Is Polysialylated NCAM Not Only a Regulator during Brain Development But also during the Formation of Other Organs?
Abstract
:1. Introduction
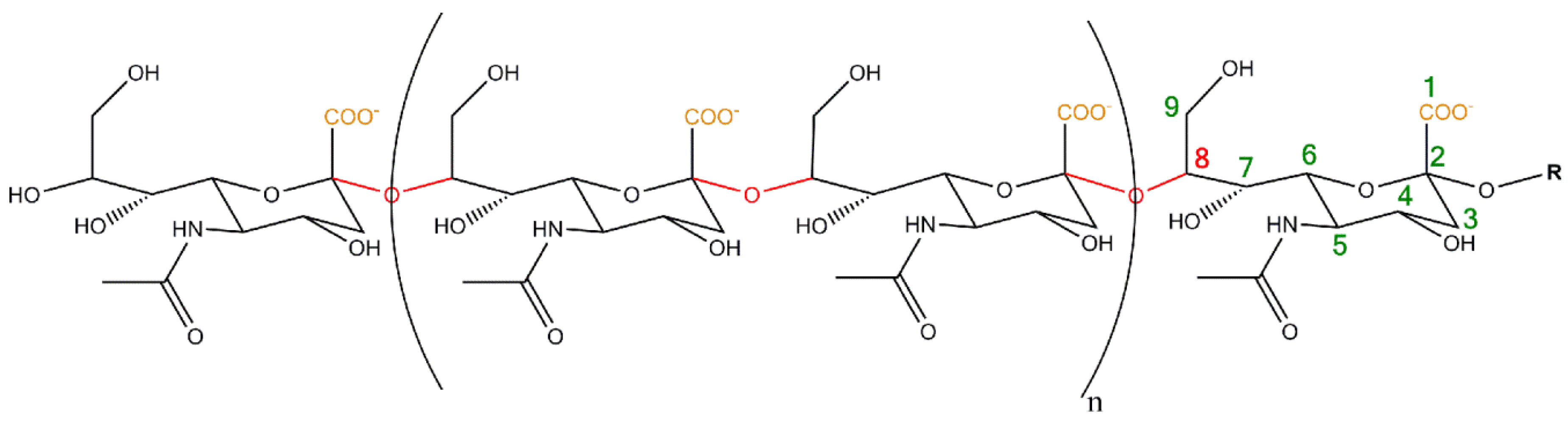
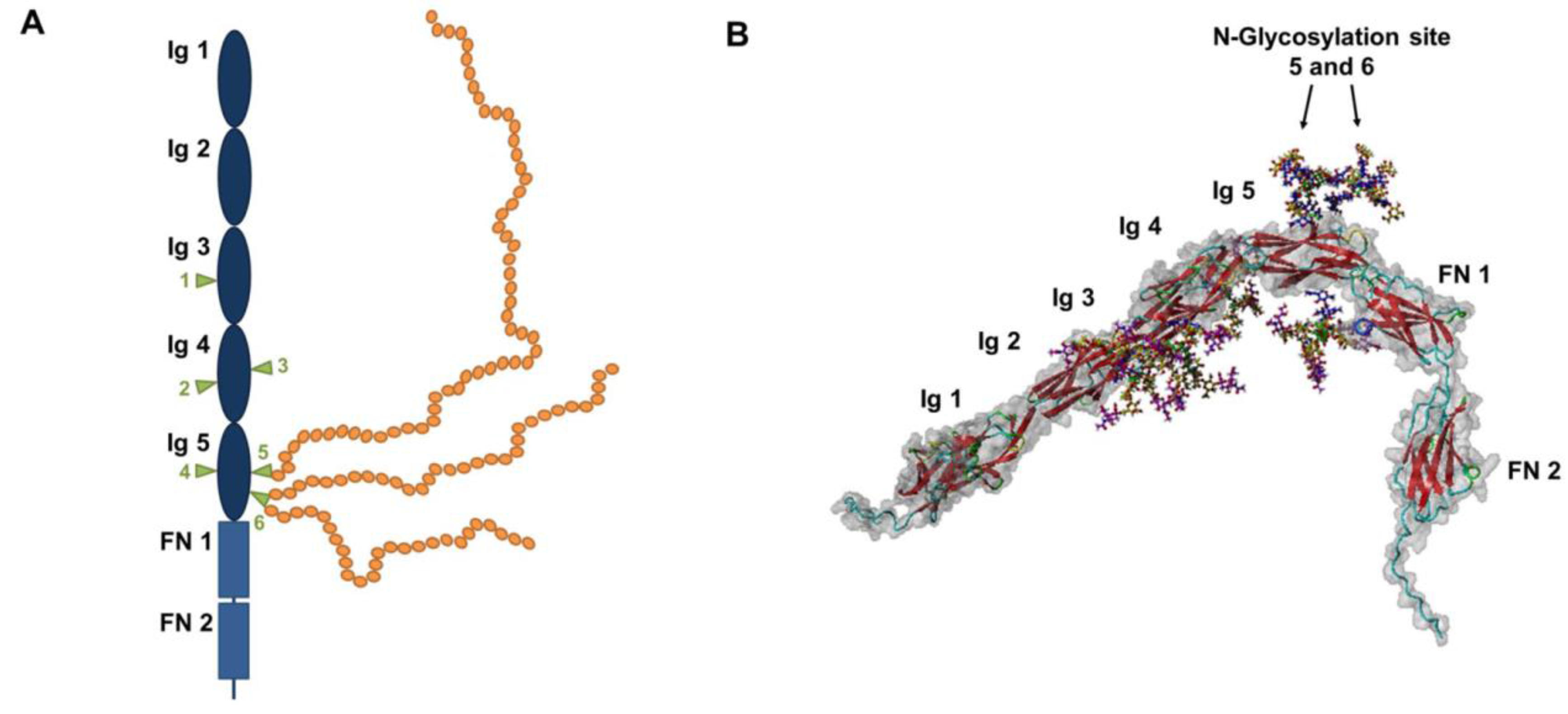
2. Polysialylation of NCAM
- A sodium channel in adult rat brain [33];
- Cluster of differentiation (CD) 36 in murine and human milk [34];
- C-C chemokine receptor type 7 (CCR7) on dendritic cells (mouse and human) [38];
- Synaptic cell adhesion molecule SynCAM-1 on polydendrocytes (NG2) cells in postnatal mouse brain [39]; and
- E-selectin ligand-1 on microglia and macrophages (mouse and human) [40].
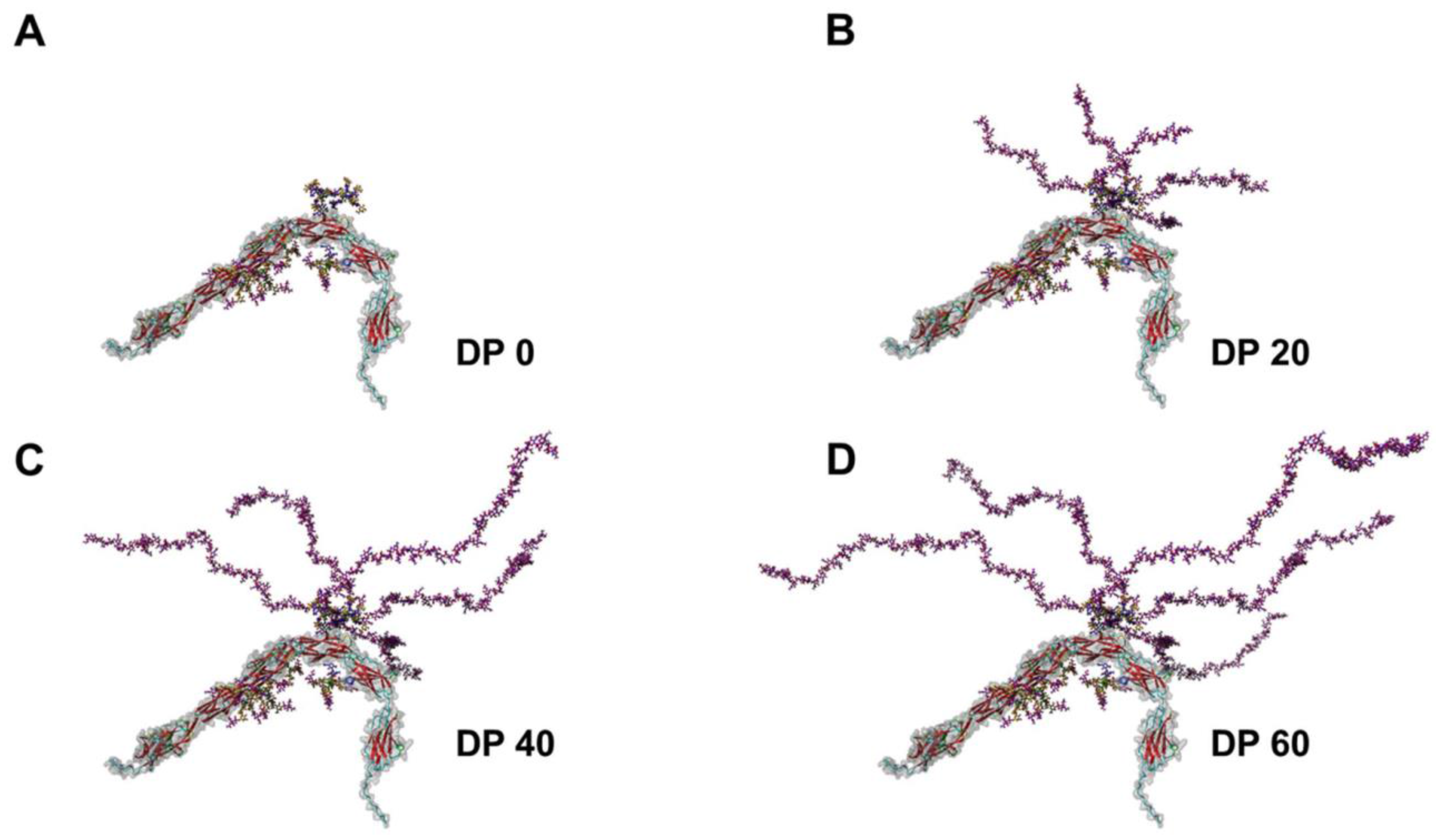
3. Impact of PolySia
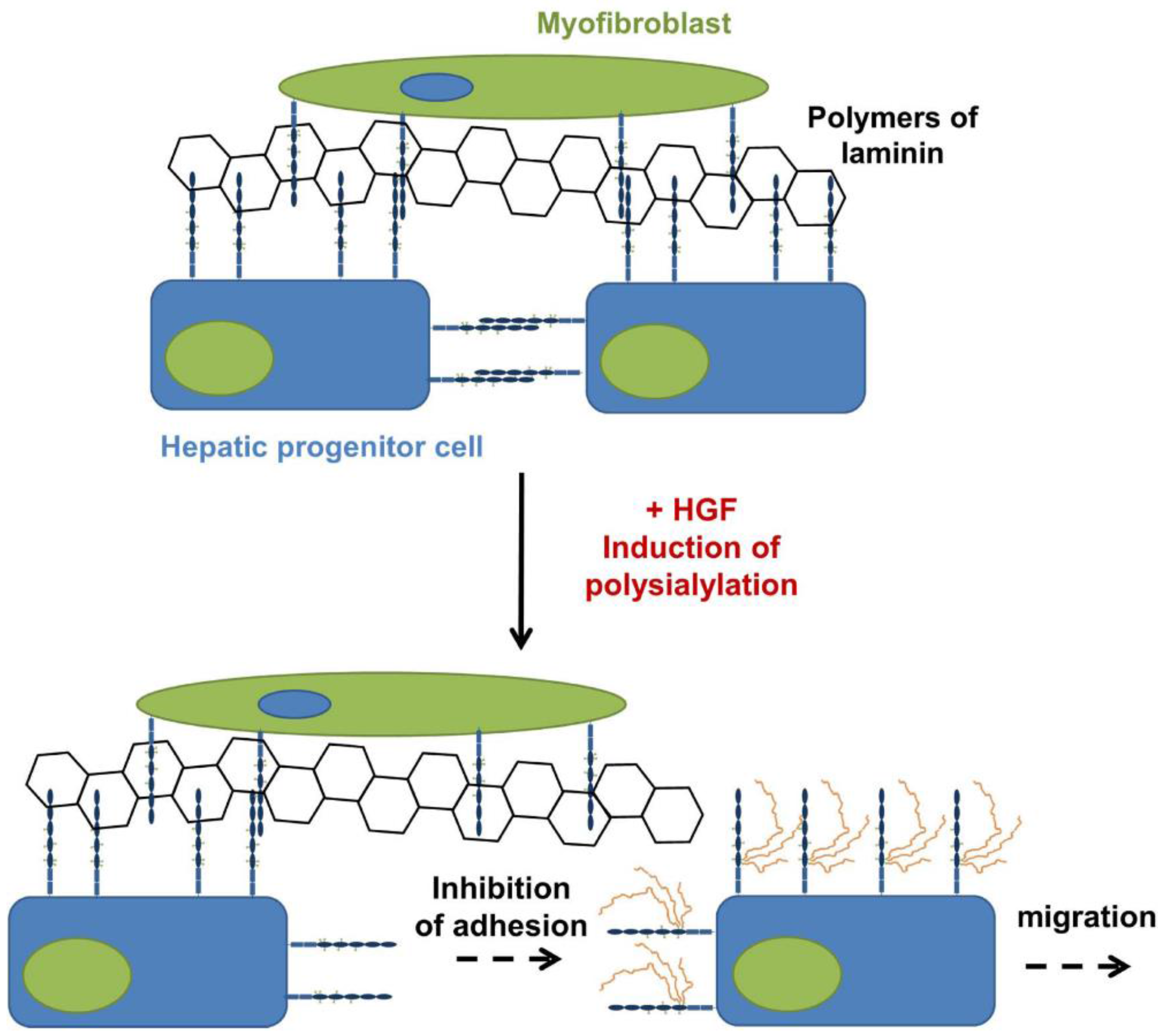
4. PolySia-NCAM during the Development of the Liver
5. PolySia-NCAM during the Development of the Heart
6. PolySia-NCAM during the Development of the Kidney
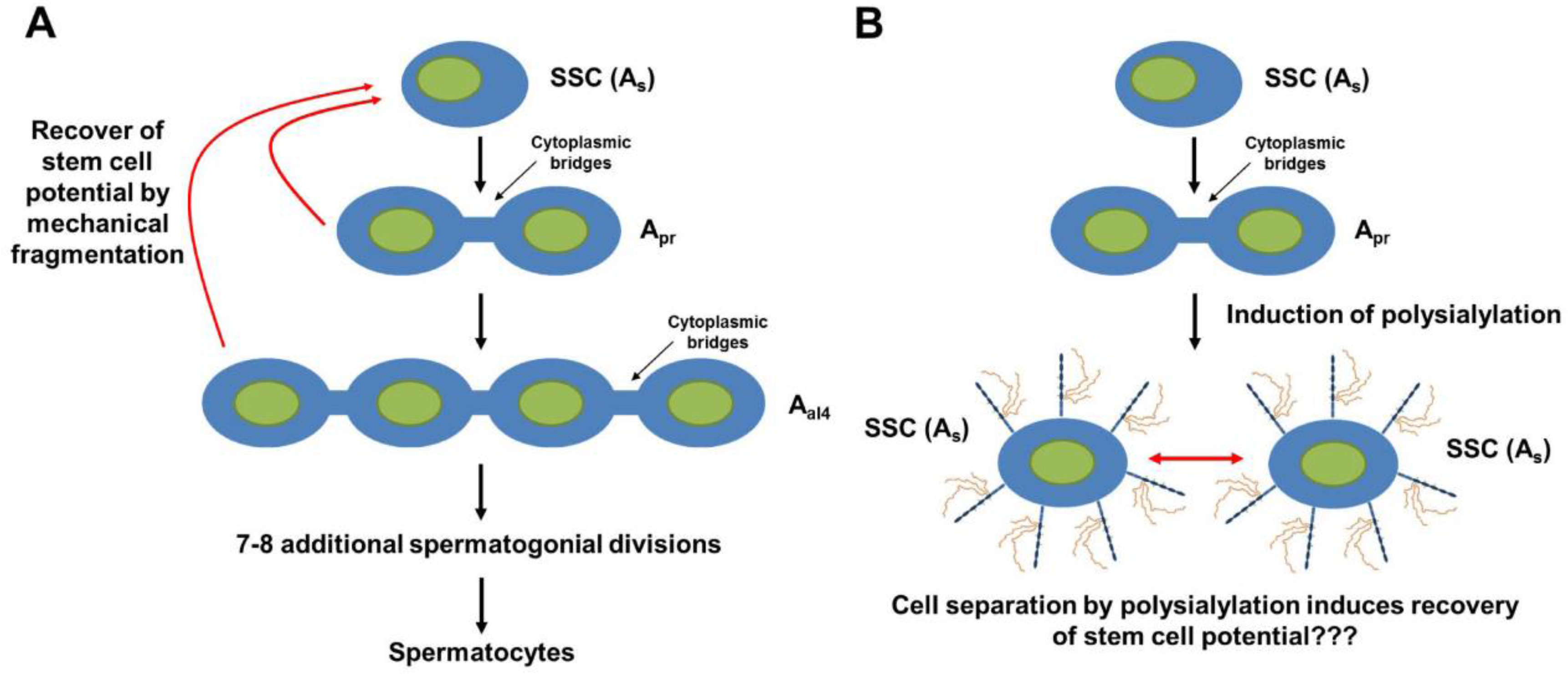
7. PolySia-NCAM during the Development of the Testis
8. PolySia-NCAM during the Development of the Epididymis
9. PolySia during the Development of the Placenta
10. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Troy, F.A. Polysialylation: From bacteria to brains. Glycobiology 1992, 2, 5–23. [Google Scholar] [CrossRef] [PubMed]
- Colley, K.J.; Kitajima, K.; Sato, C. Polysialic acid: Biosynthesis, novel functions and applications. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 498–532. [Google Scholar] [CrossRef] [PubMed]
- Miyata, S.; Sato, C.; Kitajima, K. Glycobiology of polysialic acid on sea urchin gametes. TIGG 2007, 19, 85–98. [Google Scholar] [CrossRef]
- Sato, C. Chain length diversity of sialic acids and its biological significance. TIGG 2004, 16, 331–344. [Google Scholar] [CrossRef]
- Schnaar, R.L.; Gerardy-Schahn, R.; Hildebrandt, H. Sialic acids in the brain: Gangliosides and polysialic acid in nervous system development, stability, disease, and regeneration. Physiol. Rev. 2014, 94, 461–518. [Google Scholar] [CrossRef] [PubMed]
- Vimr, E.; Lichtensteiger, C. To sialylate, or not to sialylate: That is the question. Trends Microbiol. 2002, 10, 254–257. [Google Scholar] [CrossRef]
- Vimr, E.R.; Kalivoda, K.A.; Deszo, E.L.; Steenbergen, S.M. Diversity of microbial sialic acid metabolism. Microbiol. Mol. Biol. Rev. 2004, 68, 132–153. [Google Scholar] [CrossRef] [PubMed]
- Cremer, H.; Lange, R.; Christoph, A.; Plomann, M.; Vopper, G.; Roes, J.; Brown, R.; Baldwin, S.; Kraemer, P.; Scheff, S.; et al. Inactivation of the N-CAM gene in mice results in size reduction of the olfactory bulb and deficits in spatial learning. Nature 1994, 367, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Finne, J. Occurrence of unique polysialosyl carbohydrate units in glycoproteins of developing brain. J. Biol. Chem. 1982, 257, 11966–11970. [Google Scholar] [PubMed]
- Finne, J.; Finne, U.; Deagostini-Bazin, H.; Goridis, C. Occurrence of alpha 2–8 linked polysialosyl units in a neural cell adhesion molecule. Biochem. Biophys. Res. Commun. 1983, 112, 482–487. [Google Scholar] [CrossRef]
- Hildebrandt, H.; Muhlenhoff, M.; Gerardy-Schahn, R. Polysialylation of NCAM. Adv. Exp. Med. Biol. 2010, 663, 95–109. [Google Scholar] [PubMed]
- Mühlenhoff, M.; Oltmann-Norden, I.; Weinhold, B.; Hildebrandt, H.; Gerardy-Schahn, R. Brain development needs sugar: The role of polysialic acid in controlling NCAM functions. Biol. Chem. 2009, 390, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Mühlenhoff, M.; Rollenhagen, M.; Werneburg, S.; Gerardy-Schahn, R.; Hildebrandt, H. Polysialic acid: Versatile modification of NCAM, SynCAM 1 and neuropilin-2. Neurochem. Res. 2013, 38, 1134–1143. [Google Scholar] [CrossRef] [PubMed]
- Weinhold, B.; Seidenfaden, R.; Rockle, I.; Mühlenhoff, M.; Schertzinger, F.; Conzelmann, S.; Marth, J.D.; Gerardy-Schahn, R.; Hildebrandt, H. Genetic ablation of polysialic acid causes severe neurodevelopmental defects rescued by deletion of the neural cell adhesion molecule. J. Biol. Chem. 2005, 280, 42971–42977. [Google Scholar] [CrossRef] [PubMed]
- Schauer, R. Sialic acids: Fascinating sugars in higher animals and man. Zoology 2004, 107, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Schauer, R. Sialic acids as regulators of molecular and cellular interactions. Curr. Opin. Struct. Biol. 2009, 19, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Angata, T.; Varki, A. Chemical diversity in the sialic acids and related alpha-keto acids: An evolutionary perspective. Chem. Rev. 2002, 102, 439–469. [Google Scholar] [CrossRef] [PubMed]
- Kleene, R.; Schachner, M. Glycans and neural cell interactions. Nat. Rev. Neurosci. 2004, 5, 195–208. [Google Scholar] [CrossRef] [PubMed]
- He, H.T.; Finne, J.; Goridis, C. Biosynthesis, membrane association, and release of N-CAM-120, a phosphatidylinositol-linked form of the neural cell adhesion molecule. J. Cell Biol. 1987, 105, 2489–2500. [Google Scholar] [CrossRef] [PubMed]
- Von Der Ohe, M.; Wheeler, S.F.; Wuhrer, M.; Harvey, D.J.; Liedtke, S.; Muhlenhoff, M.; Gerardy-Schahn, R.; Geyer, H.; Dwek, R.A.; Geyer, R.; et al. Localization and characterization of polysialic acid-containing N-linked glycans from bovine NCAM. Glycobiology 2002, 12, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Galuska, S.P.; Oltmann-Norden, I.; Geyer, H.; Weinhold, B.; Kuchelmeister, K.; Hildebrandt, H.; Gerardy-Schahn, R.; Geyer, R.; Mühlenhoff, M. Polysialic acid profiles of mice expressing variant allelic combinations of the polysialyltransferases ST8SiaII and ST8SiaIV. J. Biol. Chem. 2006, 281, 31605–31615. [Google Scholar] [CrossRef] [PubMed]
- Ulm, C.; Saffarzadeh, M.; Mahavadi, P.; Müller, S.; Prem, G.; Saboor, F.; Simon, P.; Middendorff, R.; Geyer, H.; Henneke, I.; et al. Soluble polysialylated NCAM: A novel player of the innate immune system in the lung. Cell Mol. Life Sci. 2013, 70, 3695–3708. [Google Scholar] [CrossRef] [PubMed]
- Eswar, N.; Webb, B.; Marti-Renom, M.A.; Madhusudhan, M.S.; Eramian, D.; Shen, M.Y.; Pieper, U.; Sali, A. Comparative protein structure modeling using Modeller. Curr. Protoc. Bioinformatics 2006. [Google Scholar] [CrossRef]
- Berman, H.; Henrick, K.; Nakamura, H. Announcing the worldwide protein data bank. Nat. Struct. Biol. 2003, 10. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Varki, A.; Cummings, R.D.; Aebi, M.; Packer, N.H.; Seeberger, P.H.; Esko, J.D.; Stanley, P.; Hart, G.; Darvill, A.; Kinoshita, T.; et al. Symbol nomenclature for graphical representations of glycans. Glycobiology. 2015, 25, 1323–1324. [Google Scholar] [CrossRef] [PubMed]
- Galuska, S.P.; Geyer, R.; Gerardy-Schahn, R.; Mühlenhoff, M.; Geyer, H. Enzyme-dependent variations in the polysialylation of the neural cell adhesion molecule (ncam) in vivo. J. Biol. Chem. 2008, 283, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Angata, K.; Suzuki, M.; Fukuda, M. Differential and cooperative polysialylation of the neural cell adhesion molecule by two polysialyltransferases, PST and STX. J. Biol. Chem. 1998, 273, 28524–28532. [Google Scholar] [CrossRef] [PubMed]
- Angata, K.; Fukuda, M. Polysialyltransferases: Major players in polysialic acid synthesis on the neural cell adhesion molecule. Biochimie 2003, 85, 195–206. [Google Scholar] [CrossRef]
- Close, B.E.; Colley, K.J. In vivo autopolysialylation and localization of the polysialyltransferases PST and STX. J. Biol. Chem. 1998, 273, 34586–34593. [Google Scholar] [CrossRef] [PubMed]
- Mühlenhoff, M.; Eckhardt, M.; Bethe, A.; Frosch, M.; Gerardy-Schahn, R. Autocatalytic polysialylation of polysialyltransferase-1. Embo. J. 1996, 15, 6943–6950. [Google Scholar] [PubMed]
- Simon, P.; Bäumner, S.; Busch, O.; Röhrich, R.; Kaese, M.; Richterich, P.; Wehrend, A.; Müller, K.; Gerardy-Schahn, R.; Mühlenhoff, M.; et al. Polysialic acid is present in mammalian semen as a post-translational modification of the neural cell adhesion molecule NCAM and the polysialyltransferase ST8SiaII. J. Biol. Chem. 2013, 288, 18825–18833. [Google Scholar] [CrossRef] [PubMed]
- Zuber, C.; Lackie, P.M.; Catterall, W.A.; Roth, J. Polysialic acid is associated with sodium channels and the neural cell adhesion molecule N-CAM in adult rat brain. J. Biol. Chem. 1992, 267, 9965–9971. [Google Scholar] [PubMed]
- Yabe, U.; Sato, C.; Matsuda, T.; Kitajima, K. Polysialic acid in human milk CD36 is a new member of mammalian polysialic acid-containing glycoprotein. J. Biol. Chem. 2003, 278, 13875–13880. [Google Scholar] [CrossRef] [PubMed]
- Curreli, S.; Arany, Z.; Gerardy-Schahn, R.; Mann, D.; Stamatos, N.M. Polysialylated neuropilin-2 is expressed on the surface of human dendritic cells and modulates dendritic cell-T lymphocyte interactions. J. Biol. Chem. 2007, 282, 30346–30356. [Google Scholar] [CrossRef] [PubMed]
- Werneburg, S.; Muhlenhoff, M.; Stangel, M.; Hildebrandt, H. Polysialic acid on SynCAM 1 in NG2 cells and on neuropilin-2 in microglia is confined to intracellular pools that are rapidly depleted upon stimulation. Glia 2015, 63, 1240–1255. [Google Scholar] [CrossRef] [PubMed]
- Stamatos, N.M.; Zhang, L.; Jokilammi, A.; Finne, J.; Chen, W.H.; El-Maarouf, A.; Cross, A.S.; Hankey, K.G. Changes in polysialic acid expression on myeloid cells during differentiation and recruitment to sites of inflammation: Role in phagocytosis. Glycobiology 2014, 24, 864–879. [Google Scholar] [CrossRef] [PubMed]
- Kiermaier, E.; Moussion, C.; Veldkamp, C.T.; Gerardy-Schahn, R.; De Vries, I.; Williams, L.G.; Chaffee, G.R.; Phillips, A.J.; Freiberger, F.; Imre, R.; et al. Polysialylation controls dendritic cell trafficking by regulating chemokine recognition. Science 2016, 351, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Galuska, S.P.; Rollenhagen, M.; Kaup, M.; Eggers, K.; Oltmann-Norden, I.; Schiff, M.; Hartmann, M.; Weinhold, B.; Hildebrandt, H.; Geyer, R.; et al. Synaptic cell adhesion molecule SynCAM 1 is a target for polysialylation in postnatal mouse brain. Proc. Natl. Acad. Sci. USA 2010, 107, 10250–10255. [Google Scholar] [CrossRef] [PubMed]
- Werneburg, S.; Buettner, F.F.; Erben, L.; Mathews, M.; Neumann, H.; Muhlenhoff, M.; Hildebrandt, H. Polysialylation and lipopolysaccharide-induced shedding of E-selectin ligand-1 and neuropilin-2 by microglia and THP-1 macrophages. Glia 2016, 64, 1314–1330. [Google Scholar] [CrossRef] [PubMed]
- Colley, K.J. Structural basis for the polysialylation of the neural cell adhesion molecule. Adv. Exp. Med. Biol. 2010, 663, 111–126. [Google Scholar] [PubMed]
- Nelson, R.W.; Bates, P.A.; Rutishauser, U. Protein determinants for specific polysialylation of the neural cell adhesion molecule. J. Biol. Chem. 1995, 270, 17171–17179. [Google Scholar] [CrossRef] [PubMed]
- Close, B.E.; Mendiratta, S.S.; Geiger, K.M.; Broom, L.J.; Ho, L.L.; Colley, K.J. The minimal structural domains required for neural cell adhesion molecule polysialylation by PST/ST8Sia IV and STX/ST8Sia II. J. Biol. Chem. 2003, 278, 30796–30805. [Google Scholar] [CrossRef] [PubMed]
- Mendiratta, S.S.; Sekulic, N.; Hernandez-Guzman, F.G.; Close, B.E.; Lavie, A.; Colley, K.J. A novel alpha-helix in the first fibronectin type III repeat of the neural cell adhesion molecule is critical for N-glycan polysialylation. J. Biol. Chem. 2006, 281, 36052–36059. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.G.; Foley, D.A.; Swartzentruber, K.G.; Colley, K.J. Sequences at the interface of the fifth immunoglobulin domain and first fibronectin type III repeat of the neural cell adhesion molecule are critical for its polysialylation. J. Biol. Chem. 2011, 286, 4525–4534. [Google Scholar] [CrossRef] [PubMed]
- Bhide, G.P.; Fernandes, N.R.; Colley, K.J. Sequence requirements for neuropilin-2 recognition by ST8SiaIV and polysialylation of its o-glycans. J. Biol. Chem. 2016, 291, 9444–9457. [Google Scholar] [CrossRef] [PubMed]
- Nakata, D.; Zhang, L.; Troy, F.A. Molecular basis for polysialylation: A novel polybasic polysialyltransferase domain (PSTD) of 32 amino acids unique to the alpha2,8-polysialyltransferases is essential for polysialylation. Glycoconj. J. 2006, 23, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Bhide, G.P.; Prehna, G.; Ramirez, B.E.; Colley, K.J. The polybasic region of the polysialyltransferase ST8Sia-IV binds directly to the neural cell adhesion molecule, NCAM. Biochemistry 2017, 56, 1504–1517. [Google Scholar] [CrossRef] [PubMed]
- Mühlenhoff, M.; Eckhardt, M.; Bethe, A.; Frosch, M.; Gerardy-Schahn, R. Polysialylation of NCAM by a single enzyme. Curr. Biol. 1996, 6, 1188–1191. [Google Scholar] [CrossRef]
- Foley, D.A.; Swartzentruber, K.G.; Colley, K.J. Identification of sequences in the polysialyltransferases, ST8Sia II and ST8Sia IV, that are required for protein specific polysialylation of the neural cell adhesion molecule, NCAM. J. Biol. Chem. 2009, 284, 15505–15516. [Google Scholar] [CrossRef] [PubMed]
- Zapater, J.L.; Colley, K.J. Sequences prior to conserved catalytic motifs of polysialyltransferase ST8Sia IV are required for substrate recognition. J. Biol. Chem. 2012, 287, 6441–6453. [Google Scholar] [CrossRef] [PubMed]
- Volkers, G.; Worrall, L.J.; Kwan, D.H.; Yu, C.C.; Baumann, L.; Lameignere, E.; Wasney, G.A.; Scott, N.E.; Wakarchuk, W.; Foster, L.J.; et al. Structure of human ST8SiaIII sialyltransferase provides insight into cell-surface polysialylation. Nat. Struct. Mol. Biol. 2015, 22, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Rutishauser, U.; Acheson, A.; Hall, A.K.; Mann, D.M.; Sunshine, J. The neural cell adhesion molecule (NCAM) as a regulator of cell-cell interactions. Science 1988, 240, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Rutishauser, U. Polysialic acid at the cell surface: Biophysics in service of cell interactions and tissue plasticity. J. Cell Biochem. 1998, 70, 304–312. [Google Scholar] [CrossRef]
- Johnson, C.P.; Fujimoto, I.; Rutishauser, U.; Leckband, D.E. Direct evidence that neural cell adhesion molecule (NCAM) polysialylation increases intermembrane repulsion and abrogates adhesion. J. Biol. Chem. 2005, 280, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Rutishauser, U. Polysialic acid in the plasticity of the developing and adult vertebrate nervous system. Nat. Rev. Neurosci. 2008, 9, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Tomasiewicz, H.; Magnuson, T.; Rutishauser, U. N-CAM mutation inhibits tangential neuronal migration and is phenocopied by enzymatic removal of polysialic acid. Neuron 1994, 13, 595–609. [Google Scholar] [CrossRef]
- Sato, C.; Kitajima, K. Disialic, oligosialic and polysialic acids: Distribution, functions and related disease. J. Biochem. 2013, 154, 115–136. [Google Scholar] [CrossRef] [PubMed]
- Kanato, Y.; Kitajima, K.; Sato, C. Direct binding of polysialic acid to a brain-derived neurotrophic factor depends on the degree of polymerization. Glycobiology 2008, 18, 1044–1053. [Google Scholar] [CrossRef] [PubMed]
- Vutskits, L.; Djebbara-Hannas, Z.; Zhang, H.; Paccaud, J.P.; Durbec, P.; Rougon, G.; Muller, D.; Kiss, J.Z. PSA-NCAM modulates BDNF-dependent survival and differentiation of cortical neurons. Eur. J. Neurosci. 2001, 13, 1391–1402. [Google Scholar] [CrossRef] [PubMed]
- Ono, S.; Hane, M.; Kitajima, K.; Sato, C. Novel regulation of fibroblast growth factor 2 (FGF2)-mediated cell growth by polysialic acid. J. Biol. Chem. 2012, 287, 3710–3722. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, H.; Dityatev, A. Polysialic acid in brain development and synaptic plasticity. Top. Curr. Chem. 2015, 366, 55–96. [Google Scholar] [PubMed]
- Lackie, P.M.; Zuber, C.; Roth, J. Polysialic acid of the neural cell adhesion molecule (N-CAM) is widely expressed during organogenesis in mesodermal and endodermal derivatives. Differentiation 1994, 57, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, A.; Lu, W.Y.; Weinhold, B.; Boulter, L.; Stutchfield, B.M.; Williams, M.J.; Guest, R.V.; Minnis-Lyons, S.E.; Mackinnon, A.C.; Schwarzer, D.; et al. PolySia-NCAM modulates the formation of ductular reactions in liver injury. Hepatology 2014. [Google Scholar] [CrossRef]
- Roskams, T.; Desmet, V. Ductular reaction and its diagnostic significance. Semin. Diagn. Pathol. 1998, 15, 259–269. [Google Scholar] [PubMed]
- Lackie, P.M.; Zuber, C.; Roth, J. Expression of polysialylated N-CAM during rat heart development. Differentiation 1991, 47, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Timm, M.; Fallah-Najmabadi, H. Cardiac expression of polysialylated NCAM in the chicken embryo: Correlation with the ventricular conduction system. Dev. Dyn. 1992, 194, 128–141. [Google Scholar] [CrossRef] [PubMed]
- Lackie, P.M.; Zuber, C.; Roth, J. Polysialic acid and N-CAM localisation in embryonic rat kidney: Mesenchymal and epithelial elements show different patterns of expression. Development 1990, 110, 933–947. [Google Scholar] [PubMed]
- Mayerhofer, A.; Seidl, K.; Lahr, G.; Bitter-Suermann, D.; Christoph, A.; Barthels, D.; Wille, W.; Gratzl, M. Leydig cells express neural cell adhesion molecules in vivo and in vitro. Biol. Reprod. 1992, 47, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Mayerhofer, A.; Lahr, G.; Seidl, K.; Eusterschulte, B.; Christoph, A.; Gratzl, M. The neural cell adhesion molecule (NCAM) provides clues to the development of testicular Leydig cells. J. Androl. 1996, 17, 223–230. [Google Scholar] [PubMed]
- Simon, P.; Feuerstacke, C.; Kaese, M.; Saboor, F.; Middendorff, R.; Galuska, S.P. Polysialylation of NCAM characterizes the proliferation period of contractile elements during postnatal development of the epididymis. PLoS ONE 2015, 10, e0123960. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.A.; Giustizieri, M.L.; Favale, A.; Fantini, M.C.; Campagnolo, L.; Konda, D.; Germano, F.; Farini, D.; Manna, C.; Siracusa, G. Spatiotemporal patterns of expression of neurotrophins and neurotrophin receptors in mice suggest functional roles in testicular and epididymal morphogenesis. Biol. Reprod. 1999, 61, 1123–1132. [Google Scholar] [CrossRef] [PubMed]
- Sumida, M.; Hane, M.; Yabe, U.; Shimoda, Y.; Pearce, O.M.; Kiso, M.; Miyagi, T.; Sawada, M.; Varki, A.; Kitajima, K.; et al. Rapid trimming of cell surface polySia by exovesicular sialidase triggers release of preexisting surface neurotrophin. J. Biol. Chem. 2015, 290, 13202–13214. [Google Scholar] [CrossRef] [PubMed]
- Hänsch, M.; Simon, P.; Schön, J.; Kaese, M.; Braun, B.C.; Jewgenow, K.; Göritz, F.; Küpper, J.; Ahmadvand, N.; Geyer, R.; et al. Polysialylation of NCAM correlates with onset and termination of seasonal spermatogenesis in roe deer. Glycobiology 2014, 24, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Bronson, F.H.; Heidemann, P.D. Seasonal regulation of reproduction in mammals. In The Physiology of Reproduction; Knobil, E., Neill, J.D., Eds.; Raven Press: New York, NY, USA, 1994; pp. 541–583. [Google Scholar]
- Klonisch, T.; Schön, J.; Hombach-Klonisch, S.; Blottner, S. The roe deer as a model for studying seasonal regulation of testis function. Int. J. Androl. 2006, 29, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Schön, J.; Goritz, F.; Streich, J.; Blottner, S. Histological organization of roe deer testis throughout the seasonal cycle: Variable and constant components of tubular and interstitial compartment. Anat. Embryol. 2004, 208, 151–159. [Google Scholar] [PubMed]
- Eddy, E.M.; Chen, L.Y. Location, location, location: How does a spermatogonium know it is a spermatogonial stem cell (SSC)? Biol. Reprod. 2013, 88. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Sharma, M.; Nabeshima, Y.; Braun, R.E.; Yoshida, S. Functional hierarchy and reversibility within the murine spermatogenic stem cell compartment. Science 2010, 328, 62–67. [Google Scholar] [CrossRef] [PubMed]
- De Rooij, D.G.; Mizrak, S.C. Deriving multipotent stem cells from mouse spermatogonial stem cells: A new tool for developmental and clinical research. Development 2008, 135, 2207–2213. [Google Scholar] [CrossRef] [PubMed]
- Robaire, B.; Hinton, B.T.; Orgebin-Crist, M.-C. The Epididymis in Knobil and Neill’ Physiology of Reproduction. Available online: https://www.elsevier.com/books/knobil-and-neills-physiology-of-reproduction/plant/978-0-12-397175-3 (accessed on 6 September 2016).
- Middendorff, R.; Muller, D.; Mewe, M.; Mukhopadhyay, A.K.; Holstein, A.F.; Davidoff, M.S. The tunica albuginea of the human testis is characterized by complex contraction and relaxation activities regulated by cyclic GMP. J. Clin. Endocrinol. Metab. 2002, 87, 3486–3499. [Google Scholar] [CrossRef] [PubMed]
- Monzo, H.J.; Park, T.I.; Dieriks, B.V.; Jansson, D.; Faull, R.L.; Dragunow, M.; Curtis, M.A. Insulin and IGF1 modulate turnover of polysialylated neural cell adhesion molecule (PSA-NCAM) in a process involving specific extracellular matrix components. J. Neurochem. 2013, 126, 758–770. [Google Scholar] [CrossRef] [PubMed]
- Rossant, J.; Cross, J.C. Placental development: Lessons from mouse mutants. Nat. Rev. Genet. 2001, 2, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Red-Horse, K.; Zhou, Y.; Genbacev, O.; Prakobphol, A.; Foulk, R.; McMaster, M.; Fisher, S.J. Trophoblast differentiation during embryo implantation and formation of the maternal-fetal interface. J. Clin. Investig. 2004, 114, 744–754. [Google Scholar] [CrossRef] [PubMed]
- Hromatka, B.S.; Drake, P.M.; Kapidzic, M.; Stolp, H.; Goldfien, G.A.; Shih Ie, M.; Fisher, S.J. Polysialic acid enhances the migration and invasion of human cytotrophoblasts. Glycobiology 2013, 23, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Kibbelaar, R.E.; Moolenaar, C.E.; Michalides, R.J.; Bitter-Suermann, D.; Addis, B.J.; Mooi, W.J. Expression of the embryonal neural cell adhesion molecule N-CAM in lung carcinoma. Diagnostic usefulness of monoclonal antibody 735 for the distinction between small cell lung cancer and non-small cell lung cancer. J. Pathol. 1989, 159, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Moolenaar, C.E.; Muller, E.J.; Schol, D.J.; Figdor, C.G.; Bock, E.; Bitter-Suermann, D.; Michalides, R.J. Expression of neural cell adhesion molecule-related sialoglycoprotein in small cell lung cancer and neuroblastoma cell lines H69 and CHP-212. Cancer Res. 1990, 50, 1102–1106. [Google Scholar] [PubMed]
- Martersteck, C.M.; Kedersha, N.L.; Drapp, D.A.; Tsui, T.G.; Colley, K.J. Unique alpha 2, 8-polysialylated glycoproteins in breast cancer and leukemia cells. Glycobiology 1996, 6, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, F.; Otake, Y.; Nakagawa, T.; Kawano, Y.; Miyahara, R.; Li, M.; Yanagihara, K.; Nakayama, J.; Fujimoto, I.; Ikenaka, K.; et al. Expression of polysialic acid and STX, a human polysialyltransferase, is correlated with tumor progression in non-small cell lung cancer. Cancer Res. 2000, 60, 3072–3080. [Google Scholar] [PubMed]
- Tanaka, F.; Otake, Y.; Nakagawa, T.; Kawano, Y.; Miyahara, R.; Li, M.; Yanagihara, K.; Inui, K.; Oyanagi, H.; Yamada, T.; et al. Prognostic significance of polysialic acid expression in resected non-small cell lung cancer. Cancer Res. 2001, 61, 1666–1670. [Google Scholar] [PubMed]
- Whitlon, D.S.; Zhang, X. Polysialic acid in the cochlea of the developing mouse. Int. J. Dev. Neurosci. 1997, 15, 657–669. [Google Scholar] [CrossRef]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galuska, C.E.; Lütteke, T.; Galuska, S.P. Is Polysialylated NCAM Not Only a Regulator during Brain Development But also during the Formation of Other Organs? Biology 2017, 6, 27. https://doi.org/10.3390/biology6020027
Galuska CE, Lütteke T, Galuska SP. Is Polysialylated NCAM Not Only a Regulator during Brain Development But also during the Formation of Other Organs? Biology. 2017; 6(2):27. https://doi.org/10.3390/biology6020027
Chicago/Turabian StyleGaluska, Christina E., Thomas Lütteke, and Sebastian P. Galuska. 2017. "Is Polysialylated NCAM Not Only a Regulator during Brain Development But also during the Formation of Other Organs?" Biology 6, no. 2: 27. https://doi.org/10.3390/biology6020027





