Reproductive Cessation and Post-Reproductive Lifespan in Honeybee Workers
Abstract
:Simple Summary
Abstract
1. Introduction
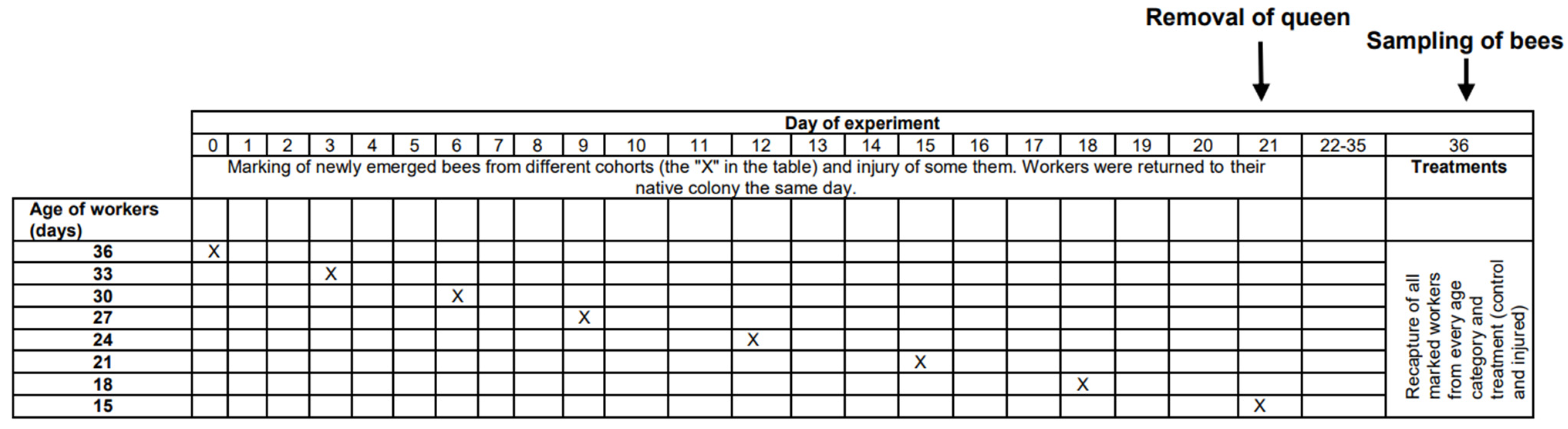
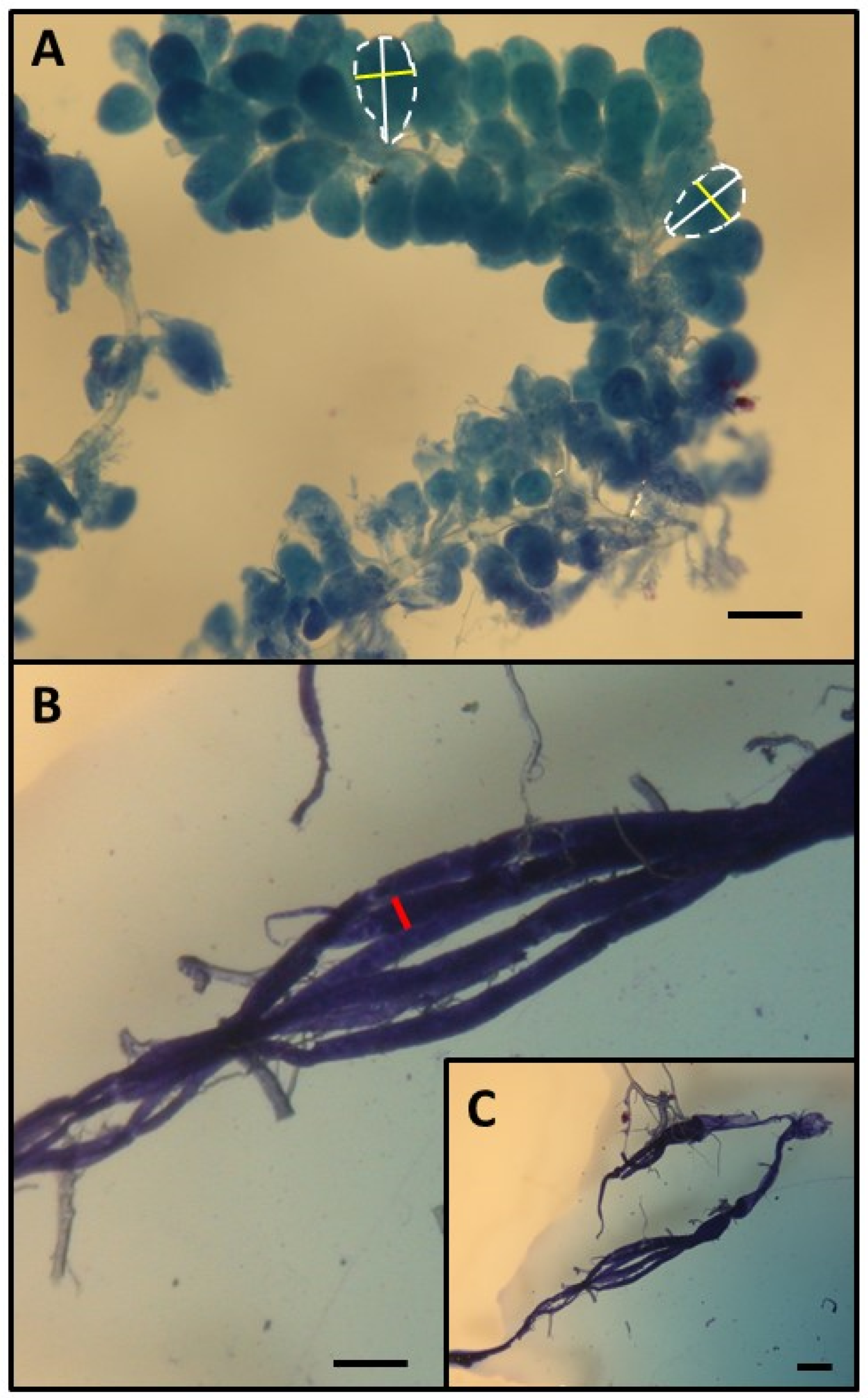
2. Methods
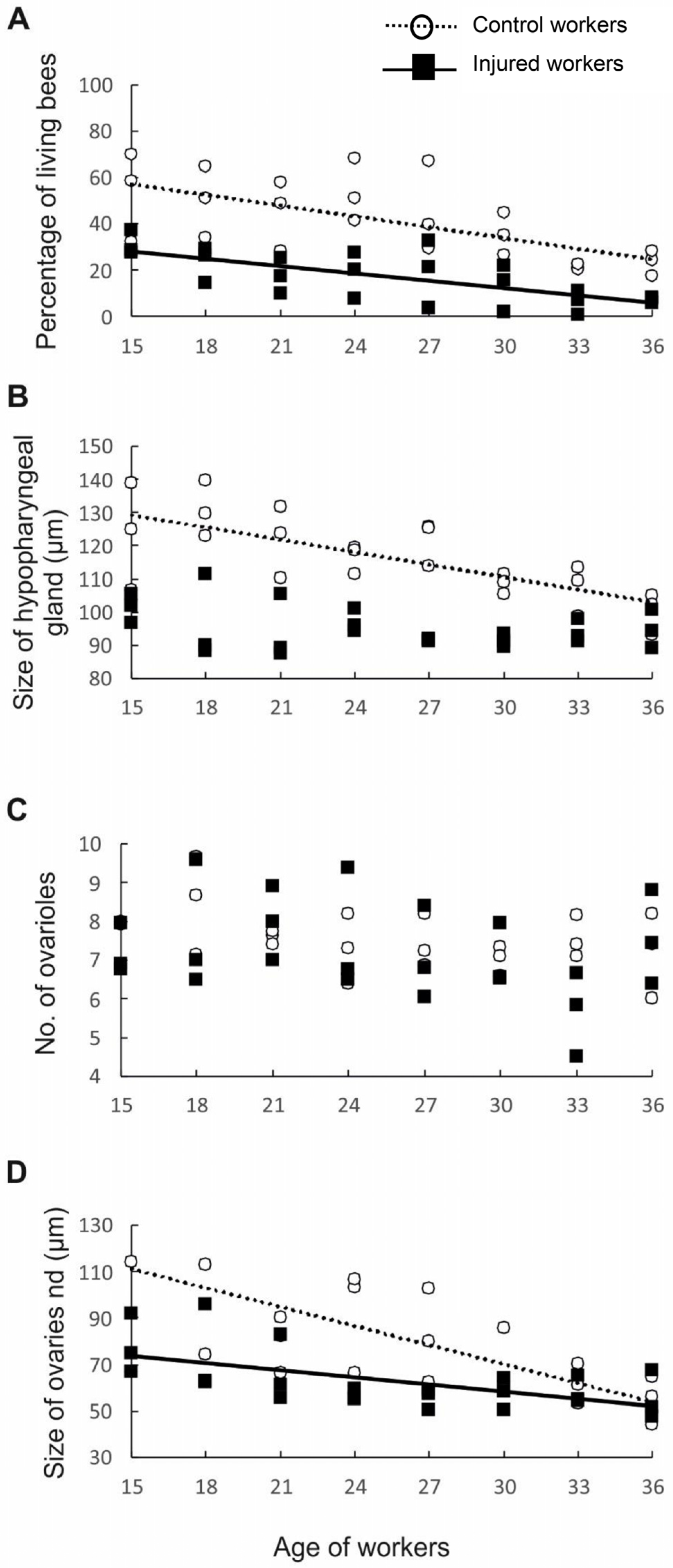
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shanley, D.P.; Kirkwood, T.B.L. Evolution of the Human Menopause. BioEssays 2001, 23, 282–287. [Google Scholar] [CrossRef]
- Lahdenperä, M.; Lummaa, V.; Helle, S.; Tremblay, M. Fitness Benefits of Prolonged Post-Reproductive Lifespan in Women. Nature 2004, 428, 2002–2005. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.L.; Herndon, J.G. Menopause in Nonhuman Primates? Biol. Reprod. 2008, 79, 398–406. [Google Scholar] [CrossRef]
- Ellis, S.; Franks, D.W.; Nattrass, S.; Cant, M.A.; Bradley, D.L.; Giles, D.; Balcomb, K.C.; Croft, D.P. Postreproductive Lifespans Are Rare in Mammals. Ecol. Evol. 2018, 8, 2482–2494. [Google Scholar] [CrossRef] [PubMed]
- Reznick, D.; Bryant, M.; Holmes, D. The Evolution of Senescence and Post-Reproductive Lifespan in Guppies (Poecilia reticulata). PLoS Biol. 2006, 4, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Uematsu, K.; Kutsukake, M.; Fukatsu, T.; Shimada, M.; Shibao, H. Altruistic Colony Defense by Menopausal Female Insects. Curr. Biol. 2010, 20, 1182–1186. [Google Scholar] [CrossRef] [PubMed]
- Tsuij, K.; Kikuchi, T. Determination of the Cost of Worker Reproduction via Diminished Life Span in the Ant diacamma sp. Evolution 2011, 66, 1322–1331. [Google Scholar] [CrossRef]
- Hamilton, W.D. The Genetical Evolution of Social Behaviour. II. J. Theor. Biol. 1964, 7, 17–52. [Google Scholar] [CrossRef] [PubMed]
- West Eberhard, M.J. The Evolution of Social Behavior by Kin Selection. Q. Rev. Biol. 1975, 50, 1–33. [Google Scholar]
- Winston, M. The Biology of Honeybee; Harvard University Press: Cambridge, MA, USA, 1987. [Google Scholar]
- Kraus, F.B.; Neumann, P.; Van Praagh, J.; Moritz, R.F.A. Sperm Limitation and the Evolution of Extreme Polyandry in Honeybees (Apis mellifera L.). Behav. Ecol. Sociobiol. 2004, 55, 494–501. [Google Scholar] [CrossRef]
- Schlüns, H.; Koeniger, G.; Koeniger, N.; Moritz, R.F.A. Sperm Utilization Pattern in the Honeybee (Apis mellifera). Behav. Ecol. Sociobiol. 2004, 56, 458–463. [Google Scholar] [CrossRef]
- Berg, S.; Koeniger, N.; Koeniger, G.; Fuchs, S. Body Size and Reproductive Success of Drones. Apidologie 1997, 28, 449–460. [Google Scholar] [CrossRef]
- Page, R.E.; Robinson, G.E. Reproductive Competition in Queenless Honey Bee Colonies (Apis mellifera L.). Behav. Ecol. Sociobiol. 1994, 35, 99–107. [Google Scholar] [CrossRef]
- Beekman, M.; Oldroyd, B.P. When Workers Disunite: Intraspecific Parasitism by Eusocial Bees. Annu. Rev. Entomol. 2008, 53, 19–37. [Google Scholar] [CrossRef] [PubMed]
- Mattila, H.R.; Reeve, H.K.; Smith, M.L. Promiscuous Honey Bee Queens Increase Colony Productivity by Suppressing Worker Selfishness. Curr. Biol. 2012, 22, 2027–2031. [Google Scholar] [CrossRef] [PubMed]
- Naeger, N.L.; Peso, M.; Even, N.; Barron, A.B.; Robinson, G.E. Altruistic Behavior by Egg-Laying Worker Honeybees. Curr. Biol. 2013, 23, 1574–1578. [Google Scholar] [CrossRef] [PubMed]
- Robinson, G.E.; Page, R.E.; Fondrk, M.K. Intracolonial Behavioral Variation in Worker Oviposition, Oophagy, and Larval Care in Queenless Honey Bee Colonies. Behav. Ecol. Sociobiol. 1990, 26, 315–323. [Google Scholar] [CrossRef]
- Beekman, M.; Calis, J.N.M.; Boot, W.J. Insect Behaviour: Parasitic Honeybee Get Royal Treatment. Nature 2000, 404, 723. [Google Scholar] [CrossRef] [PubMed]
- Makert, G.R.; Paxton, R.J.; Hartfelder, K. Ovariole Number—A Predictor of Differential Reproductive Success among Worker Subfamilies in Queenless Honeybee (Apis mellifera L.) Colonies. Behav. Ecol. Sociobiol. 2006, 60, 815–825. [Google Scholar] [CrossRef]
- Ratnieks, F.L.W. Egg-Laying, Egg-Removal, and Ovary Development by Workers in Queenright Honey Bee Colonies. Behav. Ecol. Sociobiol. 1993, 32, 191–198. [Google Scholar] [CrossRef]
- Scheiner, R.; Page, R.E.; Erber, J. The Effects of Genotype, Foraging Role, and Sucrose Responsiveness on the Tactile Learning Performance of Honey Bees (Apis mellifera L.). Neurobiol. Learn. Mem. 2001, 76, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Kuszewska, K.; Miler, K.; Rojek, W.; Woyciechowski, M. Honeybee Workers with Higher Reproductive Potential Live Longer Lives. Exp. Gerontol. 2017, 98, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Woyciechowski, M.; Moroń, D. Life Expectancy and Onset of Foraging in the Honeybee (Apis mellifera). Insectes Sociaux 2009, 56, 193–201. [Google Scholar] [CrossRef]
- Woyciechowski, M.; Kozłowski, J. Division of Labor by Division of Risk According to Worker Life Expectancy in the Honey Bee (Apis mellifera L.). Apidologie 1998, 29, 109–205. [Google Scholar] [CrossRef]
- Kuszewska, K.; Woyciechowski, M. Reversion in Honeybee, Apis mellifera, Workers with Different Life Expectancies. Anim. Behav. 2013, 85, 247–253. [Google Scholar] [CrossRef]
- Woyciechowski, M.; Kuszewska, K. Swarming Generates Rebel Workers in Honeybees. Curr. Biol. 2012, 22, 707–711. [Google Scholar] [CrossRef] [PubMed]
- Kuszewska, K.; Miler, K.; Woyciechowski, M. Honeybee Rebel Workers Preferentially Respond to High Concentrations of Sucrose. Apidologie 2019, 50, 253–261. [Google Scholar] [CrossRef]
- Moroń, D.; Lenda, M.; Skórka, P.; Woyciechowski, M. Short-Lived Ants Take Greater Risks during Food Collection. Am. Nat. 2012, 180, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Velthuis, H.H.W. Ovarian Development in Apis Mellifera Worker Bees. Entomol. Exp. Appl. 1970, 13, 377–394. [Google Scholar] [CrossRef]
- Galbraith, D.A.; Wang, Y.; Amdam, G.V.; Page, R.E.; Grozinger, C.M. Reproductive Physiology Mediates Honey Bee (Apis mellifera) Worker Responses to Social Cues. Behav. Ecol. Sociobiol. 2015, 69, 1511–1518. [Google Scholar] [CrossRef]
- Nakaoka, T.; Takeuchi, H.; Kubo, T. Laying Workers in Queenless Honeybee (Apis mellifera L.) Colonies Have Physiological States Similar to That of Nurse Bees but Opposite That of Foragers. J. Insect Physiol. 2008, 54, 806–812. [Google Scholar] [CrossRef] [PubMed]
- Amdam, G.V.; Csondes, A.; Fondrk, M.K.; Page, R.E. Complex Social Behaviour Derived from Maternal Reproductive Traits. Nature 2006, 439, 76–78. [Google Scholar] [CrossRef] [PubMed]
- Kuszewska, K.; Woyciechowski, M. Risky Robbing Is a Job for Short-Lived and Infected Worker Honeybees. Apidologie 2014, 45, 537–544. [Google Scholar] [CrossRef]
- Li-Byarlay, H.; Cleare, X.L. Current Trends in the Oxidative Stress and Ageing of Social Hymenopterans. In Advances in Insect Physiology; Elsevier: Amsterdam, The Netherlands, 2020; Volume 59, pp. 43–69. ISBN 978-0-12-820367-5. [Google Scholar]
- Cervoni, M.S.; Cardoso-Júnior, C.A.M.; Craveiro, G.; Souza, A.D.O.; Alberici, L.C.; Hartfelder, K. Mitochondrial Capacity, Oxidative Damage and Hypoxia Gene Expression Are Associated with Age-Related Division of Labor in Honey Bee, Apis Mellifera L., Workers. J. Exp. Biol. 2017, 220 Pt 2, 4035–4046. [Google Scholar] [CrossRef] [PubMed]
- Dziechciarz, P.; Strachecka, A.; Borsuk, G.; Olszewski, K. Effect of Rearing in Small-Cell Combs on Activities of Catalase and Superoxide Dismutase and Total Antioxidant Capacity in the Hemolymph of Apis Mellifera Workers. Antioxidants 2023, 12, 709. [Google Scholar] [CrossRef] [PubMed]
- Yazlovytska, L.S.; Karavan, V.V.; Domaciuk, M.; Panchuk, I.I.; Borsuk, G.; Volkov, R.A. Increased Survival of Honey Bees Consuming Pollen and Beebread Is Associated with Elevated Biomarkers of Oxidative Stress. Front. Ecol. Evol. 2023, 11, 1098350. [Google Scholar] [CrossRef]
- Corona, M.; Robinson, G.E. Genes of the Antioxidant System of the Honey Bee: Annotation and Phylogeny. Insect Mol. Biol. 2006, 15, 687–701. [Google Scholar] [CrossRef] [PubMed]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Otis, G. Factors Determining Hypopharyngeal Gland Activity of Worker Honey Bees (Apis mellifera L.). Insectes Sociaux 1989, 36, 264–276. [Google Scholar] [CrossRef]
- Huang, Z.; Robinson, G. Regulation of Honey Bee Division of Labor by Colony Age Demography. Behav. Ecol. Sociobiol. 1996, 39, 147–158. [Google Scholar] [CrossRef]
- Huang, Z.Y.; Robinson1, G.E.; Borst, D.W. Physiological Correlates of Division of Labor among Similarly Aged Honey Bees. J. Comp. Physiol. A 1994, 174, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Woyciechowski, M.; Kuszewska, K.; Pitorak, J.; Kierat, J. Honeybee Worker Larvae Perceive Queen Pheromones in Their Food. Apidologie 2017, 48, 144–149. [Google Scholar] [CrossRef]
- Ronai, I.; Allsopp, M.H.; Tan, K.; Dong, S.; Liu, X.; Vergoz, V.; Oldroyd, B.P. The Dynamic Association between Ovariole Loss and Sterility in Adult Honeybee Workers. Proc. R. Soc. B Biol. Sci. 2017, 284, 20162693. [Google Scholar] [CrossRef] [PubMed]
- de Groot, A.P.; Voogd, S. On the Ovary Development in Queenless Worker Bees (Apis mellifica L.). Experientia 1954, 10, 384–385. [Google Scholar] [CrossRef] [PubMed]
- Jaycox, E.R. Honey Bee Queen Pheromones and Worker Foraging Behavior. Ann. Entomol. Soc. Am. 1970, 63, 222–228. [Google Scholar] [CrossRef]
- Abou-Shaara, H.F. The Foraging Behaviour of Honey Bees, Apis Mellifera: A Review. Vet. Med. 2014, 59, 1–10. [Google Scholar] [CrossRef]
- Tsuji, K. Reproductive Division of Labour Related to Age in the Japanese Queenless Ant, Pristomyrmex pungens. Anim. Behav. 1990, 39, 843–849. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuszewska, K.; Woloszczuk, A.; Woyciechowski, M. Reproductive Cessation and Post-Reproductive Lifespan in Honeybee Workers. Biology 2024, 13, 287. https://doi.org/10.3390/biology13050287
Kuszewska K, Woloszczuk A, Woyciechowski M. Reproductive Cessation and Post-Reproductive Lifespan in Honeybee Workers. Biology. 2024; 13(5):287. https://doi.org/10.3390/biology13050287
Chicago/Turabian StyleKuszewska, Karolina, Anna Woloszczuk, and Michal Woyciechowski. 2024. "Reproductive Cessation and Post-Reproductive Lifespan in Honeybee Workers" Biology 13, no. 5: 287. https://doi.org/10.3390/biology13050287





