The Golgi Apparatus as an Anticancer Therapeutic Target
Abstract
:Simple Summary
Abstract
1. Introduction
Why Is the Golgi Apparatus a Relevant Organelle for Cancer Therapy?
2. Pharmacological Approaches
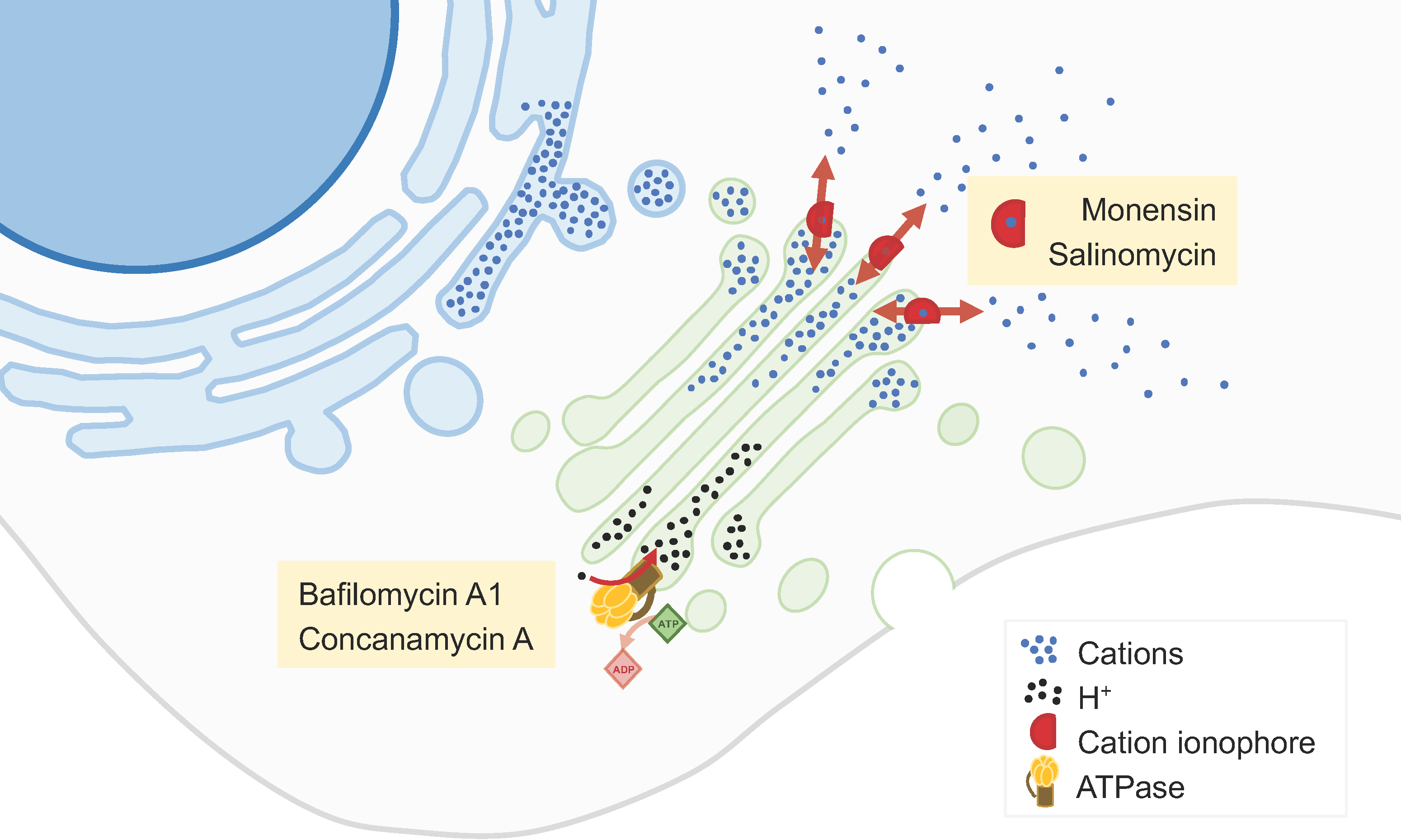
2.1. Golgi Apparatus Trafficking Disrupting Agents
2.2. Golgi-Milieu-Disrupting Agents
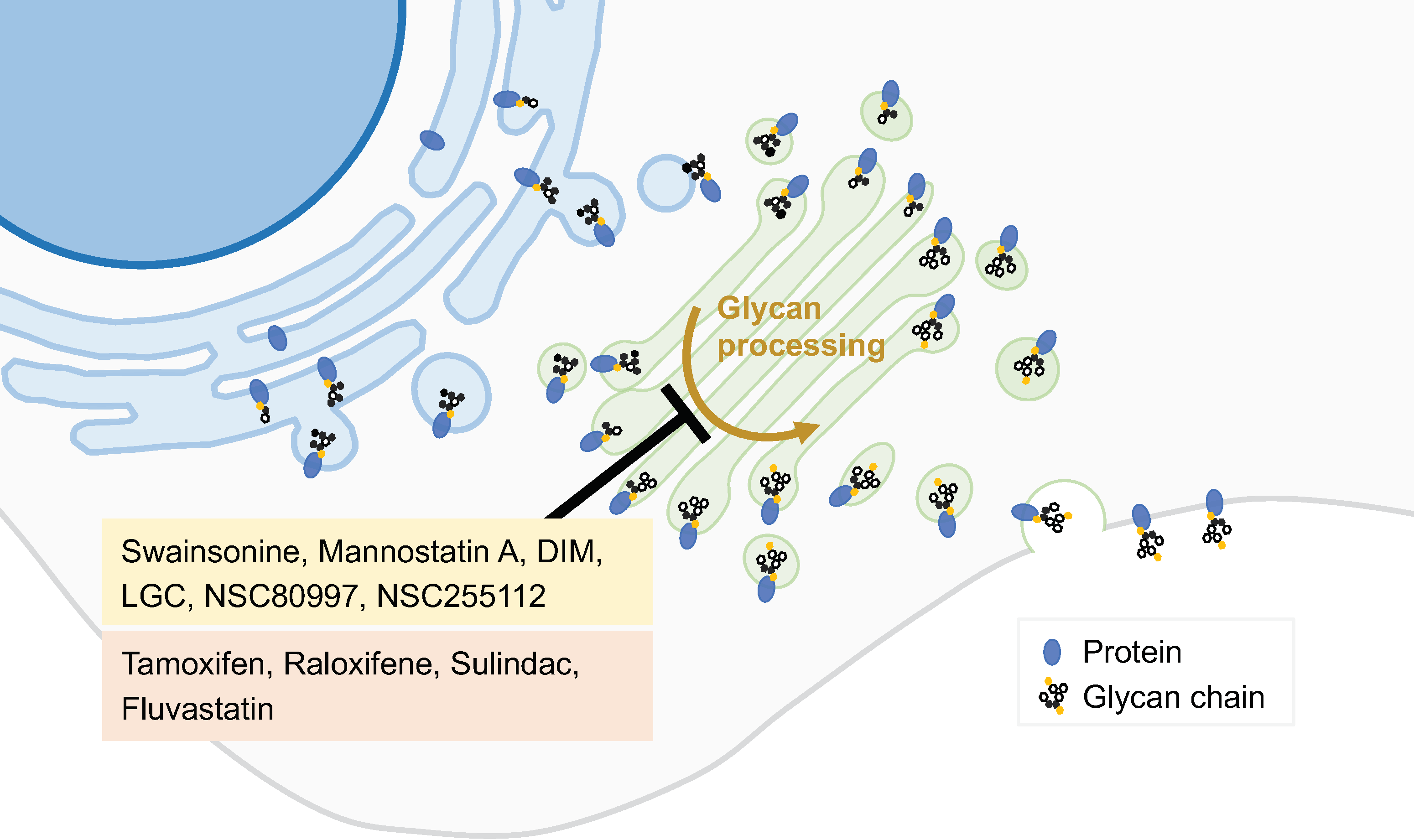
2.3. Inhibitors of the Synthetic Glycosilation Machinery
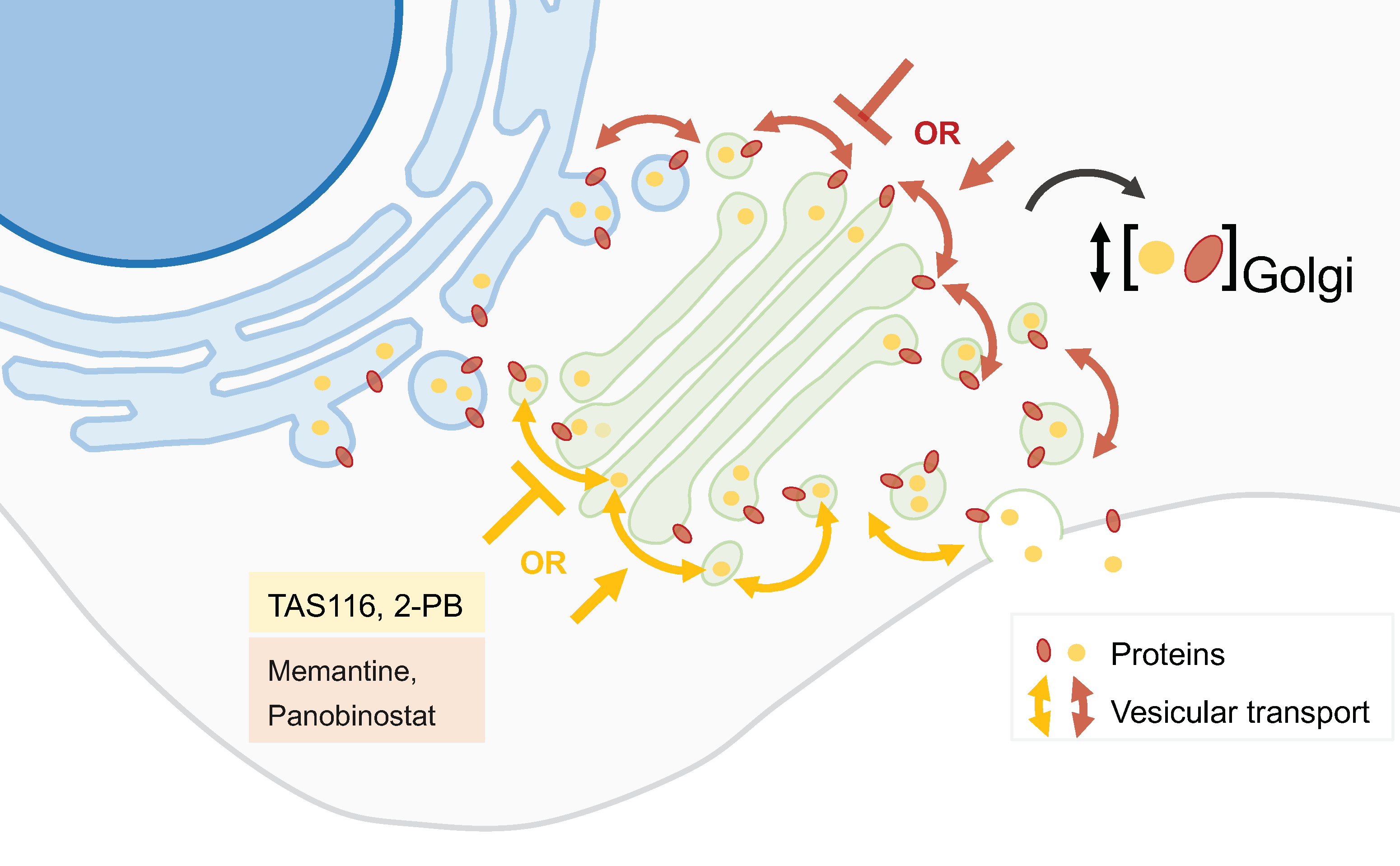
2.4. Inhibitors of Peripheral Golgi-Associated Proteins
2.5. Drug Repurposing
3. Nanotechnological Approaches
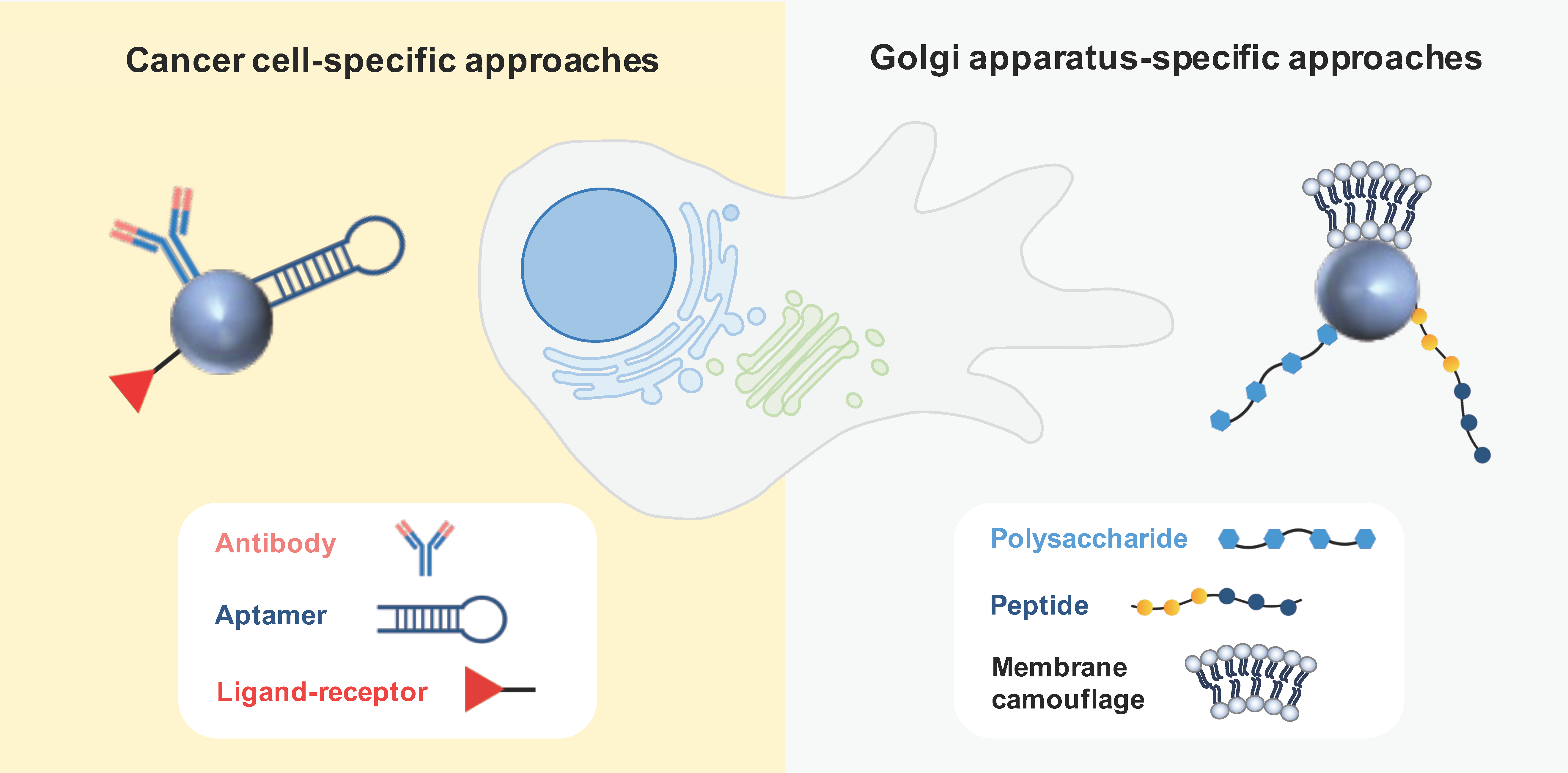
3.1. Passive Targeting
3.2. Active Targeting
3.2.1. Cancer Cell-Specific Approaches
3.2.2. Golgi-Apparatus-Specific Approaches
3.3. Physical Targeting
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klute, M.J.; Melancon, P.; Dacks, J.B. Evolution and Diversity of the Golgi. Cold Spring Harb. Perspect. Biol. 2011, 3, a007849. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Mayr, C. A Membraneless Organelle Associated with the Endoplasmic Reticulum Enables 3′UTR-Mediated Protein-Protein Interactions. Cell 2018, 175, 1492–1506.e19. [Google Scholar] [CrossRef] [PubMed]
- Kremmidiotis, G. The Batten disease gene product (CLN3p) is a Golgi integral membrane protein. Hum. Mol. Genet. 1999, 8, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Crawford, K.; Leonenko, G.; Baker, E.; Grozeva, D.; Lan-Leung, B.; Holmans, P.; Williams, J.; O’Donovan, M.C.; Escott-Price, V.; Ivanov, D.K. Golgi apparatus, endoplasmic reticulum and mitochondrial function implicated in Alzheimer’s disease through polygenic risk and RNA sequencing. Mol. Psychiatry 2023, 28, 1327–1336. [Google Scholar] [CrossRef] [PubMed]
- Almeida, N.; Carrara, G.; Palmeira, C.M.; Fernandes, A.S.; Parsons, M.; Smith, G.L.; Saraiva, N. Stimulation of cell invasion by the Golgi Ion Channel GAAP/TMBIM4 via an H2O2-Dependent Mechanism. Redox Biol. 2020, 28, 101361. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.; Fernandes, A.S.; Saraiva, N. GOLGI: Cancer cell fate control. Int. J. Biochem. Cell Biol. 2022, 145, 106174. [Google Scholar] [CrossRef] [PubMed]
- Kajiho, H.; Kajiho, Y.; Frittoli, E.; Confalonieri, S.; Bertalot, G.; Viale, G.; Di Fiore, P.P.; Oldani, A.; Garre, M.; Beznoussenko, G.V.; et al. RAB2A controls MT1-MMP endocytic and E-cadherin polarized Golgi trafficking to promote invasive breast cancer programs. EMBO Rep. 2016, 17, 1061–1080. [Google Scholar] [CrossRef]
- Magalhães, A.; Duarte, H.O.; Reis, C.A. Aberrant Glycosylation in Cancer: A Novel Molecular Mechanism Controlling Metastasis. Cancer Cell 2017, 31, 733–735. [Google Scholar] [CrossRef]
- Carrara, G.; Saraiva, N.; Parsons, M.; Byrne, B.; Prole, D.L.; Taylor, C.W.; Smith, G.L. Golgi Anti-apoptotic Proteins Are Highly Conserved Ion Channels That Affect Apoptosis and Cell Migration. J. Biol. Chem. 2015, 290, 11785–11801. [Google Scholar] [CrossRef]
- Galenkamp, K.M.O.; Sosicka, P.; Jung, M.; Recouvreux, M.V.; Zhang, Y.; Moldenhauer, M.R.; Brandi, G.; Freeze, H.H.; Commisso, C. Golgi Acidification by NHE7 Regulates Cytosolic pH Homeostasis in Pancreatic Cancer Cells. Cancer Discov. 2020, 10, 822–835. [Google Scholar] [CrossRef]
- Zeng, Z.; Lin, H.; Zhao, X.; Liu, G.; Wang, X.; Xu, R.; Chen, K.; Li, J.; Song, L. Overexpression of GOLPH3 promotes proliferation and tumorigenicity in breast cancer via suppression of the FOXO1 transcription factor. Clin. Cancer Res. 2012, 18, 4059–4069. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, R.; Russo, D.; Kurokawa, K.; Sahu, P.; Lombardi, B.; Supino, D.; Zhukovsky, M.A.; Vocat, A.; Pothukuchi, P.; Kunnathully, V.; et al. Golgi maturation-dependent glycoenzyme recycling controls glycosphingolipid biosynthesis and cell growth via GOLPH3. EMBO J. 2021, 40, e107238. [Google Scholar] [CrossRef]
- Xing, M.; Peterman, M.C.; Davis, R.L.; Oegema, K.; Shiau, A.K.; Field, S.J. GOLPH3 drives cell migration by promoting Golgi reorientation and directional trafficking to the leading edge. Mol. Biol. Cell 2016, 27, 3828–3840. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-Z.; Cui, S.-Z.; Zeng, L.-S.; Cheng, T.-T.; Li, X.-X.; Chi, J.; Wang, R.; Zheng, X.F.S.; Wang, H.-Y. Overexpression of Rab1B and MMP9 predicts poor survival and good response to chemotherapy in patients with colorectal cancer. Aging 2017, 9, 914–931. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Wang, L.; Leung, P.S.C.; Li, Y.; Liu, S.; Wang, L.; Guo, X.; Zhou, G.; Yan, Y.; Guan, G.; et al. The Clinical Significance of GP73 in Immunologically Mediated Chronic Liver Diseases: Experimental Data and Literature Review. Clin. Rev. Allergy Immunol. 2018, 54, 282–294. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.-Y.; Xing, L.; Cui, P.-F.; Qiao, J.-B.; He, Y.-J.; Chang, X.; Zhou, T.-J.; Jin, Q.-R.; Jiang, H.-L.; Xiao, Y. Regulating the Golgi apparatus by co-delivery of a COX-2 inhibitor and Brefeldin A for suppression of tumor metastasis. Biomater. Sci. 2018, 6, 2144–2155. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, J.G.; Jackson, C.L. ARF family G proteins and their regulators: Roles in membrane transport, development and disease. Nat. Rev. Mol. Cell Biol. 2011, 12, 362–375. [Google Scholar] [CrossRef]
- Mishev, K.; Dejonghe, W.; Russinova, E. Small Molecules for Dissecting Endomembrane Trafficking: A Cross-Systems View. Chem. Biol. 2013, 20, 475–486. [Google Scholar] [CrossRef]
- Ohashi, Y.; Iijima, H.; Yamaotsu, N.; Yamazaki, K.; Sato, S.; Okamura, M.; Sugimoto, K.; Dan, S.; Hirono, S.; Yamori, T. AMF-26, a Novel Inhibitor of the Golgi System, Targeting ADP-ribosylation Factor 1 (Arf1) with Potential for Cancer Therapy. J. Biol. Chem. 2012, 287, 3885–3897. [Google Scholar] [CrossRef]
- Shao, R.-G.; Shimizu, T.; Pommier, Y. Brefeldin A Is a Potent Inducer of Apoptosis in Human Cancer Cells Independently of p53. Exp. Cell Res. 1996, 227, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Satoh, M.; Ito, A.; Nojiri, H.; Handa, K.; Numahata, K.; Ohyama, C.; Saito, S.; Hoshi, S.; Hakomori, S.-I. Enhanced GM3 expression, associated with decreased invasiveness, is induced by brefeldin A in bladder cancer cells. Int. J. Oncol. 2001, 19, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.; Bebawy, M.; Kable, E.P.W.; Roufogalis, B.D. Dynamic and intracellular trafficking of P-glycoprotein-EGFP fusion protein: Implications in multidrug resistance in cancer. Int. J. Cancer 2004, 109, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Wu, M.; Zheng, X.; Yang, L.; Zhang, Z.; Li, X.; Chen, J. ERGIC3 Silencing Additively Enhances the Growth Inhibition of BFA on Lung Adenocarcinoma Cells. Curr. Cancer Drug Targets 2020, 20, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.-M.; Ji, Y.; Zhang, C.; Dong, S.-S.; Yang, S.; Xiong, Z.; Ge, M.-K.; Yu, Y.; Xia, L.; Guo, M.; et al. Nuclear PTEN safeguards pre-mRNA splicing to link Golgi apparatus for its tumor suppressive role. Nat. Commun. 2018, 9, 2392. [Google Scholar] [CrossRef] [PubMed]
- Sáenz, J.B.; Sun, W.J.; Chang, J.W.; Li, J.; Bursulaya, B.; Gray, N.S.; Haslam, D.B. Golgicide A reveals essential roles for GBF1 in Golgi assembly and function. Nat. Chem. Biol. 2009, 5, 157–165. [Google Scholar] [CrossRef]
- Shiina, I.; Umezaki, Y.; Ohashi, Y.; Yamazaki, Y.; Dan, S.; Yamori, T. Total Synthesis of AMF-26, an Antitumor Agent for Inhibition of the Golgi System, Targeting ADP-Ribosylation Factor 1. J. Med. Chem. 2013, 56, 150–159. [Google Scholar] [CrossRef]
- Alborzinia, H.; Ignashkova, T.I.; Dejure, F.R.; Gendarme, M.; Theobald, J.; Wölfl, S.; Lindemann, R.K.; Reiling, J.H. Golgi stress mediates redox imbalance and ferroptosis in human cells. Commun. Biol. 2018, 1, 210. [Google Scholar] [CrossRef]
- Del Giudice, S.; De Luca, V.; Parizadeh, S.; Russo, D.; Luini, A.; Di Martino, R. Endogenous and Exogenous Regulatory Signaling in the Secretory Pathway: Role of Golgi Signaling Molecules in Cancer. Front. Cell Dev. Biol. 2022, 10, 611. [Google Scholar] [CrossRef]
- Tan, X.; Banerjee, P.; Pham, E.A.; Rutaganira, F.U.N.; Basu, K.; Bota-Rabassedas, N.; Guo, H.-F.; Grzeskowiak, C.L.; Liu, X.; Yu, J.; et al. PI4KIIIβ is a therapeutic target in chromosome 1q–amplified lung adenocarcinoma. Sci. Transl. Med. 2020, 12, eaax3772. [Google Scholar] [CrossRef]
- Janardhanan, P.; Somasundaran, A.K.; Balakrishnan, A.J.; Pilankatta, R. Sensitization of cancer cells towards Cisplatin and Carboplatin by protein kinase D inhibitors through modulation of ATP7A/B (copper transport ATPases). Cancer Treat. Res. Commun. 2022, 32, 100613. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.Q.; Hsu, I.; Sanford, T.; Railkar, R.; Balaji, N.; Sourbier, C.; Vocke, C.; Balaji, K.C.; Agarwal, P.K. Protein kinase D inhibitor CRT0066101 suppresses bladder cancer growth in vitro and xenografts via blockade of the cell cycle at G2/M. Cell. Mol. Life Sci. 2018, 75, 939–963. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Xu, N.; Xu, W.; Ling, G.; Zhang, P. Potential therapies and diagnosis based on Golgi-targeted nano drug delivery systems. Pharmacol. Res. 2022, 175, 105861. [Google Scholar] [CrossRef] [PubMed]
- Vanneste, M.; Huang, Q.; Li, M.; Moose, D.; Zhao, L.; Stamnes, M.A.; Schultz, M.; Wu, M.; Henry, M.D. High content screening identifies monensin as an EMT-selective cytotoxic compound. Sci. Rep. 2019, 9, 1200. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, H.; Zhu, Y.; Wu, Z.; Cui, C.; Cai, F. Anticancer Mechanisms of Salinomycin in Breast Cancer and Its Clinical Applications. Front. Oncol. 2021, 11, 654428. [Google Scholar] [CrossRef] [PubMed]
- Marjanović, M.; Mikecin Dražić, A.-M.; Mioč, M.; Paradžik, M.; Kliček, F.; Novokmet, M.; Lauc, G.; Kralj, M. Salinomycin disturbs Golgi function and specifically affects cells in epithelial-to-mesenchymal transition. J. Cell Sci. 2023, 136, jcs260934. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, S.L.T.; Nguyen, T.C.; Do, T.T.; Vu, T.L.; Nguyen, T.T.; Do, T.T.; Nguyen, T.H.T.; Le, T.H.; Trinh, D.K.; Nguyen, T.A.T. Study on the Anticancer Activity of Prodigiosin from Variants of Serratia Marcescens QBN VTCC 910026. Biomed Res. Int. 2022, 2022, 4053074. [Google Scholar] [CrossRef]
- Berning, L.; Lenz, T.; Bergmann, A.K.; Poschmann, G.; Brass, H.U.C.; Schlütermann, D.; Friedrich, A.; Mendiburo, M.J.; David, C.; Akgün, S.; et al. The Golgi stacking protein GRASP55 is targeted by the natural compound prodigiosin. Cell Commun. Signal. 2023, 21, 275. [Google Scholar] [CrossRef]
- Chen, F.; Kang, R.; Liu, J.; Tang, D. The V-ATPases in cancer and cell death. Cancer Gene Ther. 2022, 29, 1529–1541. [Google Scholar] [CrossRef]
- Lu, X.; Chen, L.; Chen, Y.; Shao, Q.; Qin, W. Bafilomycin A1 inhibits the growth and metastatic potential of the BEL-7402 liver cancer and HO-8910 ovarian cancer cell lines and induces alterations in their microRNA expression. Exp. Ther. Med. 2015, 10, 1829–1834. [Google Scholar] [CrossRef]
- Lee, Z.Y.; Loo, J.S.E.; Wibowo, A.; Mohammat, M.F.; Foo, J.B. Targeting cancer via Golgi α-mannosidase II inhibition: How far have we come in developing effective inhibitors? Carbohydr. Res. 2021, 508, 108395. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg-Lerner, A.; Benyair, R.; Hizkiahou, N.; Nudel, N.; Maor, R.; Kramer, M.P.; Shmueli, M.D.; Zigdon, I.; Cherniavsky Lev, M.; Ulman, A.; et al. Golgi organization is regulated by proteasomal degradation. Nat. Commun. 2020, 11, 409. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, D.M.; Büll, C.; Madsen, T.D.; Lira-Navarrete, E.; Clausen, T.M.; Clark, A.E.; Garretson, A.F.; Karlsson, R.; Pijnenborg, J.F.A.; Yin, X.; et al. Identification of global inhibitors of cellular glycosylation. Nat. Commun. 2023, 14, 948. [Google Scholar] [CrossRef] [PubMed]
- Obata, Y.; Horikawa, K.; Takahashi, T.; Akieda, Y.; Tsujimoto, M.; Fletcher, J.A.; Esumi, H.; Nishida, T.; Abe, R. Oncogenic signaling by Kit tyrosine kinase occurs selectively on the Golgi apparatus in gastrointestinal stromal tumors. Oncogene 2017, 36, 3661–3672. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Takahashi, T.; Obata, Y.; Nishida, T.; Ohkubo, S.; Nakagawa, F.; Serada, S.; Fujimoto, M.; Ohkawara, T.; Nishigaki, T.; et al. TAS-116 inhibits oncogenic KIT signalling on the Golgi in both imatinib-naïve and imatinib-resistant gastrointestinal stromal tumours. Br. J. Cancer 2020, 122, 658–667. [Google Scholar] [CrossRef] [PubMed]
- Lio, S.C.; Johnson, J.; Chatterjee, A.; Ludwig, J.W.; Millis, D.; Banie, H.; Sircar, J.C.; Sinha, A.; Richards, M.L. Disruption of Golgi processing by 2-phenyl benzimidazole analogs blocks cell proliferation and slows tumor growth. Cancer Chemother. Pharmacol. 2008, 61, 1045–1058. [Google Scholar] [CrossRef] [PubMed]
- Koyama, R.; Hakamata, W.; Hirano, T.; Nishio, T. Identification of Small-Molecule Inhibitors of Human Golgi Mannosidase via a Drug Repositioning Screen. Chem. Pharm. Bull. 2018, 66, 678–681. [Google Scholar] [CrossRef]
- Yu, R.; Longo, J.; van Leeuwen, J.E.; Zhang, C.; Branchard, E.; Elbaz, M.; Cescon, D.W.; Drake, R.R.; Dennis, J.W.; Penn, L.Z. Mevalonate Pathway Inhibition Slows Breast Cancer Metastasis via Reduced N -glycosylation Abundance and Branching. Cancer Res. 2021, 81, 2625–2635. [Google Scholar] [CrossRef]
- Xu, B.; Muramatsu, T.; Inazawa, J. Suppression of MET Signaling Mediated by Pitavastatin and Capmatinib Inhibits Oral and Esophageal Cancer Cell Growth. Mol. Cancer Res. 2021, 19, 585–597. [Google Scholar] [CrossRef]
- Yamaguchi, F.; Hayakawa, S.; Kawashima, S.; Asakura, T.; Oishi, Y. Antitumor effect of memantine is related to the formation of the splicing isoform of GLG1, a decoy FGF-binding protein. Int. J. Oncol. 2022, 61, 80. [Google Scholar] [CrossRef]
- Gendarme, M.; Baumann, J.; Ignashkova, T.I.; Lindemann, R.K.; Reiling, J.H. Image-based drug screen identifies HDAC inhibitors as novel Golgi disruptors synergizing with JQ1. Mol. Biol. Cell 2017, 28, 3756–3772. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, H.; Rai, N.; Singh, S.; Gupta, P.; Verma, A.; Singh, A.K.; Kajal; Salvi, P.; Singh, S.K.; Gautam, V. Recent Advances in Nanomaterials-Based Targeted Drug Delivery for Preclinical Cancer Diagnosis and Therapeutics. Bioengineering 2023, 10, 760. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wei, J.; Huo, P.; Lu, Y.; Chen, Y.; Wei, Y. Controlled release of brefeldin A from electrospun PEG–PLLA nanofibers and their in vitro antitumor activity against HepG2 cells. Mater. Sci. Eng. C 2013, 33, 2513–2518. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Griffin, T.; Salimi, A.; Micetich, R.G.; Atwal, H. Potentiation of ricin A immunotoxin by monoclonal antibody targeted monensin containing small unilamellar vesicles. Cancer Lett. 1994, 84, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Ferdous, A. Role of monensin PLGA polymer nanoparticles and liposomes as potentiator of ricin A immunotoxins in vitro. J. Control Release 1998, 50, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Shaik, M.S.; Ikediobi, O.; Turnage, V.D.; McSween, J.; Kanikkannan, N.; Singh, M. Long-circulating monensin nanoparticles for the potentiation of immunotoxin and anticancer drugs. J. Pharm. Pharmacol. 2010, 53, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.M.; Yadava, S.K.; Singh, R.; Giri, J. Lipid nanocapsules co-encapsulating paclitaxel and salinomycin for eradicating breast cancer and cancer stem cells. Colloids Surfaces B Biointerfaces 2021, 204, 111775. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Liu, J.; Xie, F.; Lu, Y.; Yin, C.; Shen, X. Co-Delivery of Docetaxel and Salinomycin to Target Both Breast Cancer Cells and Stem Cells by PLGA/TPGS Nanoparticles. Int. J. Nanomed. 2019, 14, 9199–9216. [Google Scholar] [CrossRef]
- Anees, M.; Mehrotra, N.; Tiwari, S.; Kumar, D.; Kharbanda, S.; Singh, H. Polylactic acid based biodegradable hybrid block copolymeric nanoparticle mediated co-delivery of salinomycin and doxorubicin for cancer therapy. Int. J. Pharm. 2023, 635, 122779. [Google Scholar] [CrossRef]
- Norouzi, M.; Abdali, Z.; Liu, S.; Miller, D.W. Salinomycin-loaded Nanofibers for Glioblastoma Therapy. Sci. Rep. 2018, 8, 9377. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Wang, X.; Wang, J.; Zhang, X.; Zhang, Q. The eradication of breast cancer and cancer stem cells using octreotide modified paclitaxel active targeting micelles and salinomycin passive targeting micelles. Biomaterials 2012, 33, 679–691. [Google Scholar] [CrossRef] [PubMed]
- Tefas, L.R.; Toma, I.; Sesarman, A.; Banciu, M.; Jurj, A.; Berindan-Neagoe, I.; Rus, L.; Stiufiuc, R.; Tomuta, I. Co-delivery of gemcitabine and salinomycin in PEGylated liposomes for enhanced anticancer efficacy against colorectal cancer. J. Liposome Res. 2023, 33, 234–250. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, S.; Mandal, T.; Mandal, D.D. Chitosan based micro and nano-particulate delivery systems for bacterial prodigiosin: Optimization and toxicity in animal model system. Int. J. Biol. Macromol. 2022, 222, 2966–2976. [Google Scholar] [CrossRef] [PubMed]
- Gugu, T.H.; Eze, C.O.; Kenechukwu, F.C.; Khumaini Mudhar Bintang, M.A.; Patil, S.B.; Basarkar, G.D.; Attama, A.A.; Ibezim, E.C.; Upasani, C.D.; Srichana, T. Mechanistic insight into the bioactivity of prodigiosin-entrapped lipid nanoparticles against triple-negative breast, lung and colon cancer cell lines. Heliyon 2023, 9, e16963. [Google Scholar] [CrossRef] [PubMed]
- Aydın, R.S.T. Herceptin-decorated salinomycin-loaded nanoparticles for breast tumor targeting. J. Biomed. Mater. Res. Part A 2013, 101, 1405–1415. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xu, W.; Yuan, X.; Chen, H.; Song, H.; Wang, B.; Han, J. Polymer–lipid hybrid anti-HER2 nanoparticles for targeted salinomycin delivery to HER2-positive breast cancer stem cells and cancer cells. Int. J. Nanomed. 2017, 12, 6909–6921. [Google Scholar] [CrossRef]
- Zeng, Y.; Yu, Z.; He, Y.; Zhang, T.; Du, L.; Dong, Y.; Chen, H.; Zhang, Y.; Wang, W. Salinomycin-loaded lipid-polymer nanoparticles with anti-CD20 aptamers selectively suppress human CD20+ melanoma stem cells. Acta Pharmacol. Sin. 2018, 39, 261–274. [Google Scholar] [CrossRef]
- Chen, F.; Zeng, Y.; Qi, X.; Chen, Y.; Ge, Z.; Jiang, Z.; Zhang, X.; Dong, Y.; Chen, H.; Yu, Z. Targeted salinomycin delivery with EGFR and CD133 aptamers based dual-ligand lipid-polymer nanoparticles to both osteosarcoma cells and cancer stem cells. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 2115–2127. [Google Scholar] [CrossRef]
- Zhu, M.; Chen, S.; Hua, L.; Zhang, C.; Chen, M.; Chen, D.; Dong, Y.; Zhang, Y.; Li, M.; Song, X.; et al. Self-targeted salinomycin-loaded DSPE-PEG-methotrexate nanomicelles for targeting both head and neck squamous cell carcinoma cancer cells and cancer stem cells. Nanomedicine 2017, 12, 295–315. [Google Scholar] [CrossRef]
- Li, H.; Zhang, P.; Luo, J.; Hu, D.; Huang, Y.; Zhang, Z.-R.; Fu, Y.; Gong, T. Chondroitin Sulfate-Linked Prodrug Nanoparticles Target the Golgi Apparatus for Cancer Metastasis Treatment. ACS Nano 2019, 13, 9386–9396. [Google Scholar] [CrossRef]
- Luo, J.; Gong, T.; Ma, L. Chondroitin-modified lipid nanoparticles target the Golgi to degrade extracellular matrix for liver cancer management. Carbohydr. Polym. 2020, 249, 116887. [Google Scholar] [CrossRef] [PubMed]
- Li, R.S.; Liu, J.; Shi, H.; Hu, P.P.; Wang, Y.; Gao, P.F.; Wang, J.; Jia, M.; Li, H.; Li, Y.F.; et al. Transformable Helical Self-Assembly for Cancerous Golgi Apparatus Disruption. Nano Lett. 2021, 21, 8455–8465. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, C.; Xiang, Y.; Lyu, J.; Zhou, Z.; Gong, T.; Gao, H.; Li, L.; Huang, Y. Exocytosis blockade of endoplasmic reticulum-targeted nanoparticle enhances immunotherapy. Nano Today 2022, 42, 101356. [Google Scholar] [CrossRef]
- Chen, L.; Jiang, P.; Shen, X.; Lyu, J.; Liu, C.; Li, L.; Huang, Y. Cascade Delivery to Golgi Apparatus and On-Site Formation of Subcellular Drug Reservoir for Cancer Metastasis Suppression. Small 2023, 19, 2204747. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yan, L.; Wang, X.; Zhu, S.; Chen, C.; Gu, Z.; Zhao, Y. Progress, challenges, and future of nanomedicine. Nano Today 2020, 35, 101008. [Google Scholar] [CrossRef]
- Xue, F.; Wen, Y.; Wei, P.; Gao, Y.; Zhou, Z.; Xiao, S.; Yi, T. A smart drug: A pH-responsive photothermal ablation agent for Golgi apparatus activated cancer therapy. Chem. Commun. 2017, 53, 6424–6427. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Zhong, H.; Chang, Y.; Li, D.; Jin, H.; Zhang, M.; Wang, H.; Jiang, C.; Shen, Y.; Huang, Y. Targeting death receptors for drug-resistant cancer therapy: Codelivery of pTRAIL and monensin using dual-targeting and stimuli-responsive self-assembling nanocomposites. Biomaterials 2018, 158, 56–73. [Google Scholar] [CrossRef]
- Ali, O.M.; El Amin, H.A.; Sharkawy, Y.L.; Mohamed Ali, A.A.; Kholef, E.F.M.; Elsewify, W.A.E. Golgi Protein 73 versus Alpha-Fetoprotein as a New Biomarker in Early Diagnosis of Hepatocellular Carcinoma. Int. J. Gen. Med. 2020, 13, 193–200. [Google Scholar] [CrossRef]
- Kristiansen, G.; Fritzsche, F.R.; Wassermann, K.; Jäger, C.; Tölle, A.; Lein, M.; Stephan, C.; Jung, K.; Pilarsky, C.; Dietel, M.; et al. GOLPH2 protein expression as a novel tissue biomarker for prostate cancer: Implications for tissue-based diagnostics. Br. J. Cancer 2008, 99, 939–948. [Google Scholar] [CrossRef]
- Varambally, S.; Laxman, B.; Mehra, R.; Cao, Q.; Dhanasekaran, S.M.; Tomlins, S.A.; Granger, J.; Vellaichamy, A.; Sreekumar, A.; Yu, J.; et al. Golgi Protein GOLM1 Is a Tissue and Urine Biomarker of Prostate Cancer. Neoplasia 2008, 10, 1285–1294. [Google Scholar] [CrossRef]
- Cheng, P.-W.; Davidson, S.; Bhat, G. Markers of malignant prostate cancer cells: Golgi localization of α-mannosidase 1A at GM130-GRASP65 site and appearance of high mannose N-glycans on cell surface. Biochem. Biophys. Res. Commun. 2020, 527, 406–410. [Google Scholar] [CrossRef]
- Hamester, F.; Legler, K.; Wichert, B.; Kelle, N.; Eylmann, K.; Rossberg, M.; Ding, Y.; Kürti, S.; Schmalfeldt, B.; Milde-Langosch, K.; et al. Prognostic relevance of the Golgi mannosidase MAN1A1 in ovarian cancer: Impact of N-glycosylation on tumour cell aggregation. Br. J. Cancer 2019, 121, 944–953. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhu, Q.; Cai, D.; Chen, L.; Xie, W.; Bai, Y.; Luo, K. Golgi phosphoprotein 3 promotes the proliferation of gallbladder carcinoma cells via regulation of the NLRP3 inflammasome. Oncol. Rep. 2021, 45, 113. [Google Scholar] [CrossRef]
- Liang, B.-B.; Liu, Q.; Liu, B.; Yao, H.-G.; He, J.; Tang, C.-F.; Peng, K.; Su, X.-X.; Zheng, Y.; Ding, J.-Y.; et al. A Golgi-Targeted Platinum Complex Plays a Dual Role in Autophagy Regulation for Highly Efficient Cancer Therapy. Angew. Chemie Int. Ed. 2023, 62, e202312170. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, P.; Zhao, T.; Jia, M.; Yin, P.; Li, W.; Zhang, Z.-R.; Fu, Y.; Gong, T. Golgi Apparatus-Targeted Chondroitin-Modified Nanomicelles Suppress Hepatic Stellate Cell Activation for the Management of Liver Fibrosis. ACS Nano 2019, 13, 3910–3923. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, M.; Vieira, J.; Pereira-Leite, C.; Saraiva, N.; Fernandes, A.S. The Golgi Apparatus as an Anticancer Therapeutic Target. Biology 2024, 13, 1. https://doi.org/10.3390/biology13010001
Martins M, Vieira J, Pereira-Leite C, Saraiva N, Fernandes AS. The Golgi Apparatus as an Anticancer Therapeutic Target. Biology. 2024; 13(1):1. https://doi.org/10.3390/biology13010001
Chicago/Turabian StyleMartins, Marta, João Vieira, Catarina Pereira-Leite, Nuno Saraiva, and Ana Sofia Fernandes. 2024. "The Golgi Apparatus as an Anticancer Therapeutic Target" Biology 13, no. 1: 1. https://doi.org/10.3390/biology13010001







