Antioxidant Responses and Growth Impairment in Cucurbita moschata Infected by Meloidogyne incognita
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Growth Parameter Measurements
2.3. Evaluation of Hydrogen Peroxide (H2O2), Superoxide Radical (O2•−), and Malondialdehyde (MDA) Levels
2.4. Analysis of Ascorbate (ASC) and Glutathione (GSH) Content
2.5. Antioxidative Enzyme Activity Assays
2.6. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) and Native Gel Enzymatic Activity Assay
3. Results
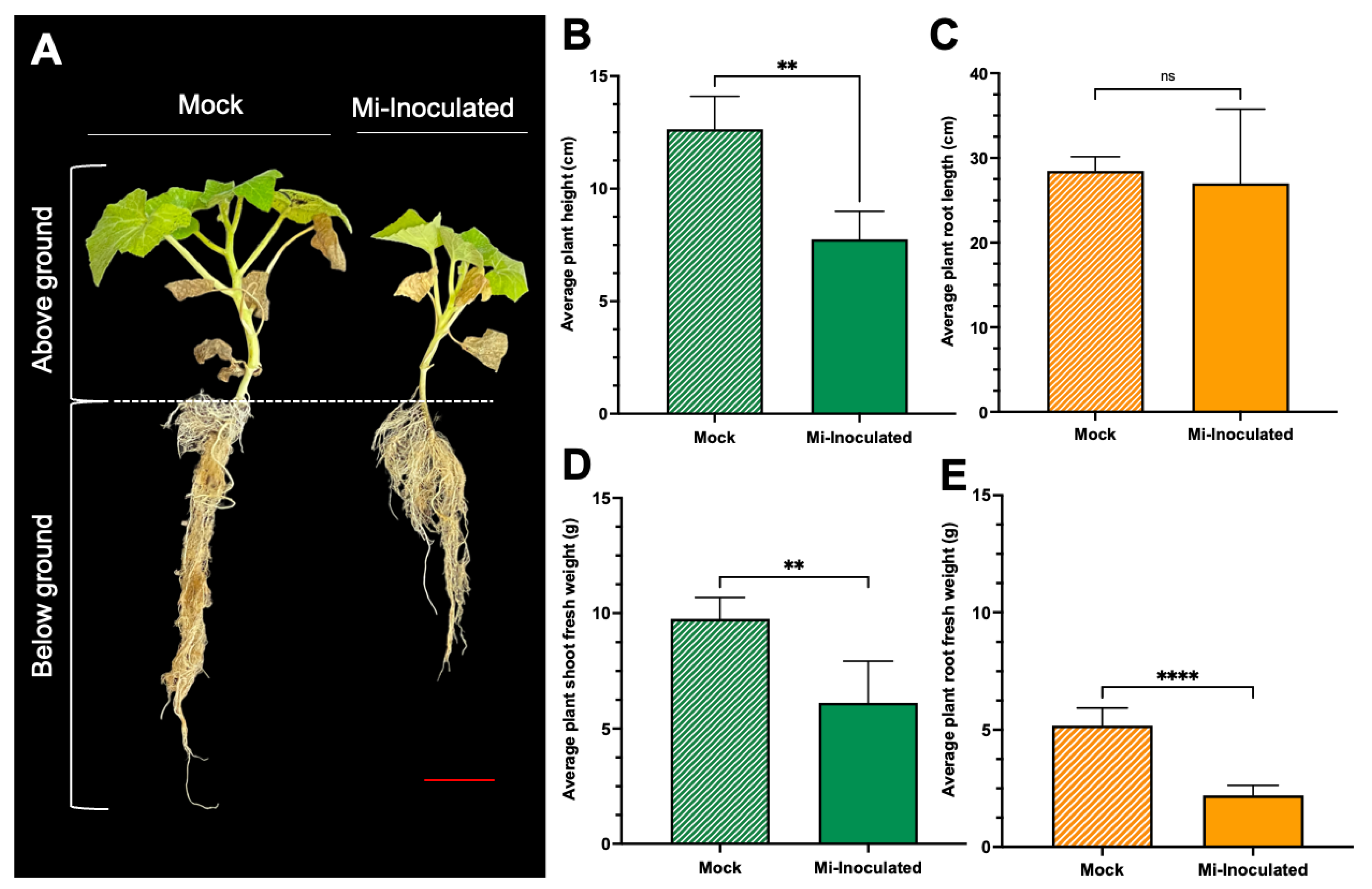
3.1. Effect of Meloidogyne incognita on Relative Growth Rate and Physiological Features of Cucurbita moschata
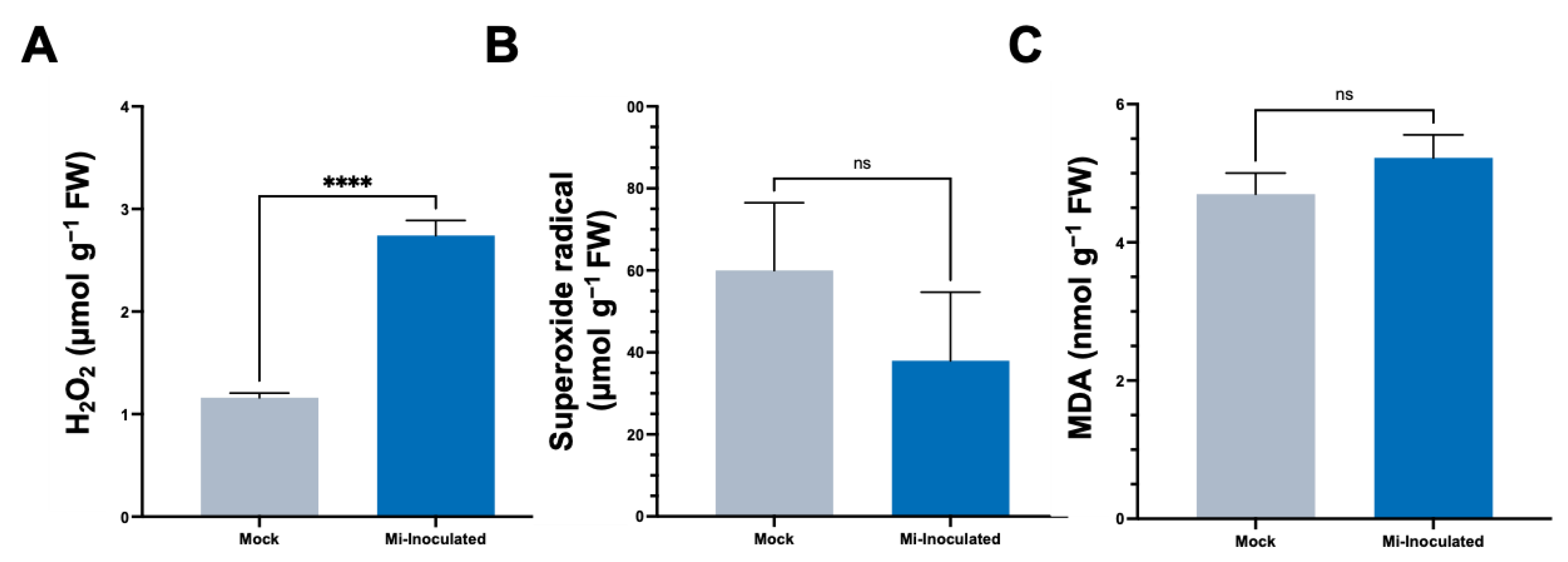
3.2. Investigating the Impact of Meloidogyne incognita on Hydrogen Peroxide (H2O2), Superoxide Radical (O2•−), and Malondialdehyde (MDA) Concentrations
3.3. Effect of Meloidogyne incognita on Ascorbate (ASC) and Glutathione (GSH) Levels in Cucurbita moschata
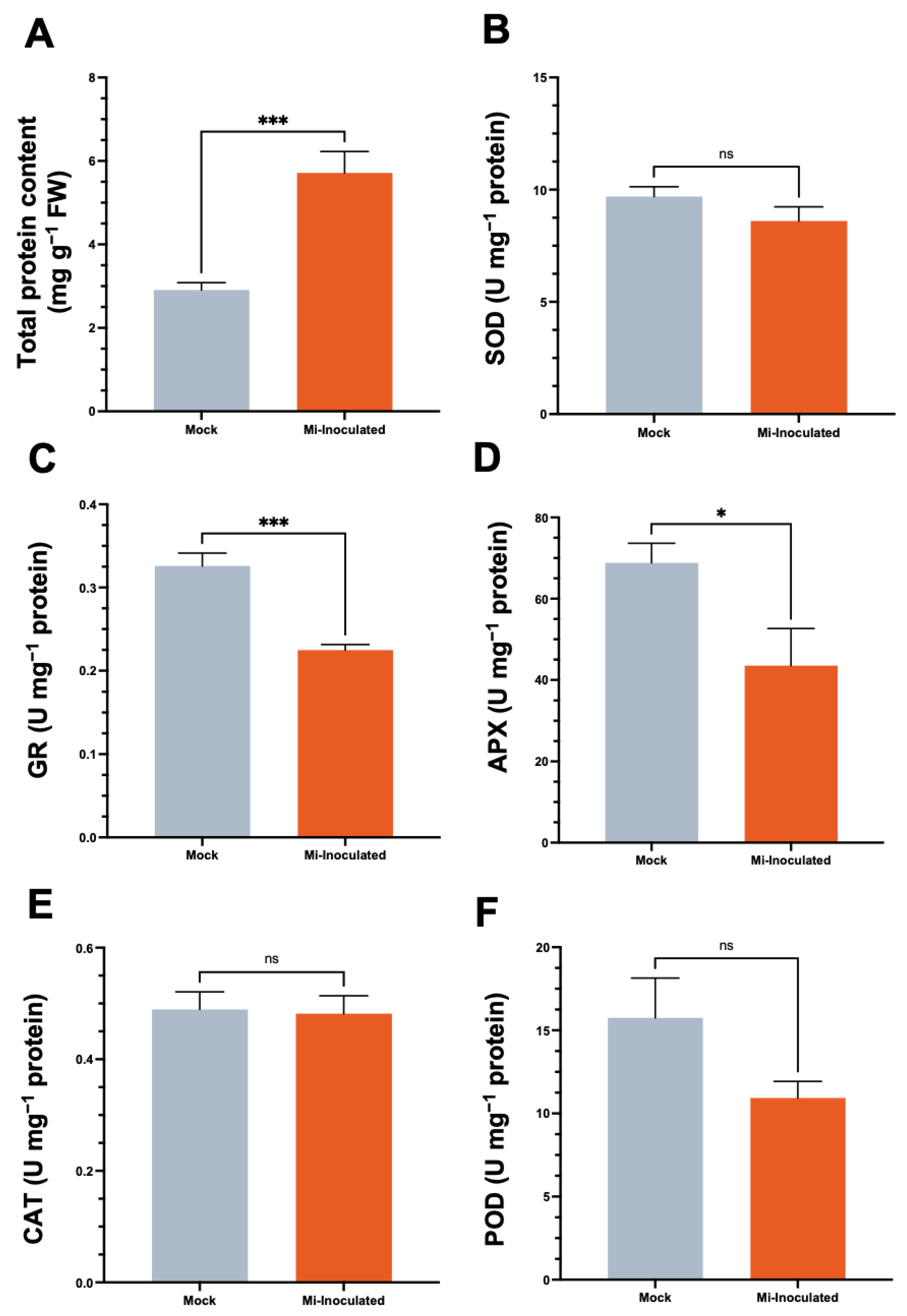
3.4. Assessment of Protein Levels and Antioxidative Enzyme Activity in Cucurbita moschata Post-Inoculation with Meloidogyne incognita
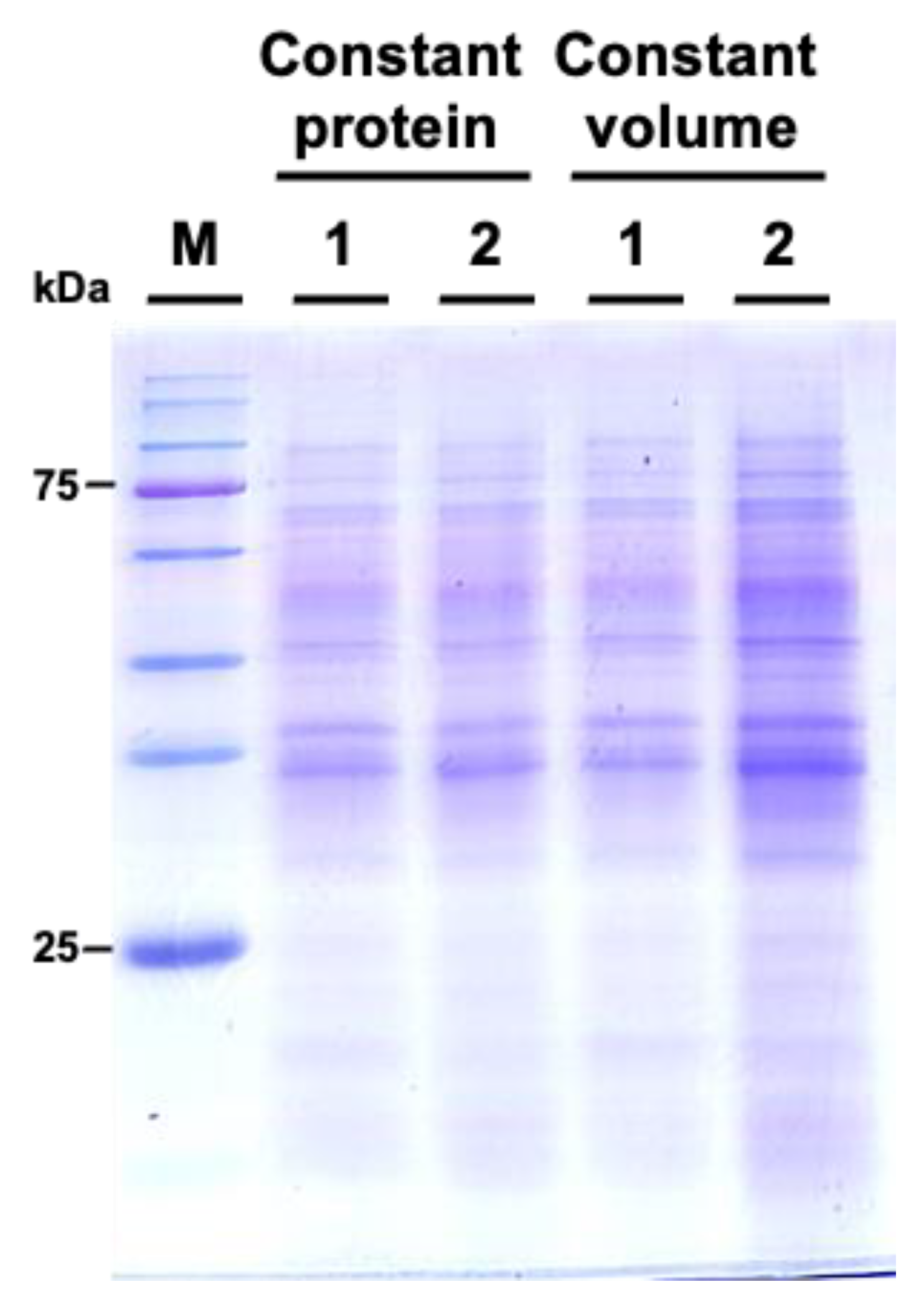
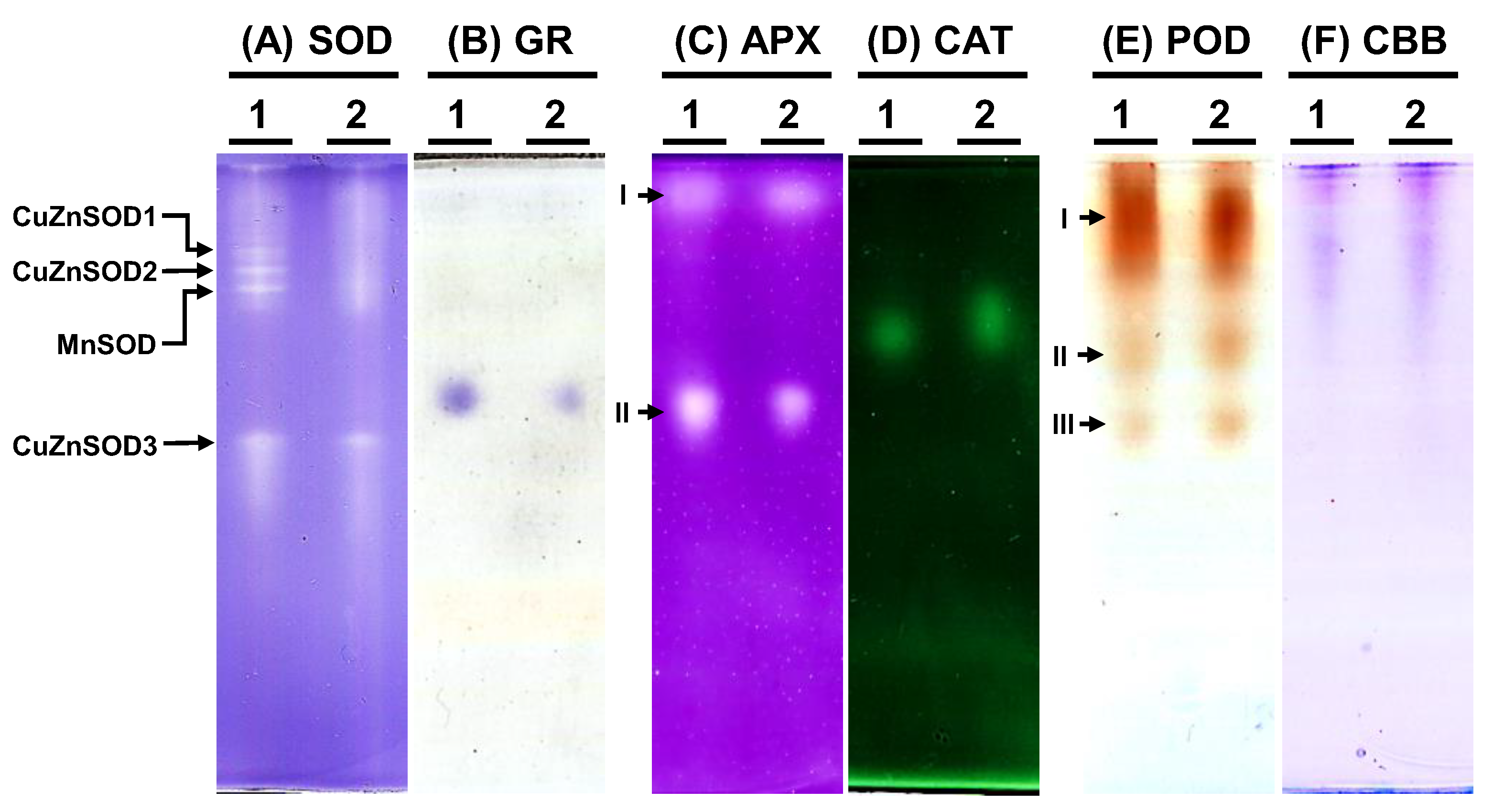
3.5. Assessment of Antioxidative Enzyme Isoforms in Cucurbita moschata via Native Gel Activity Assay following Meloidogyne incognita Inoculation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lebeda, A.; Widrlechner, M.; Staub, J.; Ezura, H.; Zalapa, J.; Kristkova, E. Cucurbits (Cucurbitaceae; Cucumis spp., Cucurbita spp., Citrullus spp.). In Genetic Resources, Chromosome Engineering, and Crop Improvement: Vegetable Crops; Iowa State University: Ames, IA, USA, 2007. [Google Scholar]
- Jeffrey, C. Systematics of the Cucurbitaceae: An overview. In Biology and utilization of the Cucurbitaceae; Cornell University Press: Ithaca, NY, USA, 1990; pp. 3–9. [Google Scholar]
- Renner, S.S.; Schaefer, H. Phylogeny and evolution of the Cucurbitaceae. In Genetics and Genomics of Cucurbitaceae; Springer: Cham, Switzerland, 2017; pp. 13–23. [Google Scholar]
- Babadoost, M. Oomycete diseases of cucurbits: History, significance, and management. In Horticultural Reviews; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 279–314. [Google Scholar]
- Brothwell, D.; Brothwell, P. Food in Antiquity: A Survey of the Diet of Early Peoples; Thames & Hudson Ltd.: London, UK, 1969. [Google Scholar]
- Sun, H.; Wu, S.; Zhang, G.; Jiao, C.; Guo, S.; Ren, Y.; Zhang, J.; Zhang, H.; Gong, G.; Jia, Z. Karyotype stability and unbiased fractionation in the paleo-allotetraploid Cucurbita genomes. Mol. Plant 2017, 10, 1293–1306. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Ma, J.; You, Z.; Weng, M.; Carballar-Lejarazú, R.; Jiao, W.; Wu, J.; Hu, X.; Wang, R.; Zhang, F. Field Efficacy of Fluopyram Suspension Concentrate against Pine Wilt Disease and Its Distribution and Persistence in Pine Tree Tissues. Forests 2023, 14, 338. [Google Scholar] [CrossRef]
- Yiblet, Y. Overview of Cucurbitaceae Families. In Biological and Abiotic Stress in Cucurbitaceae Crops; IntechOpen: London, UK, 2023. [Google Scholar]
- McCreight, J.D. Cultivation and uses of cucurbits. In Genetics and genomics of Cucurbitaceae; Springer: Cham, Switzerland, 2017; pp. 1–12. [Google Scholar]
- Caili, F.; Huan, S.; Quanhong, L. A review on pharmacological activities and utilization technologies of pumpkin. Plant Foods Hum. Nutr. 2006, 61, 70–77. [Google Scholar] [CrossRef]
- Batool, M.; Ranjha, M.M.A.N.; Roobab, U.; Manzoor, M.F.; Farooq, U.; Nadeem, H.R.; Nadeem, M.; Kanwal, R.; AbdElgawad, H.; Al Jaouni, S.K. Nutritional value, phytochemical potential, and therapeutic benefits of pumpkin (Cucurbita sp.). Plants 2022, 11, 1394. [Google Scholar] [CrossRef] [PubMed]
- Bisognin, D.A. Origin and evolution of cultivated cucurbits. Ciência Rural. 2002, 32, 715–723. [Google Scholar] [CrossRef]
- Nakazibwe, I.; Olet, E.A.; Rugunda, G.K. Nutritional physico-chemical composition of pumpkin pulp for value addition: Case of selected cultivars grown in Uganda. Afr. J. Food Sci. 2020, 14, 233–243. [Google Scholar]
- Lestari, B.; Meiyanto, E. A review: The emerging nutraceutical potential of pumpkin seeds. Indones. J. Cancer Chemoprevention 2018, 9, 92–101. [Google Scholar] [CrossRef]
- Decraemer, W.; Hunt, D.J. Structure and classification. In Plant Nematology; CABI: Wallingford, UK, 2006; pp. 3–32. [Google Scholar]
- Abad, P.; Gouzy, J.; Aury, J.-M.; Castagnone-Sereno, P.; Danchin, E.G.; Deleury, E.; Perfus-Barbeoch, L.; Anthouard, V.; Artiguenave, F.; Blok, V.C. Genome sequence of the metazoan plant-parasitic nematode Meloidogyne incognita. Nat. Biotechnol. 2008, 26, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.T.; Haegeman, A.; Danchin, E.G.; Gaur, H.S.; Helder, J.; Jones, M.G.; Kikuchi, T.; Manzanilla-López, R.; Palomares-Rius, J.E.; Wesemael, W.M. Top 10 plant-parasitic nematodes in molecular plant pathology. Mol. Plant Pathol. 2013, 14, 946–961. [Google Scholar] [CrossRef]
- Caillaud, M.-C.; Dubreuil, G.; Quentin, M.; Perfus-Barbeoch, L.; Lecomte, P.; de Almeida Engler, J.; Abad, P.; Rosso, M.-N.; Favery, B. Root-knot nematodes manipulate plant cell functions during a compatible interaction. J. Plant Physiol. 2008, 165, 104–113. [Google Scholar] [CrossRef]
- Gheysen, G.; Mitchum, M.G. How nematodes manipulate plant development pathways for infection. Curr. Opin. Plant Biol. 2011, 14, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Favery, B.; Quentin, M.; Jaubert-Possamai, S.; Abad, P. Gall-forming root-knot nematodes hijack key plant cellular functions to induce multinucleate and hypertrophied feeding cells. J. Insect Physiol. 2016, 84, 60–69. [Google Scholar] [PubMed]
- Perry, R.N.; Moens, M.; Starr, J.L. Root-Knot Nematodes; CABI: Wallingford, UK, 2009. [Google Scholar]
- Subedi, S.; Thapa, B.; Shrestha, J. Root-knot nematode (Meloidogyne incognita) and its management: A review. J. Agric. Nat. Resour. 2020, 3, 21–31. [Google Scholar] [CrossRef]
- Ralmi, N.; Khandaker, M.M.; Mat, N. Occurrence and control of root knot nematode in crops: A review. Aust. J. Crop Sci. 2016, 11, 1649. [Google Scholar]
- Guzmán-Piedrahita, Ó.A.; Zamorano-Montañez, C.; López-Nicora, H.D. Interacciones fisiológicas de plantas con nematodos fitoparásitos: Una revisión. Boletín Científico Cent. Museos. Mus. Hist. Nat. 2020, 24, 190–205. [Google Scholar] [CrossRef]
- El-Eslamboly, A.; Abd El-Wanis, M.M.; Amin, A. Algal application as a biological control method of root-knot nematode Meloidogyne incognita on cucumber under protected culture conditions and its impact on yield and fruit quality. Egypt. J. Biol. Pest Control 2019, 29, 18. [Google Scholar] [CrossRef]
- García-Bastidas, F.A.; Van der Veen, A.J.; Nakasato-Tagami, G.; Meijer, H.J.; Arango-Isaza, R.E.; Kema, G.H. An improved phenotyping protocol for Panama disease in banana. Front. Plant Sci. 2019, 10, 462822. [Google Scholar] [CrossRef]
- Torres, M.A.; Dangl, J.L. Functions of the respiratory burst oxidase in biotic interactions, abiotic stress and development. Curr. Opin. Plant Biol. 2005, 8, 397–403. [Google Scholar] [CrossRef]
- Kadota, Y.; Shirasu, K.; Zipfel, C. Regulation of the NADPH oxidase RBOHD during plant immunity. Plant Cell Physiol. 2015, 56, 1472–1480. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Fujita, M.; Hasanuzzaman, M. Approaches to enhancing antioxidant defense in plants. Antioxidants 2022, 11, 925. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Zeck, W. A rating scheme for field evaluation of root-knot nematode infestations. Pflanzenschutz-Nachrichten ‘Bayer’ 1971, 24, 141–144. [Google Scholar]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [PubMed]
- Jana, S.; Choudhuri, M.A. Glycolate metabolism of three submersed aquatic angiosperms during ageing. Aquat. Bot. 1982, 12, 345–354. [Google Scholar] [CrossRef]
- Elstner, E.F.; Heupel, A. Inhibition of nitrite formation from hydroxylammoniumchloride: A simple assay for superoxide dismutase. Anal. Biochem. 1976, 70, 616–620. [Google Scholar] [CrossRef] [PubMed]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Hodges, D.M.; Andrews, C.J.; Johnson, D.A.; Hamilton, R.I. Antioxidant compound responses to chilling stress in differentially sensitive inbred maize lines. Physiol. Plant. 1996, 98, 685–692. [Google Scholar] [CrossRef]
- Anderson, M.E. [70] Determination of glutathione and glutathione disulfide in biological samples. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1985; Volume 113, pp. 548–555. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Foster, J.G.; Hess, J.L. Responses of superoxide dismutase and glutathione reductase activities in cotton leaf tissue exposed to an atmosphere enriched in oxygen. Plant Physiol. 1980, 66, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Shimizu, S. Chlorophyll metabolism in higher plants. VII. Chlorophyll degradation in senescing tobacco leaves; phenolic-dependent peroxidative degradation. Can. J. Bot. 1987, 65, 729–735. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [PubMed]
- Mittler, R.; Zilinskas, B.A. Detection of ascorbate peroxidase activity in native gels by inhibition of the ascorbate-dependent reduction of nitroblue tetrazolium. Anal. Biochem. 1993, 212, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Köksal, E.; Gülçin, İ. Purification and characterization of peroxidase from cauliflower (Brassica oleracea L. var. botrytis) buds. Protein Pept. Lett. 2008, 15, 320–326. [Google Scholar] [CrossRef]
- Foyer, C.; Lelandais, M.; Galap, C.; Kunert, K.J. Effects of elevated cytosolic glutathione reductase activity on the cellular glutathione pool and photosynthesis in leaves under normal and stress conditions. Plant Physiol. 1991, 97, 863–872. [Google Scholar] [PubMed]
- Bui, H.X.; Desaeger, J.A. Susceptibility and host potential of six cucurbit crops to Meloidogyne enterolobii, M. floridensis, M. hapla, M. incognita and M. javanica. Nematology 2022, 24, 1121–1130. [Google Scholar] [CrossRef]
- López-Gómez, M.; Verdejo-Lucas, S. Penetration and reproduction of root-knot nematodes on cucurbit species. Eur. J. Plant Pathol. 2014, 138, 863–871. [Google Scholar] [CrossRef]
- Ayala-Doñas, A.; Cara-García, M.d.; Talavera-Rubia, M.; Verdejo-Lucas, S. Management of soil-borne fungi and root-knot nematodes in cucurbits through breeding for resistance and grafting. Agronomy 2020, 10, 1641. [Google Scholar] [CrossRef]
- Khan, M.R.; Ruiu, L.; Akram, M.; Mohammed, R.K.A. Nematode problems in cucurbits and their sustainable management. In Nematode Diseases of Crops and their Sustainable Management; Elsevier: Amsterdam, The Netherlands, 2023; pp. 279–296. [Google Scholar]
- Williamson, V.M.; Gleason, C.A. Plant–nematode interactions. Curr. Opin. Plant Biol. 2003, 6, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Kayani, M.Z.; Mukhtar, T.; Hussain, M.A. Effects of southern root knot nematode population densities and plant age on growth and yield parameters of cucumber. Crop Prot. 2017, 92, 207–212. [Google Scholar] [CrossRef]
- Aydinli, G.; Kurtar, E.S.; Mennan, S. Screening of Cucurbita maxima and Cucurbita moschata Genotypes for Resistance Against Meloidogyne arenaria, M. incognita, M. javanica, and M. luci. J. Nematol. 2019, 51, e2019-57. [Google Scholar] [CrossRef] [PubMed]
- Verdejo-Lucas, S.; Talavera, M. Root-knot nematodes on zucchini (Cucurbita pepo subsp. pepo): Pathogenicity and management. Crop Prot. 2019, 126, 104943. [Google Scholar] [CrossRef]
- Talavera-Rubia, M.; De Luque, A.P.; López-Gómez, M.; Verdejo-Lucas, S. Differential feeding site development and reproductive fitness of Meloidogyne incognita and M. javanica on zucchini, a source of resistance to M. incognita. Nematology 2018, 20, 187–199. [Google Scholar] [CrossRef]
- Verdejo-Lucas, S.; Gómez, P.; Talavera, M. Pathogenicity of Meloidogyne incognita and M. javanica on recombinant inbred lines from a crossing of Cucurbita pepo subsp. pepo× C. pepo subsp. ovifera. Plant Pathol. 2019, 68, 1225–1232. [Google Scholar] [CrossRef]
- Walters, S.; Wehner, T.; Barker, K. A Single recessive gene for resistance to the Root-knot nematode (Meloidogyne javanica) in Cucumis sativus var. hardwlckii. J. Hered. 1997, 88, 66–69. [Google Scholar]
- Walters, S.A.; Wehner, T.C.; Barker, K.R. NC-42 and NC-43: Root-knot nematode–resistant cucumber germplasm. HortScience 1996, 31, 1246–1247. [Google Scholar] [CrossRef]
- Williamson, V.M. Plant nematode resistance genes. Curr. Opin. Plant Biol. 1999, 2, 327–331. [Google Scholar] [CrossRef]
- Anthony, F.; Topart, P.; Martinez, A.; Silva, M.; Nicole, M. Hypersensitive-like reaction conferred by the Mex-1 resistance gene against Meloidogyne exigua in coffee. Plant Pathol. 2005, 54, 476–482. [Google Scholar] [CrossRef]
- Pegard, A.; Brizzard, G.; Fazari, A.; Soucaze, O.; Abad, P.; Djian-Caporalino, C. Histological characterization of resistance to different root-knot nematode species related to phenolics accumulation in Capsicum annuum. Phytopathology 2005, 95, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, D.; Thomason, I.; Van Gundy, S. Histological study of the compatible and incompatible interaction of soybeans and Meloidogyne incognita. J. Nematol. 1979, 11, 338. [Google Scholar]
- Selvi, N.T.; Pugalendhi, L.; Sivakumar, M. Screening of cucurbitaceous rootstocks against root knot nematode Meloidogyne incognita Kofoid and White. Asian J. Hortic. 2013, 8, 720–725. [Google Scholar]
- Zhou, J.; Jia, F.; Yu, J. Involvement of nitric oxide in the jasmonate-dependent basal defense against root-knot nematode in tomato plants. Front. Plant Sci. 2015, 6, 128695. [Google Scholar] [CrossRef] [PubMed]
- Karanastasi, E.; Kostara, T.; Malamos, N.; Zervoudakis, G. Catalase activity, lipid peroxidation, and protein concentration in leaves of tomato infected with Meloidogyne javanica. Nematropica 2018, 48, 15–20. [Google Scholar]
- Gillet, F.-X.; Bournaud, C.; Antonino de Souza Júnior, J.D.; Grossi-de-Sa, M.F. Plant-parasitic nematodes: Towards understanding molecular players in stress responses. Ann. Bot. 2017, 119, 775–789. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Vasil’Eva, I.; Vanyushkin, S.; Zinov’eva, S.; Udalova, Z.V.; Paseshnichenko, V.; Sonin, M. Effect of furastanol glycosides of Dioscorea on lipid peroxidation in tomatoes infected with gall nematode. Dokl. Biochem. Biophys. 2004, 397, 207–209. [Google Scholar] [CrossRef] [PubMed]
- Durner, J.; Shah, J.; Klessig, D.F. Salicylic acid and disease resistance in plants. Trends Plant Sci. 1997, 2, 266–274. [Google Scholar] [CrossRef]
- Durrant, W.E.; Dong, X. Systemic acquired resistance. Annu. Rev. Phytopathol. 2004, 42, 185–209. [Google Scholar] [CrossRef]
- Dubreuil, G.; Magliano, M.; Deleury, E.; Abad, P.; Rosso, M.-N. Transcriptome analysis of root-knot nematode functions induced in the early stages of parasitism. New Phytol. 2007, 176, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Zechmann, B. Subcellular roles of glutathione in mediating plant defense during biotic stress. Plants 2020, 9, 1067. [Google Scholar] [CrossRef] [PubMed]
- Zacheo, G.; Bleve-Zacheo, T.; Melillo, M.T. Biochemistry of plant defence responses to nematode infection. In Cellular and Molecular Aspects of Plant-Nematode Interactions; Springer: Dordrecht, The Netherlands, 1997; pp. 201–213. [Google Scholar]
- Yang, J.-W.; Park, S.-U.; Lee, H.-U.; Nam, K.J.; Lee, K.-L.; Lee, J.J.; Kim, J.H.; Kwak, S.-S.; Kim, H.S.; Kim, Y.-H. Differential responses of antioxidant enzymes and lignin metabolism in susceptible and resistant sweetpotato cultivars during root-knot nematode infection. Antioxidants 2023, 12, 1164. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.A.; Jones, J.D.; Dangl, J.L. Reactive oxygen species signaling in response to pathogens. Plant Physiol. 2006, 141, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Olabiyi, T.; Ogunniran, T.; Ojo, O.; Atungwu, J.; Abolusoro, S. Changes in Antioxidative Enzymes in Resistant and Susceptible Genotypes of Tomato Infected with Root-Knot Nematode (Meloidogyne incognita). Indian J. Nematol. 2013, 43, 29–33. [Google Scholar]
- Kaur, R.; Ohri, P.; Bhardwaj, R. Effect of 28-homobrassinolide on susceptible and resistant cultivars of tomato after nematode inoculation. Plant Growth Regul. 2013, 71, 199–205. [Google Scholar]
- Molinari, S.; Fanelli, E.; Leonetti, P. Expression of tomato salicylic acid (SA)-responsive pathogenesis-related genes in Mi-1-mediated and SA-induced resistance to root-knot nematodes. Mol. Plant Pathol. 2014, 15, 255–264. [Google Scholar] [CrossRef]
- Gautam, S.K.; Poddar, A.N. Study on protein and sugar content in Meloidogyne incognita infested roots of bitter gourd. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 470–478. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tzean, Y.; Wang, K.-T.; Gamboa Chen, E.; Wang, H.-W.; Wu, T.-M.; Liu, C.-A. Antioxidant Responses and Growth Impairment in Cucurbita moschata Infected by Meloidogyne incognita. Biology 2024, 13, 267. https://doi.org/10.3390/biology13040267
Tzean Y, Wang K-T, Gamboa Chen E, Wang H-W, Wu T-M, Liu C-A. Antioxidant Responses and Growth Impairment in Cucurbita moschata Infected by Meloidogyne incognita. Biology. 2024; 13(4):267. https://doi.org/10.3390/biology13040267
Chicago/Turabian StyleTzean, Yuh, Kuang-Teng Wang, Elena Gamboa Chen, Hung-Wen Wang, Tsung-Meng Wu, and Chia-An Liu. 2024. "Antioxidant Responses and Growth Impairment in Cucurbita moschata Infected by Meloidogyne incognita" Biology 13, no. 4: 267. https://doi.org/10.3390/biology13040267





