Effect of Different Broad Waveband Lights on Membrane Lipids of a Cyanobacterium, Synechococcus sp., as Determined by UPLC-QToF-MS and Vibrational Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. High-Performance Liquic Chromatography with Photodiode Array Detection (HPLC-DAD) Measurements
2.3. Ultra-Performance Liquid Chromatography (UPLC)-Quadrupole Time-of-Flight Mass Spectrometry (QToF-MS) Measurements
2.4. Fourier-Transform Raman (FT-Raman) and ATR-Fourier-Transform Infrared (ATR-FTIR) Spectroscopies
2.5. Statistical Analyses
3. Results and discussion
3.1. UV-Vis Spectra
3.2. UPLC-Mass Spectrometry (MS) Analysis
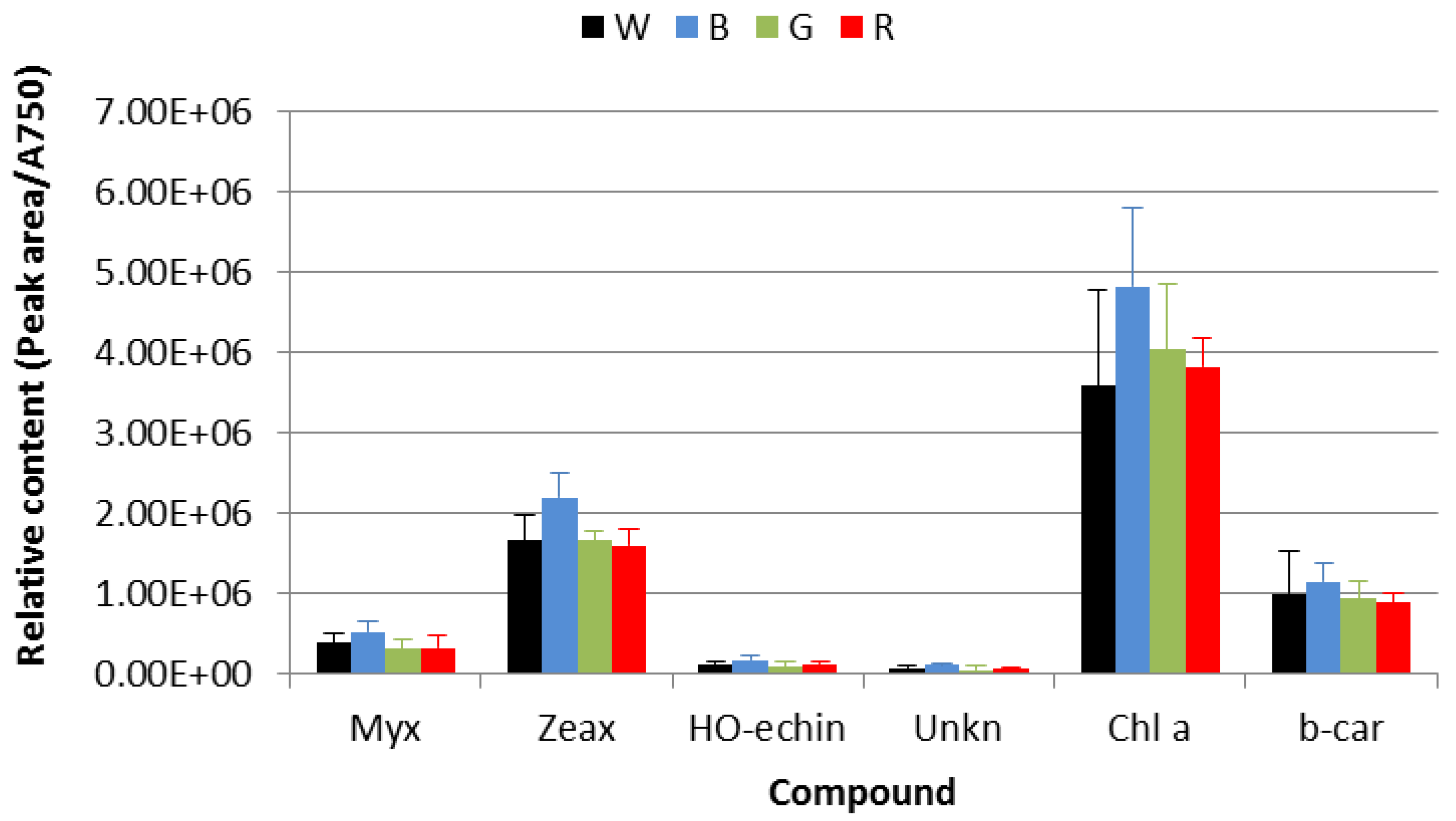
3.3. HPLC-DAD Pigments
3.4. Raman and Attenuated Reflectance Infrared (ATR-IR) Spectroscopy
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
References
- Partensky, F.; Blanchot, J.; Vaulot, D. Differential distribution and ecology of Phrochlorococcus and Synechococcus in oceanic waters: A review. In Marine Cyanobacteria; Charpy, L., Larkum, A., Eds.; Bulletin de l'Institut Océanographique de Monaco: Montpellier, France, 1999; pp. 457–475. [Google Scholar]
- García-Pichel, F.; Johnson, S.L.; Youngkin, D.; Belnap, J. Small-scale vertical distribution of bacterial biomass and diversity in biological soil crusts from arid lands in the Colorado Plateau. Microb. Ecol. 2003, 46, 312–381. [Google Scholar] [CrossRef] [PubMed]
- Morel, A. Optical properties of pure water and pure sea water. In Optical aspects of Oceanography; Jerlov, N.G., Nielsen, E.S., Eds.; Academic: London, UK, 1974; pp. 1–24. [Google Scholar]
- Whitehead, R.F.; de Mora, S.J.; Demers, S. Enhanced UV radiation—A new problem for the marine environment. In The Effects of UV Radiation in the Marine Environment; de Mora, S., Demers, S., Vernet, M., Eds.; Cambridge University Press: Cambridge, UK, 2000; pp. 1–34. [Google Scholar]
- Ferris, M.J.; Palenik, B. Niche adaptation in ocean cyanobacteria. Nature 1998, 396, 227–228. [Google Scholar] [CrossRef]
- Gutu, A.; Kehoe, D.M. Emerging perspectives on the mechanisms, regulation, and distribution of light color acclimation in Cyanobacteria. Mol. Plant 2012, 5, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.V. Antioxidants, oxidative damage and oxygen deprivation stress: A review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Bennett, A.; Bogorad, L. Complementary chromatic adaptation in a filamentous blue-green alga. J. Cell Biol. 1973, 58, 419–435. [Google Scholar] [CrossRef] [PubMed]
- Grossman, A.R.; Bhaya, D.; He, Q. Tracking the light environment by Cyanobacteria and the dynamic nature of light harvesting. J. Biol. Chem. 2001, 276, 11449–11452. [Google Scholar] [CrossRef] [PubMed]
- Vogelmann, T.C.; Scheibe, J. Action spectra of chromatic adaptation in blue-green Fremyella diplosiphon. Planta 1978, 143, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, H.C.; Kanopka, A.; Melnicki, M.R.; Hill, E.A.; Kucek, L.A.; Zhang, S.; Shen, G.; Bryant, D.A.; Beliaev, A.S. Effect of mono- and dichromatic light quality on growth rates and photosynthetic performance of Synechococcus sp. PCC 7002. Frontiers Microbiol. 2014, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ikeuchi, M.; Ishizuka, T. Cyanobacteriochromes: A new superfamily of tetrapyrrole-binding photoreceptors in cyanobacteria. Photochem. Photobiol. Sci. 2008, 7, 1159–1167. [Google Scholar] [CrossRef] [PubMed]
- Hirose, Y.; Rockwell, N.C.; Nishiyama, K.; Narikawa, R.; Ukaji, Y.; Inomata, K.; Lagarias, J.C.; Ikeuchi, M. Green/red cyanobacteriochromes regulate complementary chromatic acclimation via a photochromic photocycle. Proc. Natl. Acad. Sci. USA 2013, 110, 4974–4979. [Google Scholar] [CrossRef] [PubMed]
- Humily, F.; Partensky, F.; Six, C.; Farrant, G.K.; Ratin, M.; Marie, D.; Garczarek, L. A gene island with two possible configurations is involved in chromatic acclimation in marine Synechococcus. PLoS ONE 2013, 8, e84459. [Google Scholar] [CrossRef] [PubMed]
- Nevo, R.; Charuvi, D.; Shimoni, E.; Schwarz, R.; Kaplan, A.; Ohad, I.; Reich, Z. Thylakoid membrane perforations and connectivity enable intracellular traffic in cyanobacteria. EMBO J. 2007, 26, 1467–1473. [Google Scholar] [CrossRef] [PubMed]
- Montero, O.; Sánchez-Guijo, A.; Lubián, L.M.; Martínez-Rodríguez, G. Changes in membrane lipids and carotenoids during light acclimation in a marine cyanobacterium Synechococcus sp. J. Biosci. 2012, 37, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Schultz, Z.D.; Levin, I.W. Vibrational spectroscopy of biomembranes. Annu. Rev. Anal. Chem. 2011, 4, 343–366. [Google Scholar] [CrossRef] [PubMed]
- Szalonatai, B.; Kota, Z.; Nonaka, H.; Murata, N. Structural consequences of genetically engineered saturation of the fatty acids of phosphatidylglycerol in tobacco thylakoid membranes. An FTIR study. Biochem. 2003, 42, 4292–4299. [Google Scholar] [CrossRef] [PubMed]
- De Gelder, J.; De Gussem, K.; Vandenabeele, P.; Moens, L. Reference database of Raman spectra of biological molecules. J. Raman Spectrosc. 2007, 38, 1133–1148. [Google Scholar] [CrossRef]
- Montero, O.; Porta, J.M.; Porta, J.; Martínez, G.; Lubián, L.M. Characterization of two Synechococcus sp. PCC7002-related cyanobacterial strains in relation to 16S rDNA, crtR gene, lipids and pigments. Phycol. Res. 2011, 59, 147–155. [Google Scholar] [CrossRef]
- Plumb, R.S.; Johnson, K.A.; Rainville, P.; Smith, B.W.; Wilson, I.D.; Castro-Pérez, J.M.; Nicholson, J.K. UPLC-MSE; a new approach for generating molecular fragment information for biomarker structure elucidation. Rapid Commun. Mass Spectrom. 2006, 20, 1989–1994. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.N.; Shin, H.S.; Park, H.G.; Lee, J.; Kil, G.S.; Choi, C.Y. Profiles of photosynthetic pigment accumulation and expression of photosynthesis-related genes in the marine cyanobacterium Synechococcus sp.: Effects of LED wavelengths. Biotechnol. Bioproc. Eng. 2014, 19, 250–256. [Google Scholar] [CrossRef]
- Ma, R.; Lu, F.; Bi, Y.; Hu, Z. Effects of light intensity and quality on phycobiliprotein accumulation in the cyanobacterium Nostoc sphaeroides Kützing. Biotechnol. Lett. 2015, 37, 1663–1669. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.P.; Richter, P.; Faddoul, J.; Braun, M.; Häder, D.-P. Effects of UV and visible light on cyanobacteria at the cellular level. Photochem. Photobiol. Sci. 2002, 1, 552–559. [Google Scholar] [CrossRef]
- Montero, O. Lipid Profiling of Synechococcus sp. PCC7002 and two related strains by HPLC coupled to ESI–(Ion Trap)–MS/MS. Z. Naturforsch. C 2011, 66, 149–158. [Google Scholar] [CrossRef]
- Guskov, A.; Kern, J.; Gabdulkhakov, A.; Broser, M.; Zouni, A.; Saenger, W. Cyanobacteral photosystem II at 2.9-Å resolution and the role of quinones, lipids, channels and chloride. Nature Struct. Mol. Biol. 2009, 16, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, I.; Shen, J.-R.; Leng, J.; Ohashi, S.; Kobayashi, M.; Wada, H. Lipids in oxygen-evolving photosystem II complexes of cyanobacteria and higher plants. J. Biochem. 2006, 140, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Los, D.A.; Mironov, K.S.; Allakhverdiev, S.I. Regulatory role of membrane fluidity in gene expression and physiological functions. Photosyn. Res. 2013, 116, 489–509. [Google Scholar] [CrossRef] [PubMed]
- McConnell, M.D.; Koop, R.; Vasil’ev, S.; Bruce, D. Regulation of the distribution of chlorophyll and phycobilin-absorbed excitation energy in cyanobacteria. A structure-based model for the light state transition. Plant Physiol. 2002, 130, 1201–1212. [Google Scholar] [CrossRef] [PubMed]
- El Bissati, K.; Delphim, E.; Murata, N.; Etienne, A.L.; Kirilovsky, D. Photosystem II fluorescence quenching in the cyanobacterium Synechocystis PCC 6803: Involvement of two different mechanisms. Biochim. Biophys. Acta 2000, 1457, 229–242. [Google Scholar] [CrossRef]
- Bailey, S.; Grossman, A. Photoprotection in cyanobacteria: Regulation of light harvesting. Photochem. Photobiol. 2008, 84, 1410–1420. [Google Scholar] [CrossRef] [PubMed]
- Kirilovsky, D.; Kerfeld, C.A. The Orange Carotenoid Protein: A blue-green light photoactive protein. Photochem. Photobiol. Sci. 2013, 12, 1135–1143. [Google Scholar]
- Wilson, A.; Ajlani, G.; Verbavatz, J.-M.; Vass, I.; Kerfeld, C.; Kirilovsky, D. A soluble carotenoid protein involved in phycobilisome-related thermal energy dissipation in cyanobacteria. Plant Cell 2006, 18, 992–1007. [Google Scholar] [CrossRef] [PubMed]
- Gounaris, K.; Barber, J. Monogalactosyldiacylglycerol: The most abundant polar lipid in nature. Trends Biochem. Sci. 1983, 8, 378–381. [Google Scholar] [CrossRef]
- Shimojima, M.; Ohta, H. Critical regulation of galactolipid synthesis controls membrane differentiation and remodeling in distinct plant organs and following environmental changes. Prog. Lipid Res. 2011, 50, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Schaller, S.; Latowski, D.; Jemiola-Rzeminska, M.; Wilhem, C.; Strzalka, K.; Goss, R. The main thylakoid membrane lipid mongalactosyldiacylglycerol (MGDG) promotes de de-epoxidation of violaxanthin associated with the light-harvesting complex of photosystem II (LHII). Biochim. Biophys. Acta—Bioenerg. 2010, 1797, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Sato, N. Roles of the acidic lipids sulfoquinovosyldiacylglycerol and phosphatidylglycerol in photosynthesis: Their specificity and evolution. J. Plant Res. 2004, 117, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Sato, N.; Hagio, M.; Wada, H.; Tsuzuki, M. Requirement of phosphatidylglycerol for photosynthetic function in thylakoid membranes. Proc. Natl. Acad. Sci. USA 2000, 97, 10655–10660. [Google Scholar] [CrossRef] [PubMed]
- Domonkos, I.; Malec, P.; Sallai, A.; Kovács, L.; Itoh, K.; Shen, G.; Ughy, B.; Bogos, B.; Sakurai, I.; Kis, M.; et al. Phosphatidylglycerol is essential for oligomerization of photosystem I reaction center. Plant Physiol. 2004, 134, 1471–1478. [Google Scholar] [CrossRef] [PubMed]
- Wada, H.; Murata, N. The essential role of phosphatidylglycerol in photosynthesis. Photosyn. Res. 2007, 92, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, J.; Nie, H.; Dong, C.; Li, Z.; Zheng, Z.; Bai, Y.; Liu, H.; Zhao, J. Study on variation of lipids during different growth phases of living cyanobacteria using easy ambient sonic-spray ionization mass spectrometry. Anal. Chem. 2014, 86, 7096–7102. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.R. Lipids in photosynthetic reaction centres: Structural roles and functional holes. Prog. Lipid Res. 2007, 45, 56–87. [Google Scholar] [CrossRef] [PubMed]
- Palsdottir, H.; Hunte, C. Lipids in membrane protein structures. Biochim. Biophys. Acta 2004, 1666, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.G. How lipids affect the activities of integral membrane proteins. Biochim. Biophys. Acta 2004, 1666, 62–87. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, H.L.; Kana, T.M.; Anning, T.; Geider, R.J. Photoacclimation of photosynthesis response curves and photosynthetic pigments in microalgae and cyanobacteria. J. Phycol. 2002, 38, 17–38. [Google Scholar] [CrossRef]
- Schäfer, L.; Vioque, A.; Sandmann, G. Functional in situ evaluation of photosynthesis-protecting carotenoids in mutants of the cyanobacterium Synechocystis PCC6803. J. Photochem. Photobiol. B 2005, 78, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Zhu, X.; Fan, Q.; Wan, X. Raman spectra of amino acids and their aqueous solutions. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2011, 78, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- El Amri, C.; Lacombe, C.; Zimmerman, K.; Ladram, A.; Amiche, M.; Nicolas, P.; Bruston, F. The plasticins: Membrane adsorption, lipid disorders, and biological activity. Biochem. 2006, 45, 14285–14297. [Google Scholar] [CrossRef] [PubMed]
- Mayers, J.J.; Flynn, K.J.; Shields, R.J. Rapid determination of bulk microalgal biochemical composition by Fourier-Transform Infrared spectroscopy. Bioresour. Technol. 2013, 148, 215–220. [Google Scholar] [CrossRef] [PubMed]





| Culture | A630/A680 | |
|---|---|---|
| 24 h | 48 h | |
| Transparent filter (W) | 1.02 ± 0.02* | 1.04 ± 0.02* |
| Blue filter (B) | 1.04 ± 0.03*,** | 1.07 ± 0.04*,** |
| Green filter (G) | 1.04 ± 0.02*,† | 1.06 ± 0.02* |
| Red filter (R) | 1.03 ± 0.02*,**,† | 1.05 ± 0.05** |
| Culture | A475/A665 | |
|---|---|---|
| 24 h | 48 h | |
| Transparent filter (W) | 1.57 ± 0.35* | 1.74 ± 0.42* |
| Blue filter (B) | 1.45 ± 0.24 | 1.54 ± 0.17** |
| Green filter (G) | 1.37 ± 0.33* | 1.33 ± 0.36* |
| Red filter (R) | 1.41 ± 0.31* | 1.31 ± 0.19*,** |
| Compound | Chemical formulae | m/z for [M+NH4]+ | m/z for [M+HCOOH-H]− | Fragment m/z from MSE in negative/positive mode |
|---|---|---|---|---|
| SQDG (18:3/16:1) | C43H74O12S | 832.5243 | 813.4850 | 225.005, 253.217, 277.220, 535.266 |
| SQDG (18:3/16:0) | C43H76O12S | 834.5406 | 815.4978 | 225.007, 255.232, 277.218 537.271, 559.256 |
| SQDG (18:2/16:0) | C43H78O12S | 836.5595 | 817.5112 | 225.008, 255.232, 277.218, 537.268, 561.272 |
| SQDG (18:1/16:0) | C43H80O12S | 838.5739 | 819.5295 | 225.005, 255.232, 281.248, 537.268, 563.291 |
| SQDG (18:0/16:0) | C43H82O12S | 840.5856 | 821.5459 | 225.006, 255.232, 283.268, 537.273, 565.307 |
| SQDG (16:1/16:0) | C41H76O12S | 810.5415 | 791.4961 | 225.009, 255.234, 253.217, 537.275, 535.356 |
| SQDG (16:0/16:0) | C41H78O12S | 812.5547 | 793.5134 | 225.006, 255.234, 537.275 |
| SQDG (18:0/18:0) | C45H86O12S | 868.6231 | 849.5748 | 225.009, 283.258, 565.312 |
| SQDG (18:0/18:1) | C45H84O12S | 866.6016 | 847.5585 | 225.009, 281.248, 283.258, 565.307, 563.276 |
| SQDG (18:0/18:2) | C45H82O12S | 864.5846 | 845.5427 | 225.001, 279.236, 283.258, 563.276 |
| SQDG (18:0/18:3) | C45H80O12S | 862.5696 | 843.5311 | 225.001, 277.214, 283.258, 563.276 |
| SQDG (17:0/16:0) | C42H80O12S | 826.5721 | 807.5271 | 225.008, 255.234, 551.284 |
| SQDG (17:1/16:0) | C42H78O12S | 824.5582 | 805.5136 | 225.006, 255.234, 549.274, 537.281 |
| SQDG (17:3/16:0) | C42H74O12S | (820.5020) | 801.4863 | 225.006, 255.234 |
| SQDG (17:0/18:0) | C44H84O12S | 854.5978 | 835.5544 | 225.006, 283.264 |
| SQDG (17:0/18:1) | C44H82O12S | 852.5907 | 833.5451 | 225.008, 281.248, 551.294, (563.287) |
| SQDG (17:0/18:3) SQDG (17:1/18:2) | C44H78O12S | 848.5558 | 829.5167 | (225.002), 277.217, 279.222 |
| PG (18:3/16:1) | C40H71O10P | (760.5097) | 741.4719 | 227.196, 253.217, 277.217, 505.261, 487.246 |
| PG (18:3/16:0) | C40H73O10P | N.D. | 743.4855 | 227.196, 255.233, 277.216, 505.261, 686.524 |
| PG (18:2/16:0) | C40H75O10P | 764.5397 | 745.5013 | 227.203, 255.233, 279.232, 507.275 |
| PG (18:1/16:0) | C40H77O10P | N.D. | 747.5164 | 227.201, 255.233, 281.248, 509.284 |
| PG (18:1/18:1) | C42H79O10P | N.D. | 773.5338 | 227.202, 281.248, 509.289 |
| PG (17:0/18:1) PG (19:1/16:0) | C41H79O10P | N.D. | 761.5334 | 227.199, 281.247, 295.259, (480.282) |
| MGDG (18:3/16:1) | C43H74O10 | 768.5635 | 795.5238 | 253.217, 277.217 / 773.517, 335.258, 311.259 |
| MGDG (18:2/16:1) | C43H76O10 | 770.5809 | 797.5407 | 253.217, 279.233 / 775.533, 337.274, 311.258 |
| MGDG (18:3/16:0) | C43H76O10 | 770.5809 | 797.5407 | 255.231, 277.217 / 775.533, 335.257, 313.274 |
| MGDG (18:1/16:1) | C43H78O10 | 772.5952 | 799.5569 | 253.217, 281.247 / 777.549, 339.288, 311.258 |
| MGDG (18:2/16:0) | C43H78O10 | 772.5952 | 799.5569 | 255.231, 279.233 / 777.549, 337.275, 313.274 |
| MGDG (18:1/16:0) | C43H78O10 | 774.6095 | 801.5728 | 255.231, 281.246 / 779.566, 339.291, 313.274 |
| MGDG (18:0/16:0) | C43H80O10 | 776.6237 | 803.5802 | 255.231, (283.255) / 781.591, 341.307, 313.273 |
| MGDG (16:0/16:0) | C41H76O10 | 746.5768 | N.D. | N.D. / 751.537, 313.274 |
| MGDG (16:1/16:0) | C41H74O10 | 744.5636 | 771.5264 | 255.230, 253.218 / 749.517, 311.259, 313.273 |
| MGDG (16:1/16:1) | C41H72O10 | 742.5435 | (769.4965) | (253.218) / 747.501, 311.257 |
| MGDG (18:1/18:2) | C45H78O10 | 796.5954 | 823.5519 | 279.227, 281.251 / 339.288 |
| MGDG (18:1/18:1) | C45H80O10 | 798.6067 | N.D. | 281.245 / (339.292) |
| MGDG (18:0/18:1) | C45H82O10 | 800.6241 | 827.5853 | 281.245, 283.255 / 805.581, 339.290 |
| MGDG (17:2/16:1) | C42H74O10 | 756.5653 | N.D. | N.D. / 761.523, 311.260, 323.261 |
| MGDG (17:3/16:0) | C42H74O10 | 756.5653 | N.D. | N.D. / 761.523, 313.274, 321.243 |
| MGDG (17:1/16:0) | C42H78O10 | 760.5928 | 787.5536 | 267.236, 255.232 / 765.550, 313.274, 325.272 |
| MGDG (17:1/18:2) | C44H78O10 | 784.5931 | 811.5625 | 267.232, 279.233 / (789.540), 325.274, 337.275 |
| MGDG (17:1/18:1) | C44H80O10 | 786.6086 | 813.5717 | 267.229, 281.252 / 791.563, 325.272, 339.290 |
| DGDG (18:3/16:1) | C49H84O15 | 930.6152 | 957.5782 | 253.217, 277.217 / 935.5710, 335.258, 311.259 |
| DGDG (18:2/16:1) | C49H86O15 | 932.6310 | 959.5905 | 255.232, 279.220 / 937.587, 337.275, 311.259 |
| DGDG (18:3/16:0) | C49H86O15 | 932.6310 | 959.5905 | 253.217, 277.217 / 937.587, 335.258, 313.274 |
| DGDG (18:1/16:1) | C49H88O15 | 934.6492 | 961.6051 | 253.215, 281.246 / 939.603, 339.287, 311.259 |
| DGDG (18:2/16:0) | C49H88O15 | 934.6492 | 961.6051 | 255.232, 279.233 / 939.603, 337.275, 313.274 |
| DGDG (18:1/16:0) | C49H90O15 | 936.6652 | 963.6223 | 255.234, 281.250 / 941.617, 339.290, 313.274 |
| DGDG (17:3/16:1) | C48H82O15 | 916.6006 | 943.5618 | 253.214, 263.201 / 921.555, 321.242, 311.258 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montero, O.; Velasco, M.; Sanz-Arranz, A.; Rull, F. Effect of Different Broad Waveband Lights on Membrane Lipids of a Cyanobacterium, Synechococcus sp., as Determined by UPLC-QToF-MS and Vibrational Spectroscopy. Biology 2016, 5, 22. https://doi.org/10.3390/biology5020022
Montero O, Velasco M, Sanz-Arranz A, Rull F. Effect of Different Broad Waveband Lights on Membrane Lipids of a Cyanobacterium, Synechococcus sp., as Determined by UPLC-QToF-MS and Vibrational Spectroscopy. Biology. 2016; 5(2):22. https://doi.org/10.3390/biology5020022
Chicago/Turabian StyleMontero, Olimpio, Marta Velasco, Aurelio Sanz-Arranz, and Fernando Rull. 2016. "Effect of Different Broad Waveband Lights on Membrane Lipids of a Cyanobacterium, Synechococcus sp., as Determined by UPLC-QToF-MS and Vibrational Spectroscopy" Biology 5, no. 2: 22. https://doi.org/10.3390/biology5020022
APA StyleMontero, O., Velasco, M., Sanz-Arranz, A., & Rull, F. (2016). Effect of Different Broad Waveband Lights on Membrane Lipids of a Cyanobacterium, Synechococcus sp., as Determined by UPLC-QToF-MS and Vibrational Spectroscopy. Biology, 5(2), 22. https://doi.org/10.3390/biology5020022






