Modeling Dynamics of the Cardiovascular System Using Fluid-Structure Interaction Methods
Abstract
:Simple Summary
Abstract
1. Introduction
1.1. FSI Modeling Techniques
1.2. Applications in Cardiovascular Research
1.3. Challenges and Limitations
1.4. Clinical Implications
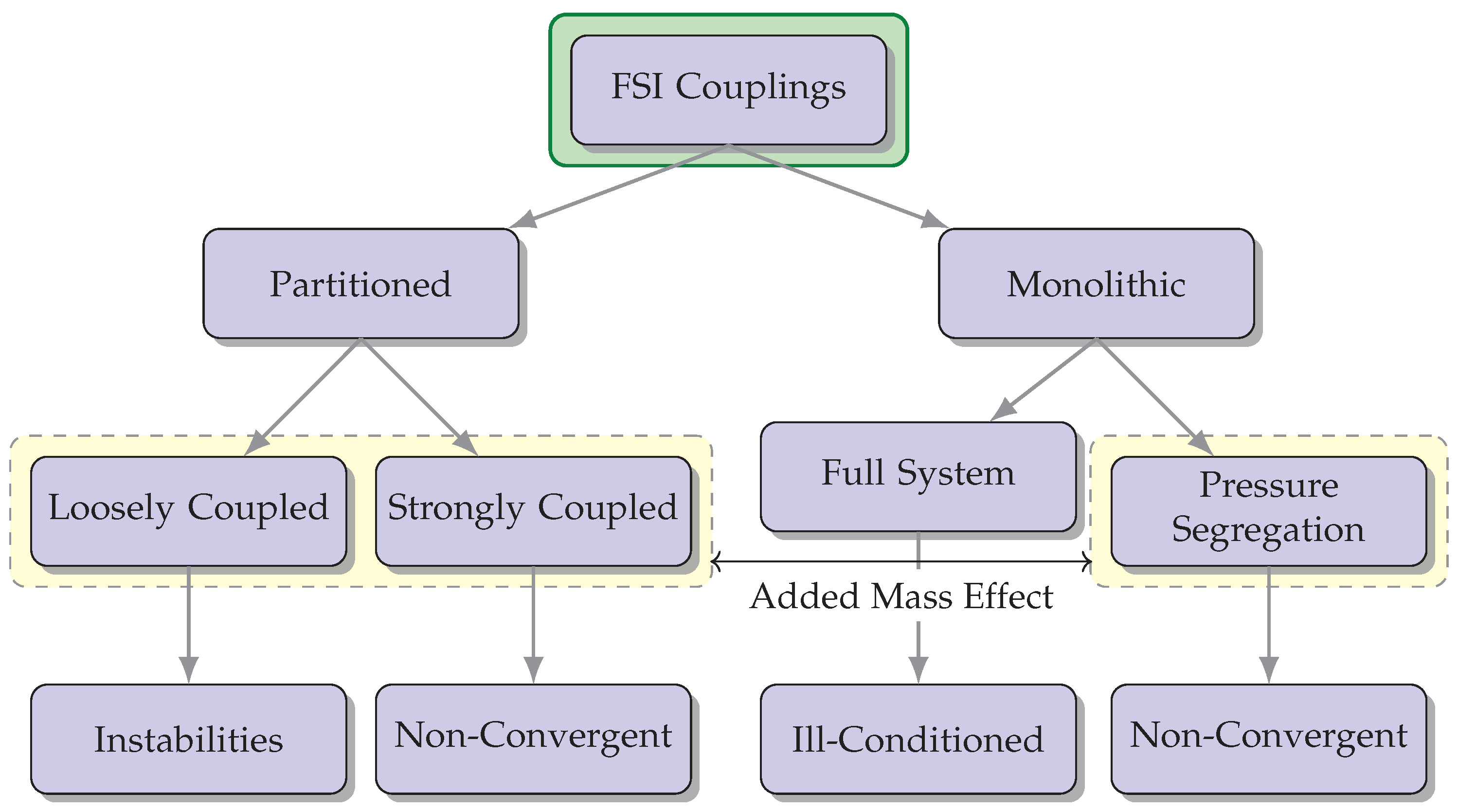
2. Fluid-Structure Interaction Techniques
- –
- Monolithic approach: In the monolithic approach, the governing equations for fluid flow and structural mechanics are solved simultaneously within a single computational framework [17]. This approach involves coupling the fluid and structural equations and solving them together, often using an iterative procedure. It provides accurate and robust predictions of the fluid-structure interaction but can be computationally demanding.
- –
- Partitioned approach: The partitioned approach separates the fluid and structural equations into two independent solvers, which are then coupled through an interface. Each solver operates independently, and the information is exchanged at the interface during the simulation. This approach allows for the use of existing specialized solvers for fluid and structural problems, making it computationally efficient [18].
- –
- One-way FSI: In one-way FSI, also known as loosely coupled FSI, the fluid and structural domains are treated as separate and independent simulations. The fluid flow is first simulated using CFD techniques, and the resulting fluid forces are applied as boundary conditions to the structural analysis. The structural deformation, in turn, influences the fluid flow indirectly through these imposed boundary conditions. The information exchange between the fluid and structural domains is performed in a one-way manner, with the fluid providing the driving forces for the structural response. This approach is computationally efficient but assumes that the structural deformation has a negligible impact on the fluid flow [19].
- –
- Two-way FSI: On the other hand, two-way FSI, also known as fully coupled FSI, considers the fluid and structure as a coupled system that interact with each other. In this approach, the fluid and structural equations are simultaneously solved in a fully coupled manner, accounting for the mutual influence between the two domains. The fluid forces affect the structural deformation, and the resulting deformation alters the fluid flow patterns. The information exchange occurs bidirectionally, allowing for a more accurate representation of the fluid-structure interaction. Two-way FSI is more computationally demanding but provides a more realistic representation of the physical phenomena involved [20].
2.1. Arbitrary Lagrangian–Eulerian Method
- –
- Partitioned approach: In the partitioned approach, the ALE method separates the fluid and structural equations into independent solvers. The fluid equations are solved using an Eulerian formulation, where the fluid mesh moves with the fluid motion. The structural equations are typically solved using a Lagrangian formulation, where the structural mesh remains fixed. The information is exchanged between the fluid and structural solvers at the fluid-structure interface, allowing for the interaction between the fluid and structure.
- –
- Monolithic approach: In the monolithic approach, the ALE method involves solving the fluid and structural equations simultaneously within a single computational framework. The fluid and structural equations are coupled and solved together in a fully coupled manner. This approach provides a more tightly coupled representation of the fluid-structure interaction, where the motion of the fluid mesh and the structural deformation are considered concurrently.
2.2. Immersed Boundary Method
2.3. Embedded or Embedded-Boundary Method
- –
- Partitioned approach: In the partitioned approach, the embedded or embedded-boundary method involves separating the fluid and structural equations into independent solvers. The fluid equations are typically solved using a traditional fluid solver, such as a finite volume or finite element method, where the fluid mesh remains fixed. The structural equations are solved separately using a structural solver. The information is exchanged at the fluid-structure interface to account for the interaction between fluid and structure.
- –
- Monolithic approach: In the monolithic approach, the embedded or embedded-boundary method solves the fluid and structural equations simultaneously within a single computational framework. The fluid and structural equations are coupled and solved together in a fully coupled manner. This approach provides a more tightly coupled representation of the fluid-structure interaction, where the fluid mesh and structural deformations are considered concurrently.
2.4. Meshless Methods
- –
- Partitioned approach: In the partitioned approach, the meshless method separates the fluid and structural equations into independent solvers. The fluid equations are typically solved using the meshless method, where the computational domain is discretized by a set of particles or scattered data points. The structural equations are solved separately using another meshless method or a different numerical technique suitable for structural analysis. The information is exchanged at the fluid-structure interface to account for the interaction between fluid and structure.
- –
- Monolithic approach: In the monolithic approach, the meshless method involves solving the fluid and structural equations simultaneously within a single computational framework. The fluid and structural equations are coupled and solved together in a fully coupled manner. This approach provides a more tightly coupled representation of the fluid-structure interaction, where the fluid particles and the structural particles or nodes interact directly and are solved concurrently.
2.5. Added Mass Effect
3. Blood Flow within Vascular Pathways
4. Blood Flow within the Heart
5. Blood Flow within Aneurysms
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| FSI | fluid-structure interaction |
| CT | computed tomography |
| MRI | magnetic resonance imaging |
| CFD | computational fluid dynamics |
| FEM | finite element method |
| FVM | finite volume method |
| IMB | immersed boundary method |
| ALE | arbitrary Lagrangian–Eulerian |
| SPH | smoothed particle hydrodynamics |
| CoA | coarctation of the aorta |
| E | elastic module |
| gPC | generalized polynomial Chaos |
| PWI | pulse wave imaging |
| PWV | pulse wave velocity |
| WSS | wall shear stress |
| LAD | left anterior descending |
| vFFR | vessel fractional flow reserve |
| TAH | total artificial heart |
| CAVS | calcified aortic valve stenosis |
| BMHV | bileaflet mechanical heart valve |
| PAS | platelet activation state |
| BAVR | bioprosthetic aortic valve replacement |
| GOA | geometric orifice area |
| TPG | transvalvular pressure gradient |
| TAAD | type A aortic dissection |
| PRR | particle residual rate |
| BRR | blood renewal rate |
| LAA | left atrial appendage |
| NVAF | non-valvular atrial fibrillation |
| TAA | thoracic aortic aneurysm |
| PIV | particle image velocimetry |
| ECAP | Endothelial cell action potential |
| PTFE | polytetrafluoroethylene |
| AI | artificial intelligence |
References
- Kamada, H.; Nakamura, M.; Ota, H.; Higuchi, S.; Takase, K. Blood flow analysis with computational fluid dynamics and 4D-flow MRI for vascular diseases. J. Cardiol. 2022, 80, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Haggerty, C.M.; Restrepo, M.; Tang, E.; de Zélicourt, D.A.; Sundareswaran, K.S.; Mirabella, L.; Bethel, J.; Whitehead, K.K.; Fogel, M.A.; Yoganathan, A.P. Fontan hemodynamics from 100 patient-specific cardiac magnetic resonance studies: A computational fluid dynamics analysis. J. Thorac. Cardiovasc. Surg. 2014, 148, 1481–1489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, E.; Wei, Z.A.; Fogel, M.A.; Veneziani, A.; Yoganathan, A.P. Fluid-Structure Interaction Simulation of an Intra-Atrial Fontan Connection. Biology 2020, 9, 412. [Google Scholar] [CrossRef] [PubMed]
- Pons, R.; Guala, A.; Rodríguez-Palomares, J.F.; Cajas, J.C.; Dux-Santoy, L.; Teixidó-Tura, G.; Molins, J.J.; Vázquez, M.; Evangelista, A.; Martorell, J. Fluid-structure interaction simulations outperform computational fluid dynamics in the description of thoracic aorta haemodynamics and in the differentiation of progressive dilation in Marfan syndrome patients. R. Soc. Open Sci. 2020, 7, 191752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.; Mirsadraee, S.; Rosendahl, U.; Pepper, J.; Xu, X.Y. Fluid-Structure Interaction Simulations of Repaired Type A Aortic Dissection: A Comprehensive Comparison with Rigid Wall Models. Front. Physiol. 2022, 13, 913457. [Google Scholar] [CrossRef]
- Martinolli, M.; Biasetti, J.; Zonca, S.; Polverelli, L.; Vergara, C. Extended finite element method for fluid-structure interaction in wave membrane blood pump. Int. J. Numer. Methods Biomed. Eng. 2021, 37, e3467. [Google Scholar] [CrossRef]
- Ames, J.; Puleri, D.F.; Balogh, P.; Gounley, J.; Draeger, E.W.; Randles, A. Multi-GPU immersed boundary method hemodynamics simulations. J. Comput. Sci. 2020, 44, 101153. [Google Scholar] [CrossRef]
- Djukic, T.; Topalovic, M.; Filipovic, N. Validation of lattice Boltzmann based software for blood flow simulations in complex patient-specific arteries against traditional CFD methods. Math. Comput. Simul. 2023, 203, 957–976. [Google Scholar] [CrossRef]
- Laudato, M.; Mosca, R.; Mihaescu, M. Buckling critical pressures in collapsible tubes relevant for biomedical flows. Sci. Rep. 2023, 13, 9298. [Google Scholar] [CrossRef]
- Sequí-Domínguez, I.; Cavero-Redondo, I.; Álvarez-Bueno, C.; Pozuelo-Carrascosa, D.P.; de Arenas-Arroyo, S.N.; Martínez-Vizcaíno, V. Accuracy of Pulse Wave Velocity Predicting Cardiovascular and All-Cause Mortality. A Systematic Review and Meta-Analysis. J. Clin. Med. 2020, 9, 2080. [Google Scholar] [CrossRef]
- Rousseau-Portalis, M.; Cymberknop, L.; Farro, I.; Armentano, R. Computational clustering reveals differentiated coronary artery calcium progression at prevalent levels of pulse wave velocity by classifying high-risk patients. Front. Cardiovasc. Med. 2023, 10, 1161914. [Google Scholar] [CrossRef]
- Bäumler, K.; Vedula, V.; Sailer, A.M.; Seo, J.; Chiu, P.; Mistelbauer, G.; Chan, F.P.; Fischbein, M.P.; Marsden, A.L.; Fleischmann, D. Fluid-structure interaction simulations of patient-specific aortic dissection. Biomech. Model. Mechanobiol. 2020, 19, 1607–1628. [Google Scholar] [CrossRef]
- Einstein, D.R.; Pin, F.D.; Jiao, X.; Kuprat, A.P.; Carson, J.P.; Kunzelman, K.S.; Cochran, R.P.; Guccione, J.M.; Ratcliffe, M.B. Fluid-structure interactions of the mitral valve and left heart: Comprehensive strategies, past, present and future. Int. J. Numer. Methods Biomed. Eng. 2010, 26, 348–380. [Google Scholar] [CrossRef] [Green Version]
- Jayendiran, R.; Nour, B.; Ruimi, A. Fluid-structure interaction (FSI) analysis of stent-graft for aortic endovascular aneurysm repair (EVAR): Material and structural considerations. J. Mech. Behav. Biomed. Mater. 2018, 87, 95–110. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Griffith, B.E. On the Lagrangian-Eulerian coupling in the immersed finite element/difference method. J. Comput. Phys. 2022, 457, 111042. [Google Scholar] [CrossRef] [PubMed]
- Bazilevs, Y.; Takizawa, K.; Tezduyar, T.E. Computational Fluid-Structure Interaction: Methods and Applications; J. Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Cetin, A.; Sahin, M. A monolithic fluid-structure interaction framework applied to red blood cells. Int. J. Numer. Methods Biomed. Eng. 2018, 35, e3171. [Google Scholar] [CrossRef]
- Wong, K.K.L.; Thavornpattanapong, P.; Cheung, S.C.P.; Tu, J. Numerical Stability of Partitioned Approach in Fluid-Structure Interaction for a Deformable Thin-Walled Vessel. Comput. Math. Methods Med. 2013, 2013, 638519. [Google Scholar] [CrossRef] [Green Version]
- Kuchumov, A.G.; Vedeneev, V.; Samartsev, V.; Khairulin, A.; Ivanov, O. Patient-specific fluid-structure interaction model of bile flow: Comparison between 1-way and 2-way algorithms. Comput. Methods Biomech. Biomed. Eng. 2021, 24, 1693–1717. [Google Scholar] [CrossRef] [PubMed]
- Athani, A.; Ghazali, N.N.N.; Badruddin, I.A.; Kamangar, S.; Anqi, A.E.; Algahtani, A. Investigation of two-way fluid-structure interaction of blood flow in a patient-specific left coronary artery. Bio-Med. Mater. Eng. 2022, 33, 13–30. [Google Scholar] [CrossRef]
- Wang, C.; Tang, H.; Zhang, X. Fluid-structure interaction of bio-inspired flexible slender structures: A review of selected topics. Bioinspir. Biomim. 2022, 17, 041002. [Google Scholar] [CrossRef]
- Souli, M.; Benson, D.J. Arbitrary Lagrangian-Eulerian and Fluid-Structure Interaction: Numerical Simulation; ISTE: Arlington, VA, USA, 2010. [Google Scholar]
- Toma, M.; Chan-Akeley, R.; Arias, J.; Kurgansky, G.D.; Mao, W. Fluid-Structure Interaction Analyses of Biological Systems Using Smoothed-Particle Hydrodynamics. Biology 2021, 10, 185. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Choi, H. Immersed boundary methods for fluid-structure interaction: A review. Int. J. Heat Fluid Flow 2019, 75, 301–309. [Google Scholar] [CrossRef]
- Griffith, B.E.; Patankar, N.A. Immersed Methods for Fluid-Structure Interaction. Annu. Rev. Fluid Mech. 2020, 52, 421–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaiser, A.D.; Schiavone, N.K.; Elkins, C.J.; McElhinney, D.B.; Eaton, J.K.; Marsden, A.L. Validation of Immersed Boundary Simulations of Heart Valve Hemodynamics against In Vitro 4D Flow MRI Data. arXiv 2021. [Google Scholar] [CrossRef]
- Ho, J.; Farhat, C. Discrete embedded boundary method with smooth dependence on the evolution of a fluid-structure interface. Int. J. Numer. Methods Eng. 2020, 122, 5353–5383. [Google Scholar] [CrossRef]
- Mazhar, F.; Javed, A.; Xing, J.T.; Shahzad, A.; Mansoor, M.; Maqsood, A.; Shah, S.I.A.; Asim, K. On the meshfree particle methods for fluid-structure interaction problems. Eng. Anal. Bound. Elem. 2021, 124, 14–40. [Google Scholar] [CrossRef]
- Sun, P.N.; Touzé, D.L.; Oger, G.; Zhang, A.M. An accurate FSI-SPH modeling of challenging fluid-structure interaction problems in two and three dimensions. Ocean. Eng. 2021, 221, 108552. [Google Scholar] [CrossRef]
- Lind, S.J.; Rogers, B.D.; Stansby, P.K. Review of smoothed particle hydrodynamics: Towards converged Lagrangian flow modelling. Proc. R. Soc. A 2020, 476, 20190801. [Google Scholar] [CrossRef]
- Souto-Iglesias, A.; Avalos, J.B.; Antuono, M.; Colagrossi, A. General isotropic micropolar fluid model in smoothed particle hydrodynamics. Phys. Rev. E 2021, 104, 015315. [Google Scholar] [CrossRef]
- Kashfi, M.; Fakhri, P.; Ghavamian, A.; Pourrabia, P.; Ghalesefid, F.S.; Kahhal, P. Crack growth pattern analysis of monolithic glass ceramic on a titanium abutment for single crown implant restorations using smooth particle hydrodynamics algorithm. J. Adv. Periodontol. Implant. Dent. 2021, 13, 7–11. [Google Scholar] [CrossRef]
- Avalos, J.B.; Antuono, M.; Colagrossi, A.; Souto-Iglesias, A. Shear-viscosity-independent bulk-viscosity term in smoothed particle hydrodynamics. Phys. Rev. E 2020, 101, 013302. [Google Scholar] [CrossRef]
- Jacob, B.; Drawert, B.; Yi, T.M.; Petzold, L. An arbitrary Lagrangian Eulerian smoothed particle hydrodynamics (ALE-SPH) method with a boundary volume fraction formulation for fluid-structure interaction. Eng. Anal. Bound. Elem. 2021, 128, 274–289. [Google Scholar] [CrossRef] [PubMed]
- Toma, M. The Emerging Use of SPH In Biomedical Applications. In Significances of Bioengineering Biosciences; Crimson: New York, NY, USA, 2017; Volume 1. [Google Scholar] [CrossRef]
- Ahmadzadeh, H.; Rausch, M.K.; Humphrey, J.D. Particle-based computational modelling of arterial disease. J. R. Soc. Interface 2018, 15, 20180616. [Google Scholar] [CrossRef] [Green Version]
- Fernandez, J.W.; Das, R.; Cleary, P.W.; Hunter, P.J.; Thomas, C.D.L.; Clement, J.G. Using smooth particle hydrodynamics to investigate femoral cortical bone remodelling at the Haversian level. Int. J. Numer. Methods Biomed. Eng. 2012, 29, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.J.; Benharash, P.; Dutson, E.P.; Eldredge, J.D. Smoothed particle hydrodynamics simulation of biphasic soft tissue and its medical applications. Med. Biol. Eng. Comput. 2021, 59, 227–242. [Google Scholar] [CrossRef]
- Caballero, A.; Mao, W.; Liang, L.; Oshinski, J.; Primiano, C.; McKay, R.; Kodali, S.; Sun, W. Modeling Left Ventricular Blood Flow Using Smoothed Particle Hydrodynamics. Cardiovasc. Eng. Technol. 2017, 8, 465–479. [Google Scholar] [CrossRef]
- Huang, W.X.; Tian, F.B. Recent trends and progress in the immersed boundary method. Proc. Inst. Mech. Eng. Part C J. Mech. Eng. Sci. 2019, 233, 7617–7636. [Google Scholar] [CrossRef]
- Toma, M.; Krdey, A.; Takagi, S.; Oshima, M. Strongly coupled fluid-structure interaction cardiovascular analysis with the effect of peripheral network. Seisan Kenkyu 2011, 63, 339–344. [Google Scholar] [CrossRef]
- Toma, M.; Oshima, M.; Takagi, S. Decomposition and parallelization of strongly coupled fluid-structure interaction linear subsystems based on the Q1/P0 discretization. Comput. Struct. 2016, 173, 84–94. [Google Scholar] [CrossRef]
- Zhao, S.Z.; Xu, X.Y.; Collins, M.W. The numerical analysis of fluid-solid interactions for blood flow in arterial structures Part 1: A review of models for arterial wall behaviour. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 1998, 212, 229–240. [Google Scholar] [CrossRef]
- Zhao, S.Z.; Xu, X.Y.; Collins, M.W. The numerical analysis of fluid-solid interactions for blood flow in arterial structures Part 2: Development of coupled fluid-solid algorithms. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 1998, 212, 241–252. [Google Scholar] [CrossRef]
- Sturla, F.; Votta, E.; Stevanella, M.; Conti, C.A.; Redaelli, A. Impact of modeling fluid-structure interaction in the computational analysis of aortic root biomechanics. Med. Eng. Phys. 2013, 35, 1721–1730. [Google Scholar] [CrossRef]
- Toma, M.; Lu, Y.; Zhou, H.; Garcia, J.D. Thresholding Segmentation Errors and Uncertainty with Patient-Specific Geometries. J. Biomed. Phys. Eng. 2021, 11, 115. [Google Scholar] [CrossRef]
- Bloodworth, C.H.; Pierce, E.L.; Easley, T.F.; Drach, A.; Khalighi, A.H.; Toma, M.; Jensen, M.O.; Sacks, M.S.; Yoganathan, A.P. Ex Vivo Methods for Informing Computational Models of the Mitral Valve. Ann. Biomed. Eng. 2016, 45, 496–507. [Google Scholar] [CrossRef] [Green Version]
- Toma, M.; Bloodworth, C.H.; Einstein, D.R.; Pierce, E.L.; Cochran, R.P.; Yoganathan, A.P.; Kunzelman, K.S. High-resolution subject-specific mitral valve imaging and modeling: Experimental and computational methods. Biomech. Model. Mechanobiol. 2016, 15, 1619–1630. [Google Scholar] [CrossRef] [PubMed]
- Hirschhorn, M.; Tchantchaleishvili, V.; Stevens, R.; Rossano, J.; Throckmorton, A. Fluid-structure interaction modeling in cardiovascular medicine—A systematic review 2017–2019. Med. Eng. Phys. 2020, 78, 1–13. [Google Scholar] [CrossRef]
- Bianchi, D.; Monaldo, E.; Gizzi, A.; Marino, M.; Filippi, S.; Vairo, G. A FSI computational framework for vascular physiopathology: A novel flow-tissue multiscale strategy. Med. Eng. Phys. 2017, 47, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Li, G.; Dai, J.; Zhang, K.; Xu, T.; Wei, L.; Zhang, X.; Ding, D.; Hou, J.; Li, J.; et al. A fluid-structure interaction model accounting arterial vessels as a key part of the blood-flow engine for the analysis of cardiovascular diseases. Front. Bioeng. Biotechnol. 2022, 10, 981187. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ghayesh, M.H.; Kotousov, A.; Zander, A.C.; Dawson, J.A.; Psaltis, P.J. Fluid-structure interaction study for biomechanics and risk factors in Stanford type A aortic dissection. Int. J. Numer. Methods Biomed. Eng. 2023. early view. [Google Scholar] [CrossRef]
- Azarnoosh, J.; Ghorbannia, A.; Ibrahim, E.S.H.; Jurkiewicz, H.; Kalvin, L.; LaDisa, J.F. Temporal evolution of mechanical stimuli from vascular remodeling in response to the severity and duration of aortic coarctation in a preclinical model. Sci. Rep. 2023, 13, 8352. [Google Scholar] [CrossRef] [PubMed]
- Fanni, B.M.; Antonuccio, M.N.; Pizzuto, A.; Berti, S.; Santoro, G.; Celi, S. Uncertainty Quantification in the In Vivo Image-Based Estimation of Local Elastic Properties of Vascular Walls. J. Cardiovasc. Dev. Dis. 2023, 10, 109. [Google Scholar] [CrossRef] [PubMed]
- Mobadersany, N.; Meshram, N.H.; Kemper, P.; Sise, C.; Karageorgos, G.M.; Liang, P.; Ateshian, G.A.; Konofagou, E.E. Pulse wave imaging of a stenotic artery model with plaque constituents of different stiffnesses: Experimental demonstration in phantoms and fluid-structure interaction simulation. J. Biomech. 2023, 149, 111502. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, H.J.; Ghayesh, M.H.; Zander, A.C.; Psaltis, P.J. On the nonlinear relationship between wall shear stress topology and multi-directionality in coronary atherosclerosis. Comput. Methods Programs Biomed. 2023, 231, 107418. [Google Scholar] [CrossRef] [PubMed]
- Fandaros, M.; Li, Y.Y.; Cao, J.J.; Yin, W. A spatiotemporal analysis of the left coronary artery biomechanics using fluid-structure interaction models. Med. Biol. Eng. Comput. 2023, 61, 1533–1548. [Google Scholar] [CrossRef]
- Toma, M.; Kuo, S.H. Computational Assessment of Risk of Subdural Hematoma Associated with Ventriculoperitoneal Shunt Placement. In Lecture Notes in Computational Vision and Biomechanics; Springer International Publishing: Cham, Switzerland, 2020; pp. 36–47. [Google Scholar] [CrossRef]
- Carmody, C.; Burriesci, G.; Howard, I.; Patterson, E. An approach to the simulation of fluid-structure interaction in the aortic valve. J. Biomech. 2006, 39, 158–169. [Google Scholar] [CrossRef]
- Hsu, M.C.; Kamensky, D.; Bazilevs, Y.; Sacks, M.S.; Hughes, T.J.R. Fluid-structure interaction analysis of bioprosthetic heart valves: Significance of arterial wall deformation. Comput. Mech. 2014, 54, 1055–1071. [Google Scholar] [CrossRef]
- Bornoff, J.; Najar, A.; Fresiello, L.; Finocchiaro, T.; Perkins, I.L.; Gill, H.; Cookson, A.N.; Fraser, K.H. Fluid-structure interaction modelling of a positive-displacement Total Artificial Heart. Sci. Rep. 2023, 13, 5734. [Google Scholar] [CrossRef]
- Cai, L.; Hao, Y.; Ma, P.; Zhu, G.; Luo, X.; Gao, H. Fluid-structure interaction simulation of calcified aortic valve stenosis. Math. Biosci. Eng. 2022, 19, 13172–13192. [Google Scholar] [CrossRef]
- Ahmed, M.; Gupta, N.; Jana, R.; Das, M.K.; Kar, K.K. Ramifications of Vorticity on Aggregation and Activation of Platelets in Bi-Leaflet Mechanical Heart Valve: Fluid-Structure-Interaction Study. J. Biomech. Eng. 2022, 144, 081002. [Google Scholar] [CrossRef]
- Oks, D.; Samaniego, C.; Houzeaux, G.; Butakoff, C.; Vázquez, M. Fluid-structure interaction analysis of eccentricity and leaflet rigidity on thrombosis biomarkers in bioprosthetic aortic valve replacements. Int. J. Numer. Methods Biomed. Eng. 2022, 38, e3649. [Google Scholar] [CrossRef]
- Khaledian, N.; Villard, P.F.; Berger, M.O. Capturing contact in mitral valve dynamic closure with fluid-structure interaction simulation. Int. J. Comput. Assist. Radiol. Surg. 2022, 17, 1391–1398. [Google Scholar] [CrossRef]
- Toma, M.; Jensen, M.Ø.; Einstein, D.R.; Yoganathan, A.P.; Cochran, R.P.; Kunzelman, K.S. Fluid-Structure Interaction Analysis of Papillary Muscle Forces Using a Comprehensive Mitral Valve Model with 3D Chordal Structure. Ann. Biomed. Eng. 2015, 44, 942–953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toma, M.; Bloodworth, C.H.; Pierce, E.L.; Einstein, D.R.; Cochran, R.P.; Yoganathan, A.P.; Kunzelman, K.S. Fluid-Structure Interaction Analysis of Ruptured Mitral Chordae Tendineae. Ann. Biomed. Eng. 2016, 45, 619–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh-Gryzbon, S.; Sadri, V.; Toma, M.; Pierce, E.L.; Wei, Z.A.; Yoganathan, A.P. Development of a Computational Method for Simulating Tricuspid Valve Dynamics. Ann. Biomed. Eng. 2019, 47, 1422–1434. [Google Scholar] [CrossRef]
- Toma, M.; Einstein, D.R.; Kohli, K.; Caroll, S.L.; Bloodworth, C.H.; Cochran, R.P.; Kunzelman, K.S.; Yoganathan, A.P. Effect of Edge-to-Edge Mitral Valve Repair on Chordal Strain: Fluid-Structure Interaction Simulations. Biology 2020, 9, 173. [Google Scholar] [CrossRef]
- Toma, M.; Einstein, D.R.; Bloodworth, C.H.; Cochran, R.P.; Yoganathan, A.P.; Kunzelman, K.S. Fluid-structure interaction and structural analyses using a comprehensive mitral valve model with 3D chordal structure. Int. J. Numer. Methods Biomed. Eng. 2016, 33, e2815. [Google Scholar] [CrossRef] [Green Version]
- Fang, R.; Wang, Z.; Zhao, X.; Wang, J.; Li, Y.; Zhang, Y.; Chen, Q.; Wang, J.; Liu, Q.; Chen, M.; et al. Stroke risk evaluation for patients with atrial fibrillation: Insights from left atrial appendage with fluid-structure interaction analysis. Comput. Biol. Med. 2022, 148, 105897. [Google Scholar] [CrossRef]
- Khan, S.; Syed, F.; Toma, M. Management of Intracranial Hemorrhage in the Setting of Mechanical Heart Valve Replacement Therapy. Appl. Mech. 2023, 4, 644–667. [Google Scholar] [CrossRef]
- Toma, M.; Singh-Gryzbon, S.; Frankini, E.; Wei, Z.A.; Yoganathan, A.P. Clinical Impact of Computational Heart Valve Models. Materials 2022, 15, 3302. [Google Scholar] [CrossRef] [PubMed]
- Lipp, S.N.; Niedert, E.E.; Cebull, H.L.; Diorio, T.C.; Ma, J.L.; Rothenberger, S.M.; Boster, K.A.S.; Goergen, C.J. Computational Hemodynamic Modeling of Arterial Aneurysms: A Mini-Review. Front. Physiol. 2020, 11, 454. [Google Scholar] [CrossRef]
- Perdikaris, P.; Insley, J.A.; Grinberg, L.; Yu, Y.; Papka, M.E.; Karniadakis, G.E. Visualizing multiphysics, fluid-structure interaction phenomena in intracranial aneurysms. Parallel Comput. 2016, 55, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Rostam-Alilou, A.A.; Jarrah, H.R.; Zolfagharian, A.; Bodaghi, M. Fluid-structure interaction (FSI) simulation for studying the impact of atherosclerosis on hemodynamics, arterial tissue remodeling, and initiation risk of intracranial aneurysms. Biomech. Model. Mechanobiol. 2022, 21, 1393–1406. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, M.; Shamloo, A.; Mohammadi, A. Fluid-Structure Interaction Simulation of Blood Flow and Cerebral Aneurysm: Effect of Partly Blocked Vessel. J. Vasc. Res. 2019, 56, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Jahed, M.; Ghalichi, F.; Farhoudi, M. Fluid-structure interaction of patient-specific Circle of Willis with aneurysm: Investigation of hemodynamic parameters. Bio-Med. Mater. Eng. 2018, 29, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Voß, S.; Glaßer, S.; Hoffmann, T.; Beuing, O.; Weigand, S.; Jachau, K.; Preim, B.; Thévenin, D.; Janiga, G.; Berg, P. Fluid-Structure Simulations of a Ruptured Intracranial Aneurysm: Constant versus Patient-Specific Wall Thickness. Comput. Math. Methods Med. 2016, 2016, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bazilevs, Y.; Hsu, M.C.; Zhang, Y.; Wang, W.; Kvamsdal, T.; Hentschel, S.; Isaksen, J.G. Computational vascular fluid-structure interaction: Methodology and application to cerebral aneurysms. Biomech. Model. Mechanobiol. 2010, 9, 481–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Razaghi, R.; Biglari, H.; Karimi, A. Risk of rupture of the cerebral aneurysm in relation to traumatic brain injury using a patient-specific fluid-structure interaction model. Comput. Methods Programs Biomed. 2019, 176, 9–16. [Google Scholar] [CrossRef]
- Suzuki, T.; Takao, H.; Suzuki, T.; Kambayashi, Y.; Watanabe, M.; Shinohara, K.; Fujiwara, H.; Nakazato, S.; Watanabe, M.; Dahmani, C.; et al. Fluid structure interaction analysis reveals facial nerve palsy caused by vertebral-posterior inferior cerebellar artery aneurysm. Comput. Biol. Med. 2015, 66, 263–268. [Google Scholar] [CrossRef]
- Kelly, S.; O’Rourke, M. Fluid, solid and fluid-structure interaction simulations on patient-based abdominal aortic aneurysm models. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2012, 226, 288–304. [Google Scholar] [CrossRef]
- Vorp, D.A.; Raghavan, M.; Webster, M.W. Mechanical wall stress in abdominal aortic aneurysm: Influence of diameter and asymmetry. J. Vasc. Surg. 1998, 27, 632–639. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Uhlmann, K.; Vedula, V.; Balzani, D.; Varnik, F. Fluid-structure interaction simulation of tissue degradation and its effects on intra-aneurysm hemodynamics. Biomech. Model. Mechanobiol. 2022, 21, 671–683. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Han, X.; Bi, Y.; Ju, S.; Gu, L. Fluid-Structure Interaction in Abdominal Aortic Aneurysm: Effect of Modeling Techniques. BioMed Res. Int. 2017, 2017, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Li, X. A fluid-structure interaction-based numerical investigation on the evolution of stress, strength and rupture potential of an abdominal aortic aneurysm. Comput. Methods Biomech. Biomed. Eng. 2013, 16, 1032–1039. [Google Scholar] [CrossRef]
- Fonken, J.H.C.; Maas, E.J.; Nievergeld, A.H.M.; van Sambeek, M.R.H.M.; van de Vosse, F.N.; Lopata, R.G.P. Ultrasound-Based Fluid-Structure Interaction Modeling of Abdominal Aortic Aneurysms Incorporating Pre-stress. Front. Physiol. 2021, 12, 1255. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Raut, S.S.; Jana, A.; Biederman, R.W.; Doyle, M.; Muluk, S.C.; Finol, E.A. Fluid-Structure Interaction Modeling of Abdominal Aortic Aneurysms: The Impact of Patient-Specific Inflow Conditions and Fluid/Solid Coupling. J. Biomech. Eng. 2013, 135, 081001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drewe, C.J.; Parker, L.P.; Kelsey, L.J.; Norman, P.E.; Powell, J.T.; Doyle, B.J. Haemodynamics and stresses in abdominal aortic aneurysms: A fluid-structure interaction study into the effect of proximal neck and iliac bifurcation angle. J. Biomech. 2017, 60, 150–156. [Google Scholar] [CrossRef] [Green Version]
- Rahmani, S.; Jarrahi, A.; Saed, B.; Navidbakhsh, M.; Farjpour, H.; Alizadeh, M. Three-dimensional modeling of Marfan syndrome with elastic and hyperelastic materials assumptions using fluid-structure interaction. Bio-Med. Mater. Eng. 2019, 30, 255–266. [Google Scholar] [CrossRef]
- Sharzehee, M.; Khalafvand, S.S.; Han, H.C. Fluid-structure interaction modeling of aneurysmal arteries under steady-state and pulsatile blood flow: A stability analysis. Comput. Methods Biomech. Biomed. Eng. 2018, 21, 219–231. [Google Scholar] [CrossRef] [Green Version]
- Simsek, F.G.; Kwon, Y.W. Investigation of material modeling in fluid-structure interaction analysis of an idealized three-layered abdominal aorta: Aneurysm initiation and fully developed aneurysms. J. Biol. Phys. 2015, 41, 173–201. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.; Zhang, Y.; Takao, H.; Murayama, Y.; Qian, Y. A fluid-structure interaction study using patient-specific ruptured and unruptured aneurysm: The effect of aneurysm morphology, hypertension and elasticity. J. Biomech. 2013, 46, 2402–2410. [Google Scholar] [CrossRef]
- Jayendiran, R.; Nour, B.; Ruimi, A. Computational analysis of Nitinol stent-graft for endovascular aortic repair (EVAR) of abdominal aortic aneurysm (AAA): Crimping, sealing and fluid-structure interaction (FSI). Int. J. Cardiol. 2020, 304, 164–171. [Google Scholar] [CrossRef]
- Ong, C.W.; Kabinejadian, F.; Xiong, F.; Wong, Y.R.; Toma, M.; Nguyen, Y.N.; Chua, K.J.; Cui, F.S.; Ho, P.; Leo, H.; et al. Pulsatile Flow Investigation in Development of Thoracic Aortic Aneurysm: An In-Vitro Validated Fluid Structure Interaction Analysis. J. Appl. Fluid Mech. 2019, 12, 1855–1872. [Google Scholar] [CrossRef]
- Gao, F.; Ueda, H.; Gang, L.; Okada, H. Fluid structure interaction simulation in three-layered aortic aneurysm model under pulsatile flow: Comparison of wrapping and stenting. J. Biomech. 2013, 46, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Aricò, C.; Sinagra, M.; Nagy, R.; Napoli, E.; Tucciarelli, T. Investigation of the hemodynamic flow conditions and blood-induced stresses inside an abdominal aortic aneurysm by means of a SPH numerical model. Int. J. Numer. Methods Biomed. Eng. 2019, 36, e3263. [Google Scholar] [CrossRef]
- Toma, M.; Einstein, D.R.; Bloodworth, C.H.; Kohli, K.; Cochran, R.P.; Kunzelman, K.S.; Yoganathan, A.P. Fluid-Structure Interaction Analysis of Subject-Specific Mitral Valve Regurgitation Treatment with an Intra-Valvular Spacer. Prosthesis 2020, 2, 65–75. [Google Scholar] [CrossRef]
- Toma, M.; Chan-Akeley, R. Biofluid-Biostructure Interaction Analyses Using Comprehensive Patient-Specific Geometries. In Advances in Intelligent Systems and Computing; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–16. [Google Scholar] [CrossRef]
- Toma, M.; Addepalli, D.; Chan-Akeley, R. The Intricacies of Computational Medical Research: An Advanced Study Approach. In Recent Developments in Medicine and Medical Research Volume 4; Book Publisher International: Houston, TX, USA, 2021; pp. 71–83. [Google Scholar] [CrossRef]
- Collin, C.B.; Gebhardt, T.; Golebiewski, M.; Karaderi, T.; Hillemanns, M.; Khan, F.M.; Salehzadeh-Yazdi, A.; Kirschner, M.; Krobitsch, S.; and, L.K. Computational Models for Clinical Applications in Personalized Medicine—Guidelines and Recommendations for Data Integration and Model Validation. J. Pers. Med. 2022, 12, 166. [Google Scholar] [CrossRef]
- Toma, M.; Guru, S.K.; Wu, W.; Ali, M.; Ong, C.W. Addressing Discrepancies between Experimental and Computational Procedures. Biology 2021, 10, 536. [Google Scholar] [CrossRef]
- Toma, M.; Wei, O.C. Predictive Modeling in Medicine. Encyclopedia 2023, 3, 590–601. [Google Scholar] [CrossRef]
- Tang, X. The role of artificial intelligence in medical imaging research. BJR Open 2020, 2, 20190031. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.A.; Sonntag, S.J.; Toma, M.; Singh-Gryzbon, S.; Sun, W. Computational Fluid Dynamics Assessment Associated with Transcatheter Heart Valve Prostheses: A Position Paper of the ISO Working Group. Cardiovasc. Eng. Technol. 2018, 9, 289–299. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Syed, F.; Khan, S.; Toma, M. Modeling Dynamics of the Cardiovascular System Using Fluid-Structure Interaction Methods. Biology 2023, 12, 1026. https://doi.org/10.3390/biology12071026
Syed F, Khan S, Toma M. Modeling Dynamics of the Cardiovascular System Using Fluid-Structure Interaction Methods. Biology. 2023; 12(7):1026. https://doi.org/10.3390/biology12071026
Chicago/Turabian StyleSyed, Faiz, Sahar Khan, and Milan Toma. 2023. "Modeling Dynamics of the Cardiovascular System Using Fluid-Structure Interaction Methods" Biology 12, no. 7: 1026. https://doi.org/10.3390/biology12071026





