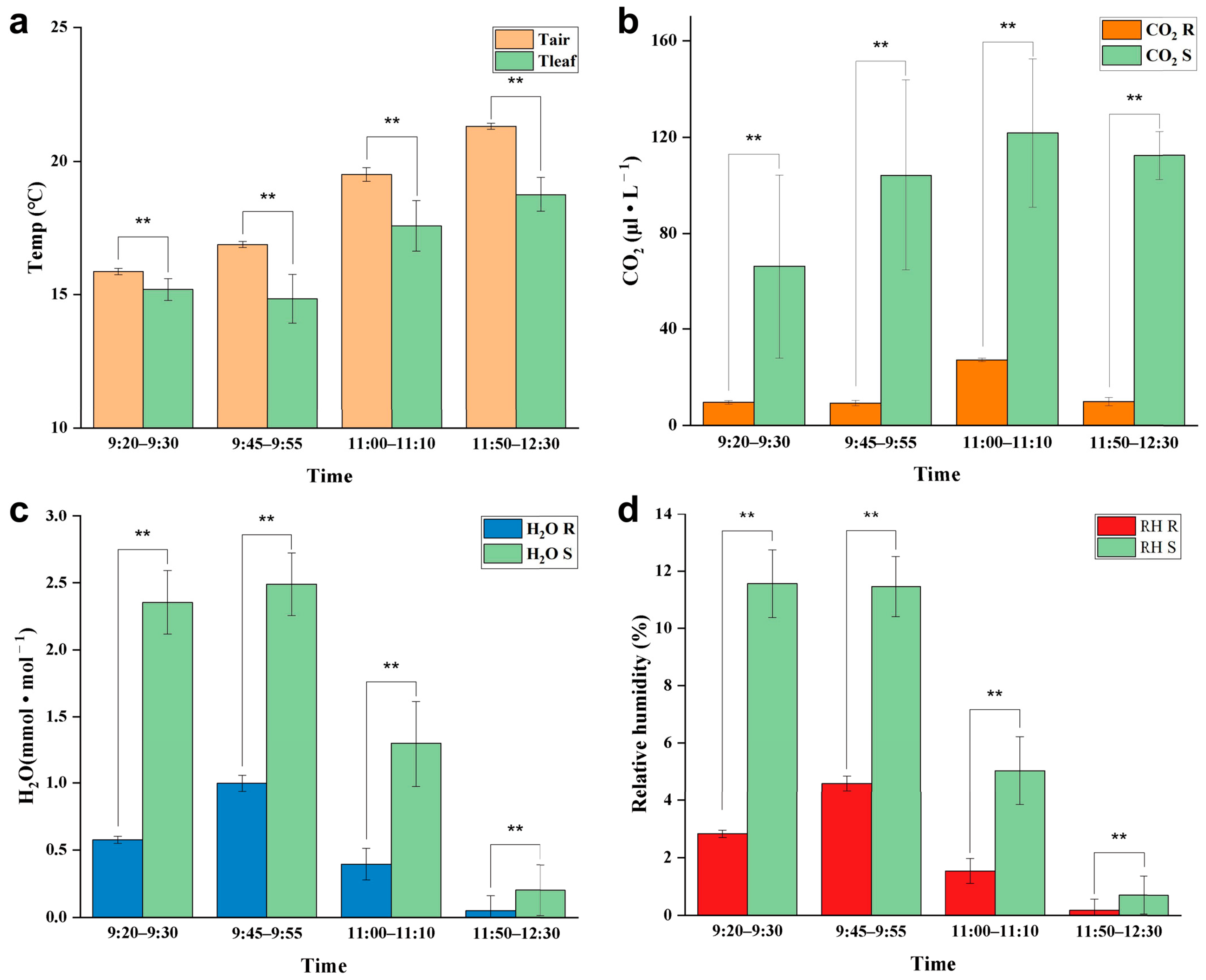
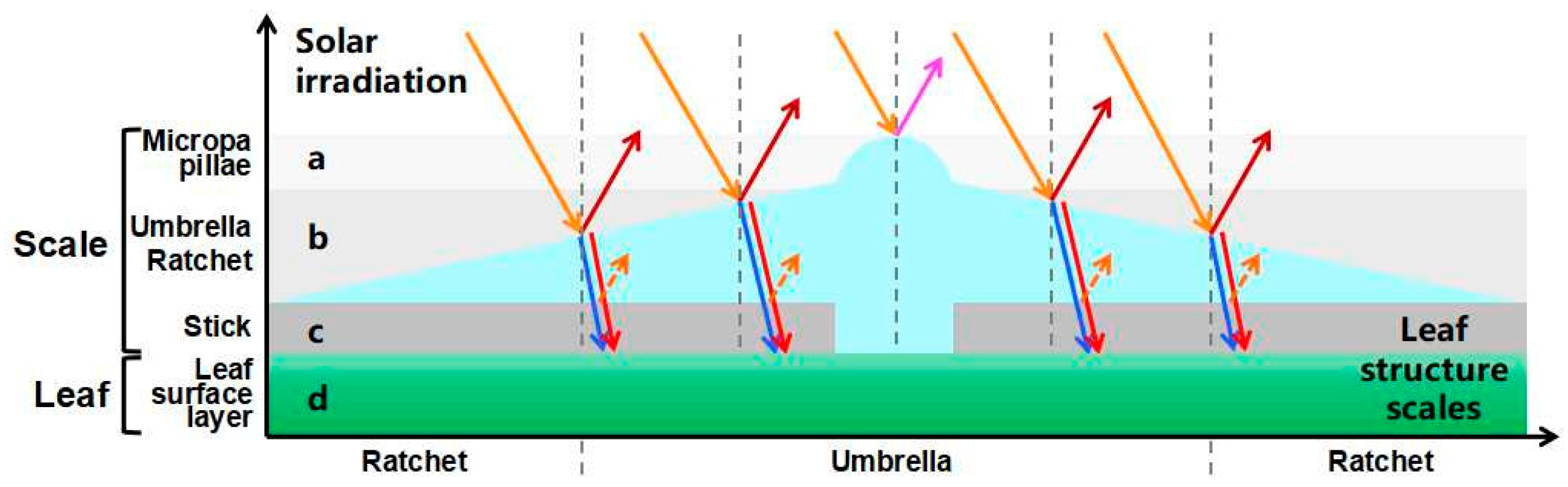
3.5. Response of Leaves to the Light
4. Discussion
4.1. Occult Precipitation Is an Important Source of Water in Arid Regions
4.2. Umbrella-Shaped Ratchet Trichomes on the E. angustifolia Leaf Surface Can Capture and Directionally Transport Water Droplets
4.3. Leaves Are the Main Plant Organ Responding to Solar Radiation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kasten, F.; Czeplak, G. Solar and terrestrial radiation dependent on the amount and type of cloud. Sol. Energy 1980, 24, 177–189. [Google Scholar] [CrossRef]
- Prăvălie, R. Drylands extent and environmental issues. A global approach. Earth-Sci. Rev. 2016, 161, 259–278. [Google Scholar] [CrossRef]
- Dregne, H.E. Land degradation in the drylands. Arid. Land Res. Manag. 2002, 16, 99–132. [Google Scholar] [CrossRef]
- Vicente-Serrano, S.M.; Gouveia, C.; Camarero, J.J.; Beguería, S.; Trigo, R.; López-Moreno, J.I.; Azorín-Molina, C.; Pasho, E.; Lorenzo-Lacruz, J.; Revuelto, J. Response of vegetation to drought time-scales across global land biomes. Proc. Natl. Acad. Sci. USA 2013, 110, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Breshears, D.D.; Cobb, N.S.; Rich, P.M.; Price, K.P.; Allen, C.D.; Balice, R.G.; Romme, W.H.; Kastens, J.H.; Floyd, M.L.; Belnap, J. Regional vegetation die-off in response to global-change-type drought. Proc. Natl. Acad. Sci. USA 2005, 102, 15144–15148. [Google Scholar] [CrossRef]
- Miriti, M.N.; Rodríguez-Buriticá, S.; Wright, S.J.; Howe, H.F. Episodic death across species of desert shrubs. Ecology 2007, 88, 32–36. [Google Scholar] [CrossRef]
- Pivovaroff, A.L.; Pasquini, S.C.; De Guzman, M.E.; Alstad, K.P.; Stemke, J.S.; Santiago, L.S. Multiple strategies for drought survival among woody plant species. Funct. Ecol. 2016, 30, 517–526. [Google Scholar] [CrossRef] [Green Version]
- Ju, J.; Bai, H.; Zheng, Y.; Zhao, T.; Fang, R.; Jiang, L. A multi-structural and multi-functional integrated fog collection system in cactus. Nat. Commun. 2012, 3, 1247. [Google Scholar] [CrossRef] [Green Version]
- Pan, Z.; Pitt, W.G.; Zhang, Y.; Wu, N.; Tao, Y.; Truscott, T.T. The upside-down water collection system of Syntrichia caninervis. Nat. Plants 2016, 2, 16076. [Google Scholar] [CrossRef]
- Ye, C.; Li, M.; Hu, J.; Cheng, Q.; Jiang, L.; Song, Y. Highly reflective superhydrophobic white coating inspired by poplar leaf hairs toward an effective “cool roof”. Energy Environ. Sci. 2011, 4, 3364–3367. [Google Scholar] [CrossRef]
- Weyl, P.; Asadi, G.A.; Cristofaro, M.; Vidovic, B.; Petanovic, R.; Marini, F.; Schaffner, U. The host range and impact of Aceria angustifoliae (Eriophyidae), a potential biological control agent against Russian olive, Elaeagnus angustifoliae (Elaeagnaceae) in North America. Biocontrol Sci. Technol. 2020, 30, 85–92. [Google Scholar] [CrossRef]
- Sun, M.; Lin, Q. A revision of Elaeagnus L. (Elaeagnaceae) in mainland China. J. Syst. Evol. 2010, 48, 356–390. [Google Scholar] [CrossRef]
- Bailey, L.H. The Standard Cyclopedia of Horticulture; Macmillan: London, UK, 1914; Volume 2. [Google Scholar]
- Collette, L.K.; Pither, J. Russian-olive (Elaeagnus angustifolia) biology and ecology and its potential to invade northern North American riparian ecosystems. Invasive Plant Sci. Manag. 2015, 8, 1–14. [Google Scholar] [CrossRef]
- Safdari, L.; Khadivi, A. Identification of the promising oleaster (Elaeagnus angustifolia L.) genotypes based on fruit quality-related characters. Food Sci. Nutr. 2021, 9, 5712–5721. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, G.; Du, S. Simulating the potential distribution of Elaeagnus angustifolia L. based on climatic constraints in China. Ecol. Eng. 2018, 113, 27–34. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, W.; Jin, B. The response of sap flow in desert shrubs to environmental variables in an arid region of China. Ecohydrology 2011, 4, 448–457. [Google Scholar] [CrossRef]
- Dong, S.; Wan, S.; Kang, Y.; Li, X. Establishing an ecological forest system of salt-tolerant plants in heavily saline wasteland using the drip-irrigation reclamation method. Agric. Water Manag. 2021, 245, 106587. [Google Scholar] [CrossRef]
- Sudnik, B.; Wójcikowska, I.M.; Slim, P.A.; Moraczewski, I.R. Impact of the Invasive Species Elaeagnus angustifolia L. on Vegetation in Pontic Desert Steppe Zone (Southern Ukraine). Pol. J. Ecol. 2009, 57, 269–281. [Google Scholar]
- Abramova, L.; Mustafina, A.; Golovanov, Y.; Zhigunov, O.Y.; Anishchenko, I.; Shigapov, Z.K. Features of the Biology and Ecology of Elaeagnus angustifolia L. in Southern Ural. Contemp. Probl. Ecol. 2021, 14, 446–455. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, J.; Yang, X.; Wu, H.; Wei, Q.; Wei, H.; Zhang, H. Growth performance, organ-level ionic relations and organic osmoregulation of Elaeagnus angustifolia in response to salt stress. PLoS ONE 2018, 13, e0191552. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Ruan, X.; Huang, J.; Xu, N.; Yan, Q. Intra-specific genetic relationship analyses of Elaeagnus angustifolia based on RP-HPLC biochemical markers. J. Zhejiang Univ. Sci. B 2006, 7, 272–278. [Google Scholar] [CrossRef] [Green Version]
- Weryszko-Chmielewska, E.; Chernetskyy, M. Structure of trichomes from the surface of leaves of some species of Kalanchoë Adans. Acta Biol. Cracoviensia Ser. Bot. 2005, 47, 15–22. [Google Scholar]
- Bacelar, E.A.; Correia, C.M.; Moutinho-Pereira, J.M.; Gonçalves, B.C.; Lopes, J.I.; Torres-Pereira, J.M. Sclerophylly and leaf anatomical traits of five field-grown olive cultivars growing under drought conditions. Tree Physiol. 2004, 24, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Klich, M.a.G. Leaf variations in Elaeagnus angustifolia related to environmental heterogeneity. Environ. Exp. Bot. 2000, 44, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Levizou, E.; Drilias, P.; Psaras, G.K.; Manetas, Y. Nondestructive assessment of leaf chemistry and physiology through spectral reflectance measurements may be misleading when changes in trichome density co-occur. New Phytol. 2005, 165, 463–472. [Google Scholar] [CrossRef]
- Andrews, H.; Eccles, E.; Schofield, W.; Badyal, J. Three-dimensional hierarchical structures for fog harvesting. Langmuir 2011, 27, 3798–3802. [Google Scholar] [CrossRef]
- Lawrence, E.H.; Stinziano, J.R.; Hanson, D.T. Using the Rapid A-Ci Response (RACiR) in the Li-Cor 6400 to Measure Developmental Gradients of Photosynthetic Capacity in Poplar; 0140-7791; Wiley Online Library: Hoboken, NJ, USA, 2019. [Google Scholar]
- Lee, A.; Moon, M.-W.; Lim, H.; Kim, W.-D.; Kim, H.-Y. Water harvest via dewing. Langmuir 2012, 28, 10183–10191. [Google Scholar] [CrossRef]
- Zangvil, A. Six years of dew observations in the Negev Desert, Israel. J. Arid. Environ. 1996, 32, 361–371. [Google Scholar] [CrossRef]
- Kidron, G.J. Dew moisture regime of endolithic and epilithic lichens inhabiting limestone cobbles and rock outcrops, Negev Highlands, Israel. Flora 2000, 195, 146–153. [Google Scholar] [CrossRef]
- Kidron, G.J.; Herrnstadt, I.; Barzilay, E. The role of dew as a moisture source for sand microbiotic crusts in the Negev Desert, Israel. J. Arid. Environ. 2002, 52, 517–533. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Downing, A.; Cheng, J.; Zhou, X.; Zhang, B. The influence of biological soil crusts on dew deposition in Gurbantunggut Desert, Northwestern China. J. Hydrol. 2009, 379, 220–228. [Google Scholar] [CrossRef]
- Moffett, M.W. An Indian ant’s novel method for obtaining water. Natl. Geogr. Res. 1985, 1, 146–149. [Google Scholar]
- Robert, N.W. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar]
- Cassie, A.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Bliznyuk, O.; Jansen, H.P.; Kooij, E.S.; Zandvliet, H.J.; Poelsema, B. Smart design of stripe-patterned gradient surfaces to control droplet motion. Langmuir 2011, 27, 11238–11245. [Google Scholar] [CrossRef]
- Lorenceau, E.; Quéré, D. Drops on a conical wire. J. Fluid Mech. 2004, 510, 29–45. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Han, Y. Shape-gradient composite surfaces: Water droplets move uphill. Langmuir 2007, 23, 6136–6141. [Google Scholar] [CrossRef]
- Yarin, A.L.; Liu, W.; Reneker, D.H. Motion of droplets along thin fibers with temperature gradient. J. Appl. Phys. 2002, 91, 4751–4760. [Google Scholar] [CrossRef]
- Mettu, S.; Chaudhury, M.K. Motion of drops on a surface induced by thermal gradient and vibration. Langmuir 2008, 24, 10833–10837. [Google Scholar] [CrossRef]
- Chaudhury, M.K.; Whitesides, G.M. How to make water run uphill. Science 1992, 256, 1539–1541. [Google Scholar] [CrossRef]
- Daniel, S.; Chaudhury, M.K.; Chen, J.C. Fast drop movements resulting from the phase change on a gradient surface. Science 2001, 291, 633–636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guzmán-Delgado, P.; Laca, E.; Zwieniecki, M.A. Unravelling foliar water uptake pathways: The contribution of stomata and the cuticle. Plant Cell Environ. 2021, 44, 1728–1740. [Google Scholar] [CrossRef]
- Emery, N.C. Foliar uptake of fog in coastal California shrub species. Oecologia 2016, 182, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; von Willert, D.J. Leaf epidermal hydathodes and the ecophysiological consequences of foliar water uptake in species of Crassula from the Namib Desert in southern Africa. Plant Biol. 2000, 2, 229–242. [Google Scholar] [CrossRef]
- Garland, J. Some fog droplet size distributions obtained by an impaction method. Q. J. R. Meteorol. Soc. 1971, 97, 483–494. [Google Scholar] [CrossRef]
- Rykaczewski, K.; Scott, J.; Fedorov, A.G. Electron beam heating effects during environmental scanning electron microscopy imaging of water condensation on superhydrophobic surfaces. Appl. Phys. Lett. 2011, 98, 093106. [Google Scholar] [CrossRef]
- Rykaczewski, K.; Scott, J.H.J.; Rajauria, S.; Chinn, J.; Chinn, A.M.; Jones, W. Three dimensional aspects of droplet coalescence during dropwise condensation on superhydrophobic surfaces. Soft Matter 2011, 7, 8749–8752. [Google Scholar] [CrossRef]
- Zheng, Y.; Bai, H.; Huang, Z.; Tian, X.; Nie, F.-Q.; Zhao, Y.; Zhai, J.; Jiang, L. Directional water collection on wetted spider silk. Nature 2010, 463, 640–643. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Wang, L.; Ju, J.; Sun, R.; Zheng, Y.; Jiang, L. Efficient water collection on integrative bioinspired surfaces with star-shaped wettability patterns. Adv. Mater. 2014, 26, 5025–5030. [Google Scholar] [CrossRef]
- Wang, X.; Zeng, J.; Li, J.; Yu, X.; Wang, Z.; Zhang, Y. Beetle and cactus-inspired surface endows continuous and directional droplet jumping for efficient water harvesting. J. Mater. Chem. A 2021, 9, 1507–1516. [Google Scholar] [CrossRef]
- Kempe, S. Carbon in the freshwater cycle. Glob. Carbon Cycle 1979, 13, 317–342. [Google Scholar]
- Brewer, C.; Smith, W.; Vogelmann, T. Functional interaction between leaf trichomes, leaf wettability and the optical properties of water droplets. Plant Cell Environ. 1991, 14, 955–962. [Google Scholar] [CrossRef]
- Roth-Nebelsick, A.; Ebner, M.; Miranda, T.; Gottschalk, V.; Voigt, D.; Gorb, S.; Stegmaier, T.; Sarsour, J.; Linke, M.; Konrad, W. Leaf surface structures enable the endemic Namib desert grass Stipagrostis sabulicola to irrigate itself with fog water. J. R. Soc. Interface 2012, 9, 1965–1974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zong, J.-Y.; Zhou, X.-J.; Hu, Y.-F.; Yang, T.-B.; Yan, D.-X.; Lin, H.; Lei, J.; Li, Z.-M. A wearable multifunctional fabric with excellent electromagnetic interference shielding and passive radiation heating performance. Compos. Part B Eng. 2021, 225, 109299. [Google Scholar] [CrossRef]
- Pakdel, E.; Xie, W.; Wang, J.; Kashi, S.; Sharp, J.; Zhang, Q.; Varley, R.J.; Sun, L.; Wang, X. Superhydrophobic natural melanin-coated cotton with excellent UV protection and personal thermal management functionality. Chem. Eng. J. 2022, 433, 133688. [Google Scholar] [CrossRef]
- Pakdel, E.; Naebe, M.; Sun, L.; Wang, X. Advanced functional fibrous materials for enhanced thermoregulating performance. ACS Appl. Mater. Interfaces 2019, 11, 13039–13057. [Google Scholar] [CrossRef]
- Bilger, H.-W.; Schreiber, U.; Lange, O. Determination of leaf heat resistance: Comparative investigation of chlorophyll fluorescence changes and tissue necrosis methods. Oecologia 1984, 63, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Henrion, W.; Tributsch, H. Optical solar energy adaptations and radiative temperature control of green leaves and tree barks. Sol. Energy Mater. Sol. Cells 2009, 93, 98–107. [Google Scholar] [CrossRef]
- Sandquist, D.R.; Ehleringer, J.R. Intraspecific variation of leaf pubescence and drought response in Encelia farinosa associated with contrasting desert environments. New Phytol. 1997, 135, 635–644. [Google Scholar] [CrossRef]
- Wang, H.; Liang, Y.; Cheng, S.; Li, B.; Li, A.; Du, G.; Hu, W. Bio-inspired nanostructures for enhanced light management. J. Vac. Sci. Technol. B 2017, 35, 06GJ02. [Google Scholar] [CrossRef]
- Zhu, L.; Raman, A.; Wang, K.X.; Abou Anoma, M.; Fan, S. Radiative cooling of solar cells. Optica 2014, 1, 32–38. [Google Scholar] [CrossRef]
- Karabourniotis, G.; Bornman, J.F. Penetration of UV-A, UV-B and blue light through the leaf trichome layers of two xeromorphic plants, olive and oak, measured by optical fibre microprobes. Physiol. Plant. 1999, 105, 655–661. [Google Scholar] [CrossRef]
- Hünig, R.; Mertens, A.; Stephan, M.; Schulz, A.; Richter, B.; Hetterich, M.; Powalla, M.; Lemmer, U.; Colsmann, A.; Gomard, G. Flower power: Exploiting plants’ epidermal structures for enhanced light harvesting in thin-film solar cells. Adv. Opt. Mater. 2016, 4, 1487–1493. [Google Scholar] [CrossRef]
- Schulte, A.J.; Mail, M.; Hahn, L.A.; Barthlott, W. Ultraviolet patterns of flowers revealed in polymer replica–caused by surface architecture. Beilstein J. Nanotechnol. 2019, 10, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Klomberg, Y.; Dywou Kouede, R.; Bartoš, M.; Mertens, J.E.; Tropek, R.; Fokam, E.B.; Janeček, Š. The role of ultraviolet reflectance and pattern in the pollination system of Hypoxis camerooniana (Hypoxidaceae). AoB Plants 2019, 11, plz057. [Google Scholar] [CrossRef] [Green Version]
- Richardson, A.D.; Aubrecht, D.M.; Basler, D.; Hufkens, K.; Muir, C.D.; Hanssen, L. Developmental changes in the reflectance spectra of temperate deciduous tree leaves and implications for thermal emissivity and leaf temperature. New Phytol. 2021, 229, 791–804. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bei, Z.; Zhang, X.; Tian, X. The Mechanism by Which Umbrella-Shaped Ratchet Trichomes on the Elaeagnus angustifolia Leaf Surface Collect Water and Reflect Light. Biology 2023, 12, 1024. https://doi.org/10.3390/biology12071024
Bei Z, Zhang X, Tian X. The Mechanism by Which Umbrella-Shaped Ratchet Trichomes on the Elaeagnus angustifolia Leaf Surface Collect Water and Reflect Light. Biology. 2023; 12(7):1024. https://doi.org/10.3390/biology12071024
Chicago/Turabian StyleBei, Zhanlin, Xin Zhang, and Xingjun Tian. 2023. "The Mechanism by Which Umbrella-Shaped Ratchet Trichomes on the Elaeagnus angustifolia Leaf Surface Collect Water and Reflect Light" Biology 12, no. 7: 1024. https://doi.org/10.3390/biology12071024