Microbial Competition in Polar Soils: A Review of an Understudied but Potentially Important Control on Productivity
Abstract
:1. Introduction
1.1. Microbial Diversity and Productivity
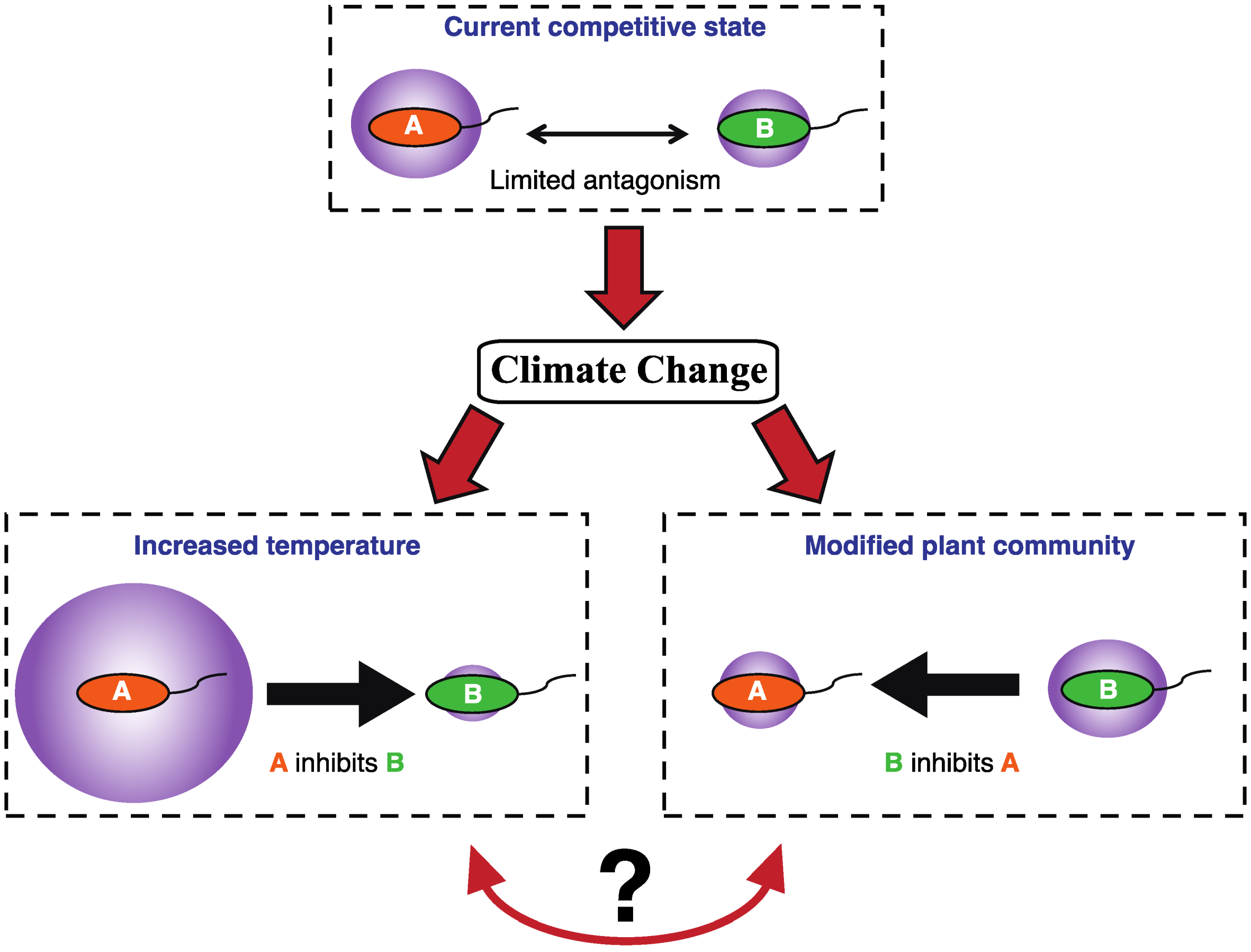
1.2. Microbial Competition in Polar Soils
| Habitat | Antagonists | Function(s) affected | Proposed mechanism(s) of competition | * Special notes | Reference |
|---|---|---|---|---|---|
| In vitro | |||||
| Moss-covered and barren soil in Svalbard, Norway | Actinobacteria (Arthrobacter), Gammaproteobacteria (Pseudomonas), Firmicutes (Paenibacillus), Bacteroidetes (Flavobacterium) | Growth of individual strains | Antimicrobial production; differential growth rates | Competition varied at different incubation temperatures | [29] |
| Various Antarctic soils | Antimicrobial producers: Actinobacteria (Arthrobacter), Firmicutes (Planococcus), Gammaproteobacteria (Pseudomonas); Affected: Firmicutes (Listeria, Staphylococcus, Brocothrix), Gammaproteobacteria (Salmonella, Escherichia, Pseudomonas) | Growth of individual strains | Antimicrobial production | Producers were Antarctic bacteria, while affected bacteria were food-borne pathogens | [11] |
| King George Island, Antarctica | Antimicrobial producers:Bacteroidetes (Pedobacter), Gammaproteobacteria (Pseudomonas); Affected:Gammaproteobacteria (Salmonella, Escherichia, Klebsiella, Enterobacter, Vibrio), Firmicutes (Bacillus) | Growth of individual strains | Antimicrobial production | Producers were Antarctic bacteria, while affected bacteria were food-borne pathogens | [30] |
| Tundra wetland soil, Ural, Russia | Methanogens and homoacetogenic Firmicutes (Acetobacterium) | H2 consumption | Differential H2 affinity | Competition was modeled based on changing H2 affinities at various temperatures; some strains isolated from pond and fen sediments | [31] |
| In situ | |||||
| Unvegetated contaminated soil in Alert, Nunavut, Canada | Alpha-, Beta-, Gammaproteobacteria, Actinobacteria | Assimilation of added monoammonium phosphate | Differential nutrient uptake | Alphaproteobacteria most effectively assimilated added nutrients | [32] |
| Soil microcosms | |||||
| Lowland soil, Devon Island, Nunavut, Canada | Archaeal and bacterial nitrifiers, fungal and bacterial denitrifiers | N2O production, nitrate availability, biomass of microbial domains | Differential nutrient uptake | Effects varied with temperature | [33] |
2. Factors Influencing the Relative Success of Polar Microorganisms
2.1. Environmental Factors
2.2. Biotic Interactions
3. Important Microbial Functions Potentially Affected by Competition in Polar Soils
3.1. Greenhouse Gas Flux
3.2. Biodegradation
3.3. Plant Productivity
3.4. Nutrient Cycling
4. The Effects of Environmental Change on Competition
5. Studying Competition in Natural Communities
6. Conclusions
- Polar soils contain large stores of organic material and nutrients. The extent to which microbial competition can limit rates of decomposition and nutrient cycling will affect climate change predictions and future management plans.
- By purposefully altering the soil environment, microbial competition may be either increased or reduced, possibly opening biotechnological opportunities such as enhanced bioremediation.
- Microbial composition and activity also affect the activity and growth of other organisms such as plants, and vice versa. Competition between these groups is also likely to affect the composition and functioning of each.
Acknowledgements
References
- Connell, J.H. The influence of interspecific competition and other factors on the distribution of the barnacle Chthamalus stellatus. Ecology 1961, 42, 710–723. [Google Scholar] [CrossRef]
- Huisman, J.; Weissing, F.J. Biodiversity of plankton by species oscillations and chaos. Nature 1999, 402, 407–410. [Google Scholar] [CrossRef]
- Van Nes, E.H.; Scheffer, M. Large species shifts triggered by small forces. Am. Nat. 2004, 164, 255–266. [Google Scholar] [CrossRef]
- Beninca, E.; Huisman, J.; Heerkloss, R.; Johnk, K.D.; Branco, P.; van Nes, E.H.; Scheffer, M.; Ellner, S.P. Chaos in a long-term experiment with a plankton community. Nature 2008, 451, 822–825. [Google Scholar] [CrossRef]
- Deslippe, J.R.; Hartmann, M.; Simard, S.W.; Mohn, W.W. Long-term warming alters the composition of arctic soil microbial communities. FEMS Microbiol. Ecol. 2012, 82, 303–315. [Google Scholar]
- Yergeau, E.; Bokhorst, S.; Kang, S.; Zhou, J.Z.; Greer, C.W.; Aerts, R.; Kowalchuk, G.A. Shifts in soil microorganisms in response to warming are consistent across a range of antarctic environments. ISME J. 2012, 6, 692–702. [Google Scholar] [CrossRef]
- Barrett, L.G.; Bell, T.; Dwyer, G.; Bergelson, J. Cheating, trade-offs and the evolution of aggressiveness in a natural pathogen population. Ecol. Lett. 2011, 14, 1149–1157. [Google Scholar] [CrossRef]
- Kreth, J.; Merritt, J.; Shi, W.Y.; Qi, F.X. Competition and coexistence between Streptococcus mutans and Streptococcus sanguinis in the dental biofilm. J. Bacteriol. 2005, 187, 7193–7203. [Google Scholar] [CrossRef]
- Lopez-Garcia, S.L.; Vazquez, T.E.E.; Favelukes, G.; Lodeiro, A.R. Rhizobial position as a main determinant in the problem of competition for nodulation in soybean. Environ. Microbiol. 2002, 4, 216–224. [Google Scholar] [CrossRef]
- van Elsas, J.D.; Chiurazzi, M.; Mallon, C.A.; Elhottova, D.; Kristufek, V.; Salles, J.F. Microbial diversity determines the invasion of soil by a bacterial pathogen. Proc. Natl. Acad. Sci. USA 2012, 109, 1159–1164. [Google Scholar]
- O'Brien, A.; Sharp, R.; Russell, N.J.; Roller, S. Antarctic bacteria inhibit growth of food-borne microorganisms at low temperatures. FEMS Microbiol. Ecol. 2004, 48, 157–167. [Google Scholar]
- Bell, T.H.; Yergeau, E.; Juck, D.; Whyte, L.G.; Greer, C.W. Alteration of microbial community structure affects diesel degradation in an arctic soil. FEMS Microbiol. Ecol. 2013, in press. [Google Scholar]
- Bullock, J.M.; Pywell, R.F.; Burke, M.J.W.; Walker, K.J. Restoration of biodiversity enhances agricultural production. Ecol. Lett. 2001, 4, 185–189. [Google Scholar] [CrossRef]
- Doherty, J.M.; Callaway, J.C.; Zedler, J.B. Diversity-function relationships changed in a long-term restoration experiment. Ecol. Appl. 2011, 21, 2143–2155. [Google Scholar] [CrossRef]
- Fargione, J.; Tilman, D.; Dybzinski, R.; Lambers, J.H.; Clark, C.; Harpole, W.S.; Knops, J.M.H.; Reich, P.B.; Loreau, M. From selection to complementarity: Shifts in the causes of biodiversity-productivity relationships in a long-term biodiversity experiment. Proc. Roy. Soc. B 2007, 274, 871–876. [Google Scholar] [CrossRef]
- Foster, K.R.; Bell, T. Competition, not cooperation, dominates interactions among culturable microbial species. Curr. Biol. 2012, 22, 1845–1850. [Google Scholar] [CrossRef]
- Peter, H.; Beier, S.; Bertilsson, S.; Lindström, E.S.; Langenheder, S.; Tranvik, L.J. Function-specific response to depletion of microbial diversity. ISME J. 2011, 5, 351–361. [Google Scholar] [CrossRef]
- Salles, J.F.; Poly, F.; Schmid, B.; Le Roux, X. Community niche predicts the functioning of denitrifying bacterial assemblages. Ecology 2009, 90, 3324–3332. [Google Scholar] [CrossRef]
- Strickland, M.S.; Lauber, C.; Fierer, N.; Bradford, M.A. Testing the functional significance of microbial community composition. Ecology 2009, 90, 441–451. [Google Scholar] [CrossRef]
- Degens, B.P. Decreases in microbial functional diversity do not result in corresponding changes in decomposition under different moisture conditions. Soil Biol. Biochem. 1998, 30, 1989–2000. [Google Scholar] [CrossRef]
- Griffiths, B.S.; Ritz, K.; Bardgett, R.D.; Cook, R.; Christensen, S.; Ekelund, F.; Sørensen, S.J.; Bååth, E.; Bloem, J.; de Ruiter, P.C.; et al. Ecosystem response of pasture soil communities to fumigation-induced microbial diversity reductions: An examination of the biodiversity-ecosystem function relationship. Oikos 2000, 90, 279–294. [Google Scholar]
- Fournier, G.; Fournier, J.C. Effect of microbial competition on the survival and activity of 2,4-d-degrading Alcaligenes xylosoxidans subsp. Denitrificans added to soil. Lett. Appl. Microbiol. 1993, 16, 178–181. [Google Scholar] [CrossRef]
- Hibbing, M.E.; Fuqua, C.; Parsek, M.R.; Peterson, S.B. Bacterial competition: Surviving and thriving in the microbial jungle. Nat. Rev. Microbiol. 2010, 8, 15–25. [Google Scholar] [CrossRef]
- Little, A.E.F.; Robinson, C.J.; Peterson, S.B.; Raffa, K.E.; Handelsman, J. Rules of engagement: Interspecies interactions that regulate microbial communities. Annu. Rev. Microbiol. 2008, 62, 375–401. [Google Scholar] [CrossRef]
- Roesch, L.F.; Fulthorpe, R.R.; Riva, A.; Casella, G.; Hadwin, A.K.M.; Kent, A.D.; Daroub, S.H.; Camargo, F.A.O.; Farmerie, W.G.; Triplett, E.W. Pyrosequencing enumerates and contrasts soil microbial diversity. ISME J. 2007, 1, 283–290. [Google Scholar]
- Chu, H.Y.; Fierer, N.; Lauber, C.L.; Caporaso, J.G.; Knight, R.; Grogan, P. Soil bacterial diversity in the arctic is not fundamentally different from that found in other biomes. Environ. Microbiol. 2010, 12, 2998–3006. [Google Scholar] [CrossRef]
- Neufeld, J.D.; Mohn, W.W. Unexpectedly high bacterial diversity in arctic tundra relative to boreal forest soils, revealed by serial analysis of ribosomal sequence tags. Appl. Environ. Microb. 2005, 71, 5710–5718. [Google Scholar] [CrossRef]
- McMahon, S.K.; Wallenstein, M.D.; Schimel, J.P. A cross-seasonal comparison of active and total bacterial community composition in arctic tundra soil using bromodeoxyuridine labeling. Soil Biol. Biochem. 2011, 43, 287–295. [Google Scholar] [CrossRef]
- Prasad, S.; Manasa, P.; Buddhi, S.; Singh, S.M.; Shivaji, S. Antagonistic interaction networks among bacteria from a cold soil environment. FEMS Microbiol. Ecol. 2011, 78, 376–385. [Google Scholar] [CrossRef]
- Wong, C.M.V.L.; Tam, H.K.; Alias, S.A.; Gonzalez, M.; Gonzalez-Rocha, G.; Dominguez-Yevenes, M. Pseudomonas and pedobacter isolates from king george island inhibited the growth of foodborne pathogens. Pol. Polar Res. 2011, 32, 3–14. [Google Scholar]
- Kotsyurbenko, O.R.; Glagolev, M.V.; Nozhevnikova, A.N.; Conrad, R. Competition between homoacetogenic bacteria and methanogenic archaea for hydrogen at low temperature. FEMS Microbiol. Ecol. 2001, 38, 153–159. [Google Scholar]
- Bell, T.H.; Yergeau, E.; Martineau, C.; Juck, D.; Whyte, L.G.; Greer, C.W. Identification of nitrogen-incorporating bacteria in petroleum-contaminated arctic soils by using [15n]DNA-based stable isotope probing and pyrosequencing. Appl. Environ. Microb. 2011, 77, 4163–4171. [Google Scholar] [CrossRef]
- Siciliano, S.D.; Ma, W.K.; Ferguson, S.; Farrell, R.E. Nitrifier dominance of arctic soil nitrous oxide emissions arises due to fungal competition with denitrifiers for nitrate. Soil Biol. Biochem. 2009, 41, 1104–1110. [Google Scholar] [CrossRef]
- Steven, B.; Niederberger, T.D.; Bottos, E.M.; Dyen, M.R.; Whyte, L.G. Development of a sensitive radiorespiration method for detecting microbial activity at subzero temperatures. J. Microbiol. Methods 2007, 71, 275–280. [Google Scholar] [CrossRef]
- D’Amico, S.; Collins, T.; Marx, J.C.; Feller, G.; Gerday, C. Psychrophilic microorganisms: Challenges for life. EMBO Rep. 2006, 7, 385–389. [Google Scholar] [CrossRef]
- Fierer, N.; Jackson, R.B. The diversity and biogeography of soil bacterial communities. Proc. Natl. Acad. Sci. USA 2006, 103, 626–631. [Google Scholar] [CrossRef]
- Yergeau, E.; Schoondermark-Stolk, S.A.; Brodie, E.L.; Dejean, S.; DeSantis, T.Z.; Goncalves, O.; Piceno, Y.M.; Andersen, G.L.; Kowalchuk, G.A. Environmental microarray analyses of antarctic soil microbial communities. ISME J. 2009, 3, 340–351. [Google Scholar] [CrossRef] [Green Version]
- Chong, C.W.; Pearce, D.A.; Convey, P.; Tan, I.K.P. The identification of environmental parameters which could influence soil bacterial community composition on the antarctic peninsula: A statistical approach. Antarct Sci. 2012, 24, 249–258. [Google Scholar] [CrossRef]
- Mannisto, M.K.; Tiirola, M.; Haggblom, M.M. Bacterial communities in arctic fjelds of finnish lapland are stable but highly ph-dependent. FEMS Microbiol. Ecol. 2007, 59, 452–465. [Google Scholar] [CrossRef]
- Ganzert, L.; Lipski, A.; Hubberten, H.W.; Wagner, D. The impact of different soil parameters on the community structure of dominant bacteria from nine different soils located on livingston island, south shetland archipelago, antarctica. FEMS Microbiol. Ecol. 2011, 76, 476–491. [Google Scholar] [CrossRef] [Green Version]
- Bell, T.H.; Yergeau, E.; Maynard, C.; Juck, D.; Whyte, L.G.; Greer, C.W. Predictable bacterial composition and hydrocarbon degradation in arctic soils following diesel and nutrient disturbance. ISME J. 2013. [Google Scholar] [CrossRef]
- Dennis, P.G.; Rushton, S.P.; Newsham, K.K.; Lauducina, V.A.; Ord, V.J.; Daniell, T.J.; O'Donnell, A.G.; Hopkins, D.W. Soil fungal community composition does not alter along a latitudinal gradient through the maritime and sub-antarctic. Fungal Ecol. 2012, 5, 403–408. [Google Scholar] [CrossRef]
- Fujimura, K.E.; Egger, K.N. Host plant and environment influence community assembly of high arctic root-associated fungal communities. Fungal Ecol. 2012, 5, 409–418. [Google Scholar] [CrossRef]
- Arenz, B.E.; Blanchette, R.A. Distribution and abundance of soil fungi in antarctica at sites on the peninsula, ross sea region and mcmurdo dry valleys. Soil Biol. Biochem. 2011, 43, 308–315. [Google Scholar] [CrossRef]
- Powell, S.M.; Bowman, J.P.; Ferguson, S.H.; Snape, I. The importance of soil characteristics to the structure of alkane-degrading bacterial communities on sub-antarctic macquarie island. Soil Biol. Biochem. 2010, 42, 2012–2021. [Google Scholar] [CrossRef]
- Ramirez, K.S.; Craine, J.M.; Fierer, N. Consistent effects of nitrogen amendments on soil microbial communities and processes across biomes. Glob. Change Biol. 2012, 18, 1918–1927. [Google Scholar] [CrossRef]
- Campbell, B.J.; Polson, S.W.; Hanson, T.E.; Mack, M.C.; Schuur, E.A.G. The effect of nutrient deposition on bacterial communities in arctic tundra soil. Environ. Microbiol. 2010, 12, 1842–1854. [Google Scholar] [CrossRef]
- Urcelay, C.; Bret-Harte, M.S.; Diaz, S.; Chapin, F.S. Mycorrhizal colonization mediated by species interactions in arctic tundra. Oecologia 2003, 137, 399–404. [Google Scholar] [CrossRef]
- Robinson, C.H.; Saunders, P.W.; Madan, N.J.; Pryce-Miller, E.J.; Pentecost, A. Does nitrogen deposition affect soil microfungal diversity and soil n and p dynamics in a high arctic ecosystem? Glob. Change Biol. 2004, 10, 1065–1079. [Google Scholar] [CrossRef]
- Stomeo, F.; Makhalanyane, T.P.; Valverde, A.; Pointing, S.B.; Stevens, M.I.; Cary, C.S.; Tuffin, M.I.; Cowan, D.A. Abiotic factors influence microbial diversity in permanently cold soil horizons of a maritime-associated antarctic dry valley. FEMS Microbiol. Ecol. 2012, 82, 326–340. [Google Scholar] [CrossRef]
- Hoj, L.; Rusten, M.; Haugen, L.E.; Olsen, R.A.; Torsvik, V.L. Effects of water regime on archaeal community composition in arctic soils. Environ. Microbiol. 2006, 8, 984–996. [Google Scholar] [CrossRef]
- Fell, J.W.; Scorzetti, G.; Connell, L.; Craig, S. Biodiversity of micro-eukaryotes in antarctic dry valley soils with <5% soil moisture. Soil Biol. Biochem. 2006, 38, 3107–3119. [Google Scholar] [CrossRef]
- Bridge, P.D.; Newsham, K.K. Soil fungal community composition at mars oasis, a southern maritime antarctic site, assessed by pcr amplification and cloning. Fungal Ecol. 2009, 2, 66–74. [Google Scholar] [CrossRef]
- Liebner, S.; Harder, J.; Wagner, D. Bacterial diversity and community structure in polygonal tundra soils from samoylov island, lena delta, siberia. Int. Microbiol. 2008, 11, 195–202. [Google Scholar]
- Aislabie, J.M.; Jordan, S.; Barker, G.M. Relation between soil classification and bacterial diversity in soils of the ross sea region, antarctica. Geoderma 2008, 144, 9–20. [Google Scholar] [CrossRef]
- Tosi, S.; Onofri, S.; Brusoni, M.; Zucconi, L.; Vishniac, H. Response of antarctic soil fungal assemblages to experimental warming and reduction of uv radiation. Polar Biol. 2005, 28, 470–482. [Google Scholar] [CrossRef]
- Feller, G.; Gerday, C. Psychrophilic enzymes: Hot topics in cold adaptation. Nat. Rev. Microbiol. 2003, 1, 200–208. [Google Scholar] [CrossRef]
- Cavicchioli, R. Cold-adapted archaea. Nat. Rev. Microbiol. 2006, 4, 331–343. [Google Scholar] [CrossRef]
- Harder, W.; Veldkamp, H. Competition of marine psychrophilic bacteria at low temperatures. Antonie Van Leeuwenhoek 1971, 37, 51–63. [Google Scholar] [CrossRef]
- Nedwell, D.B.; Rutter, M. Influence of temperature on growth rate and competition between two psychrotolerant antarctic bacteria: Low temperature diminishes affinity for substrate uptake. Appl. Environ. Microb. 1994, 60, 1984–1992. [Google Scholar]
- Knoblauch, C.; Jorgensen, B.B. Effect of temperature on sulphate reduction, growth rate and growth yield in five psychrophilic sulphate-reducing bacteria from arctic sediments. Environ. Microbiol. 1999, 1, 457–467. [Google Scholar] [CrossRef]
- Margesin, R. Effect of temperature on growth parameters of psychrophilic bacteria and yeasts. Extremophiles 2009, 13, 257–262. [Google Scholar] [CrossRef]
- Hillebrand, H. On the generality of the latitudinal diversity gradient. Am. Nat. 2004, 163, 192–211. [Google Scholar] [CrossRef] [Green Version]
- Hogg, I.D.; Cary, S.C.; Convey, P.; Newsham, K.K.; O’Donnell, A.G.; Adams, B.J.; Aislabie, J.; Frati, F.; Stevens, M.I.; Wall, D.H. Biotic interactions in antarctic terrestrial ecosystems: Are they a factor? Soil Biol. Biochem. 2006, 38, 3035–3040. [Google Scholar] [CrossRef]
- Teixeira, L.C.R.S.; Peixoto, R.S.; Cury, J.C.; Sul, W.J.; Pellizari, V.H.; Tiedje, J.; Rosado, A.S. Bacterial diversity in rhizosphere soil from antarctic vascular plants of admiralty bay, maritime antarctica. ISME J. 2010, 4, 989–1001. [Google Scholar] [CrossRef]
- Allen, B.; Willner, D.; Oechel, W.C.; Lipson, D. Top-down control of microbial activity and biomass in an arctic soil ecosystem. Environ. Microbiol. 2010, 12, 642–648. [Google Scholar] [CrossRef]
- Newsham, K.K.; Rolf, J.; Pearce, D.A.; Strachan, R.J. Differing preferences of antarctic soil nematodes for microbial prey. Eur. J. Soil Biol. 2004, 40, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Williamson, L.L.; Borlee, B.R.; Schloss, P.D.; Guan, C.H.; Allen, H.K.; Handelsman, J. Intracellular screen to identify metagenomic clones that induce or inhibit a quorum-sensing biosensor. Appl. Environ. Microb. 2005, 71, 6335–6344. [Google Scholar] [CrossRef]
- Deming, J.W. Psychrophiles and polar regions. Curr. Opin. Microbiol. 2002, 5, 301–309. [Google Scholar] [CrossRef]
- Lifshitz, R.; Kloepper, J.W.; Scher, F.M.; Tipping, E.M.; Laliberte, M. Nitrogen-fixing pseudomonads isolated from roots of plants grown in the canadian high arctic. Appl. Environ. Microb. 1986, 51, 251–255. [Google Scholar]
- D’Costa, V.M.; King, C.E.; Kalan, L.; Morar, M.; Sung, W.W.L.; Schwarz, C.; Froese, D.; Zazula, G.; Calmels, F.; Debruyne, R.; et al. Antibiotic resistance is ancient. Nature 2011, 477, 457–461. [Google Scholar]
- Ma, Y.F.; Wang, L.; Shao, Z.Z. Pseudomonas, the dominant polycyclic aromatic hydrocarbon-degrading bacteria isolated from antarctic soils and the role of large plasmids in horizontal gene transfer. Environ. Microbiol. 2006, 8, 455–465. [Google Scholar] [CrossRef]
- Martinez-Rosales, C.; Fullana, N.; Musto, H.; Castro-Sowinski, S. Antarctic DNA moving forward: Genomic plasticity and biotechnological potential. FEMS Microbiol. Lett. 2012, 331, 1–9. [Google Scholar] [CrossRef]
- Fujiyoshi, M.; Yoshitake, S.; Watanabe, K.; Murota, K.; Tsuchiya, Y.; Uchida, M.; Nakatsubo, T. Successional changes in ectomycorrhizal fungi associated with the polar willow salix polaris in a deglaciated area in the high arctic, svalbard. Polar Biol. 2011, 34, 667–673. [Google Scholar] [CrossRef]
- Sundqvist, M.K.; Giesler, R.; Graae, B.J.; Wallander, H.; Fogelberg, E.; Wardle, D.A. Interactive effects of vegetation type and elevation on aboveground and belowground properties in a subarctic tundra. Oikos 2011, 120, 128–142. [Google Scholar] [CrossRef]
- Deslippe, J.R.; Simard, S.W. Below-ground carbon transfer among betula nana may increase with warming in arctic tundra. New Phytol. 2011, 192, 689–698. [Google Scholar] [CrossRef]
- Chu, H.Y.; Neufeld, J.D.; Walker, V.K.; Grogan, P. The influence of vegetation type on the dominant soil bacteria, archaea, and fungi in a low arctic tundra landscape. Soil Sci. Soc. Am. J. 2011, 75, 1756–1765. [Google Scholar] [CrossRef]
- Reed, H.E.; Martiny, J.B.H. Testing the functional significance of microbial composition in natural communities. FEMS Microbiol. Ecol. 2007, 62, 161–170. [Google Scholar] [CrossRef]
- Singh, B.K.; Bardgett, R.D.; Smith, P.; Reay, D.S. Microorganisms and climate change: Terrestrial feedbacks and mitigation options. Nat. Rev. Microbiol. 2010, 8, 779–790. [Google Scholar] [CrossRef]
- Wagner, D.; Liebner, S. Global warming and carbon dynamics in permafrost soils: Methane production and oxidation. In Permafrost Soils; Margesin, R., Ed.; Springer Berlin Heidelberg: Berlin, Heidelberg, Germany, 2009; pp. 219–236. [Google Scholar]
- Wagner, D.; Gattinger, A.; Embacher, A.; Pfeiffer, E.M.; Schloter, M.; Lipski, A. Methanogenic activity and biomass in holocene permafrost deposits of the lena delta, siberian arctic and its implication for the global methane budge. Glob. Change Biol. 2007, 13, 1089–1099. [Google Scholar] [CrossRef]
- Ho, A.; Luke, C.; Frenzel, P. Recovery of methanotrophs from disturbance: Population dynamics, evenness and functioning. ISME J. 2011, 5, 750–758. [Google Scholar] [CrossRef]
- Martineau, C.; Whyte, L.G.; Greer, C.W. Stable isotope probing analysis of the diversity and activity of methanotrophic bacteria in soils from the canadian high arctic. Appl. Environ. Microb. 2010, 76, 5773–5784. [Google Scholar] [CrossRef]
- Achtnich, C.; Bak, F.; Conrad, R. Competition for electron donors among nitrate reducers, ferric iron reducers, sulfate reducers, and methanogens in anoxic paddy soil. Biol. Fertil. Soils 1995, 19, 65–72. [Google Scholar] [CrossRef]
- Stibal, M.; Wadham, J.L.; Lis, G.P.; Telling, J.; Pancost, R.D.; Dubnick, A.; Sharp, M.J.; Lawson, E.C.; Butler, C.E.H.; Hasan, F.; et al. Methanogenic potential of arctic and antarctic subglacial environments with contrasting organic carbon sources. Glob. Change Biol. 2012, 18, 3332–3345. [Google Scholar] [CrossRef]
- IPCC, Climate Change 2007: The Physical Science Basis; Cambridge University Press: Cambridge, UK, 2007.
- Wrage, N.; Velthof, G.L.; van Beusichem, M.L.; Oenema, O. Role of nitrifier denitrification in the production of nitrous oxide. Soil Biol. Biochem. 2001, 33, 1723–1732. [Google Scholar] [CrossRef]
- Fierer, N.; Bradford, M.A.; Jackson, R.B. Toward an ecological classification of soil bacteria. Ecology 2007, 88, 1354–1364. [Google Scholar] [CrossRef]
- Tarnocai, C.; Canadell, J.G.; Schuur, E.A.G.; Kuhry, P.; Mazhitova, G.; Zimov, S. Soil organic carbon pools in the northern circumpolar permafrost region. Glob. Biogeochem. Cycles 2009, 23. [Google Scholar] [CrossRef]
- Tveit, A.; Schwacke, R.; Svenning, M.M.; Urich, T. Organic carbon transformations in high-arctic peat soil: Key functions and microorganisms. ISME J. 2013, 7, 299–311. [Google Scholar] [CrossRef]
- Meidute, S.; Demoling, F.; Bååth, E. Antagonistic and synergistic effects of fungal and bacterial growth in soil after adding different carbon and nitrogen sources. Soil Biol. Biochem. 2008, 40, 2334–2343. [Google Scholar] [CrossRef]
- Zak, D.R.; Kling, G.W. Microbial community composition and function across an arctic tundra landscape. Ecology 2006, 87, 1659–1670. [Google Scholar] [CrossRef]
- Greer, C.W.; Whyte, L.G.; Niederberger, T.D. Microbial communities in hydrocarbon-contaminated temperate, tropical, alpine, and polar soils. In Handbook of Hydrocarbon and Lipid Microbiology; Timmis, K.N., Ed.; Springer Berlin Heidelberg: Berlin, Germany; Heidelberg, Germany, 2010; pp. 2313–2328. [Google Scholar]
- Aislabie, J.; Saul, D.J.; Foght, J.M. Bioremediation of hydrocarbon-contaminated polar soils. Extremophiles 2006, 10, 171–179. [Google Scholar] [CrossRef]
- Ciric, L.; Philp, J.C.; Whiteley, A.S. Hydrocarbon utilization within a diesel-degrading bacterial consortium. FEMS Microbiol. Lett. 2010, 303, 116–122. [Google Scholar] [CrossRef]
- Sorkhoh, N.A.; Ghannoum, M.A.; Ibrahim, A.S.; Stretton, R.J.; Radwan, S.S. Crude-oil and hydrocarbon-degrading strains of rhodococcus-rhodochrous isolated from soil and marine environments in kuwait. Environ. Pollut. 1990, 65, 1–17. [Google Scholar] [CrossRef]
- Whyte, L.G.; Hawari, J.; Zhou, E.; Bourbonnière, L.; Inniss, W.E.; Greer, C.W. Biodegradation of variable-chain-length alkanes at low temperatures by a psychrotrophic rhodococcus sp. Appl. Environ. Microb. 1998, 64, 2578–2584. [Google Scholar]
- Yergeau, E.; Sanschagrin, S.; Beaumier, D.; Greer, C.W. Metagenomic analysis of the bioremediation of diesel-contaminated canadian high arctic soils. PLoS One 2012, 7, e30058. [Google Scholar]
- Beschta, R.L.; Ripple, W.J. Large predators and trophic cascades in terrestrial ecosystems of the western united states. Biol. Conserv. 2009, 142, 2401–2414. [Google Scholar] [CrossRef]
- Falkowski, P.; Scholes, R.J.; Boyle, E.; Canadell, J.; Canfield, D.; Elser, J.; Gruber, N.; Hibbard, K.; Hogberg, P.; Linder, S.; et al. The global carbon cycle: A test of our knowledge of earth as a system. Science 2000, 290, 291–296. [Google Scholar] [CrossRef]
- Hobbie, J.E.; Hobbie, E.A. 15n in symbiotic fungi and plants estimates nitrogen and carbon flux rates in arctic tundra. Ecology 2006, 87, 816–822. [Google Scholar] [CrossRef]
- Jonasson, S.; Michelsen, A.; Schmidt, I.K. Coupling of nutrient cycling and carbon dynamics in the arctic, integration of soil microbial and plant processes. Appl. Soil Ecol. 1999, 11, 135–146. [Google Scholar] [CrossRef]
- Nordin, A.; Schmidt, I.K.; Shaver, G.R. Nitrogen uptake by arctic soil microbes and plants in relation to soil nitrogen supply. Ecology 2004, 85, 955–962. [Google Scholar] [CrossRef]
- Schmidt, I.K.; Michelsen, A.; Jonasson, S. Effects of labile soil carbon on nutrient partitioning between an arctic graminoid and microbes. Oecologia 1997, 112, 557–565. [Google Scholar] [CrossRef]
- Hodge, A.; Robinson, D.; Fitter, A. Are microorganisms more effective than plants at competing for nitrogen? Trends Plant Sci. 2000, 5, 304–308. [Google Scholar] [CrossRef]
- Buckeridge, K.M.; Jefferies, R.L. Vegetation loss alters soil nitrogen dynamics in an arctic salt marsh. J. Ecol. 2007, 95, 283–293. [Google Scholar] [CrossRef]
- Clemmensen, K.E.; Sorensen, P.L.; Michelsen, A.; Jonasson, S.; Strom, L. Site-dependent n uptake from n-form mixtures by arctic plants, soil microbes and ectomycorrhizal fungi. Oecologia 2008, 155, 771–783. [Google Scholar] [CrossRef]
- Edwards, K.A.; McCulloch, J.; Kershaw, G.P.; Jefferies, R.L. Soil microbial and nutrient dynamics in a wet arctic sedge meadow in late winter and early spring. Soil Biol. Biochem. 2006, 38, 2843–2851. [Google Scholar] [CrossRef]
- Hill, P.W.; Farrar, J.; Roberts, P.; Farrell, M.; Grant, H.; Newsham, K.K.; Hopkins, D.W.; Bardgett, R.D.; Jones, D.L. Vascular plant success in a warming antarctic may be due to efficient nitrogen acquisition. Nat. Clim. Change 2011, 1, 50–53. [Google Scholar] [CrossRef]
- Henry, H.A.L.; Jefferies, R.L. Plant amino acid uptake, soluble n turnover and microbial n capture in soils of a grazed arctic salt marsh. J. Ecol. 2003, 91, 627–636. [Google Scholar] [CrossRef]
- Chapin, F.S.; Moilanen, L.; Kielland, K. Preferential use of organic nitrogen for growth by a nonmycorrhizal arctic sedge. Nature 1993, 361, 150–153. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Howarth, R.W. Nitrogen limitation on land and in the sea: How can it occur. Biogeochemistry 1991, 13, 87–115. [Google Scholar]
- Vitousek, P.M.; Aber, J.D.; Howarth, R.W.; Likens, G.E.; Matson, P.A.; Schindler, D.W.; Schlesinger, W.H.; Tilman, D. Human alteration of the global nitrogen cycle: Sources and consequences. Ecol. Appl. 1997, 7, 737–750. [Google Scholar]
- Imsenecki, A.A.; Popova, L.S.; Kirillova, N.F. Effect of nitrogen source on growth of arthrobacter simplex and its biosynthesis of cholinesterase. Mikrobiologiâ 1976, 45, 614–619. [Google Scholar]
- Rice, C.W.; Tiedje, J.M. Regulation of nitrate assimilation by ammonium in soils and in isolated soil microorganisms. Soil Biol. Biochem. 1989, 21, 597–602. [Google Scholar] [CrossRef]
- Recous, S.; Mary, B.; Faurie, G. Microbial immobilization of ammonium and nitrate in cultivated soils. Soil Biol. Biochem. 1990, 22, 913–922. [Google Scholar] [CrossRef]
- Hill, P.W.; Farrell, M.; Roberts, P.; Farrar, J.; Grant, H.; Newsham, K.K.; Hopkins, D.W.; Bardgett, R.D.; Jones, D.L. Soil- and enantiomer-specific metabolism of amino acids and their peptides by antarctic soil microorganisms. Soil Biol. Biochem. 2011, 43, 2410–2416. [Google Scholar] [CrossRef]
- Fierer, N.; Lauber, C.L.; Ramirez, K.S.; Zaneveld, J.; Bradford, M.A.; Knight, R. Comparative metagenomic, phylogenetic and physiological analyses of soil microbial communities across nitrogen gradients. ISME J. 2012, 6, 1007–1017. [Google Scholar] [CrossRef]
- Powell, S.M.; Ferguson, S.H.; Snape, I.; Siciliano, S.D. Fertilization stimulates anaerobic fuel degradation of antarctic soils by denitrifying microorganisms. Environ. Sci. Technol. 2006, 40, 2011–2017. [Google Scholar] [CrossRef]
- Roy, R.; Greer, C.W. Hexadecane mineralization and denitrification in two diesel fuel-contaminated soils. FEMS Microbiol. Ecol. 2000, 32, 17–23. [Google Scholar] [CrossRef]
- Callaghan, T.V.; Bjorn, L.O.; Chernov, Y.; Chapin, T.; Christensen, T.R.; Huntley, B.; Ims, R.A.; Johansson, M.; Jolly, D.; Jonasson, S.; et al. Biodiversity, distributions and adaptations of arctic species in the context of environmental change. AMBIO 2004, 33, 404–417. [Google Scholar]
- Allison, S.D.; Martiny, J.B.H. Resistance, resilience, and redundancy in microbial communities. Proc. Natl. Acad. Sci. USA 2008, 105, 11512–11519. [Google Scholar] [CrossRef]
- Lawrence, D.; Fiegna, F.; Behrends, V.; Bundy, J.G.; Phillimore, A.B.; Bell, T.; Barraclough, T.G. Species interactions alter evolutionary responses to a novel environment. PLoS Biol. 2012, 10, e1001330. [Google Scholar]
- Olsson, P.A.; Eriksen, B.E.; Dahlberg, A. Colonization by arbuscular mycorrhizal and fine endophytic fungi in herbaceous vegetation in the canadian high arctic. Can. J. Bot. 2004, 82, 1547–1556. [Google Scholar] [CrossRef]
- Schmidt, I.K.; Jonasson, S.; Shaver, G.R.; Michelsen, A.; Nordin, A. Mineralization and distribution of nutrients in plants and microbes in four arctic ecosystems: Responses to warming. Plant Soil 2002, 242, 93–106. [Google Scholar] [CrossRef]
- Lamb, E.G.; Han, S.; Lanoil, B.D.; Henry, G.H.R.; Brummell, M.E.; Banerjee, S.; Siciliano, S.D. A high arctic soil ecosystem resists long-term environmental manipulations. Glob. Change Biol. 2011, 17, 3187–3194. [Google Scholar] [CrossRef]
- Barbéran, A.; Bates, S.T.; Casamayor, E.O.; Fierer, N. Using network analysis to explore co-occurrence patterns in soil microbial communities. ISME J. 2012, 6, 343–351. [Google Scholar] [CrossRef] [Green Version]
- Pan, C.L.; Fischer, C.R.; Hyatt, D.; Bowen, B.P.; Hettich, R.L.; Banfield, J.F. Quantitative tracking of isotope flows in proteomes of microbial communities. Mol. Cell. Proteomics 2011, 10, M110.006049. [Google Scholar] [CrossRef]
- Griffiths, B.S.; Kuan, H.L.; Ritz, K.; Glover, L.A.; McCaig, A.E.; Fenwick, C. The relationship between microbial community structure and functional stability, tested experimentally in an upland pasture soil. Microb. Ecol. 2004, 47, 104–113. [Google Scholar]
- Deni, J.; Penninckx, M.J. Nitrification and autotrophic nitrifying bacteria in a hydrocarbon-polluted soil. Appl. Environ. Microb. 1999, 65, 4008–4013. [Google Scholar]
- Powell, S.J.; Prosser, J.I. Inhibition of ammonium oxidation by nitrapyrin in soil and liquid culture. Appl. Environ. Microb. 1986, 52, 782–787. [Google Scholar]
- Bremner, J.M.; McCarty, G.W.; Yeomans, J.C.; Chai, H.S. Effects of phosphoroamides on nitrification, denitrification, and mineralization of organic nitrogen in soil. Commun. Soil Sci. Plant 1986, 17, 369–384. [Google Scholar] [CrossRef]
- Myrold, D.D.; Posavatz, N.R. Potential importance of bacteria and fungi in nitrate assimilation in soil. Soil Biol. Biochem. 2007, 39, 1737–1743. [Google Scholar] [CrossRef]
- Bremner, J.M.; Yeomans, J.C. Effects of nitrification inhibitors on denitrification of nitrate in soil. Biol. Fertil. Soils 1986, 2, 173–179. [Google Scholar]
- Yeomans, J.C.; Bremner, J.M. Effects of urease inhibitors on denitrification in soil. Commun. Soil Sci. Plant 1986, 17, 63–73. [Google Scholar] [CrossRef]
- Winfrey, M.R.; Ward, D.M. Substrates for sulfate reduction and methane production in intertidal sediments. Appl. Environ. Microb. 1983, 45, 193–199. [Google Scholar]
- Shen, N.; Ko, J.H.; Xiao, G.P.; Wesolowski, D.; Shan, G.; Geller, B.; Izadjoo, M.; Altman, S. Inactivation of expression of several genes in a variety of bacterial species by egs technology. Proc. Natl. Acad. Sci. USA 2009, 106, 8163–8168. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bell, T.H.; Callender, K.L.; Whyte, L.G.; Greer, C.W. Microbial Competition in Polar Soils: A Review of an Understudied but Potentially Important Control on Productivity. Biology 2013, 2, 533-554. https://doi.org/10.3390/biology2020533
Bell TH, Callender KL, Whyte LG, Greer CW. Microbial Competition in Polar Soils: A Review of an Understudied but Potentially Important Control on Productivity. Biology. 2013; 2(2):533-554. https://doi.org/10.3390/biology2020533
Chicago/Turabian StyleBell, Terrence H., Katrina L. Callender, Lyle G. Whyte, and Charles W. Greer. 2013. "Microbial Competition in Polar Soils: A Review of an Understudied but Potentially Important Control on Productivity" Biology 2, no. 2: 533-554. https://doi.org/10.3390/biology2020533
APA StyleBell, T. H., Callender, K. L., Whyte, L. G., & Greer, C. W. (2013). Microbial Competition in Polar Soils: A Review of an Understudied but Potentially Important Control on Productivity. Biology, 2(2), 533-554. https://doi.org/10.3390/biology2020533




