Lipid Production in Nannochloropsis gaditana during Nitrogen Starvation
Abstract
:1. Introduction
2. Triacylglycerol (TAG)
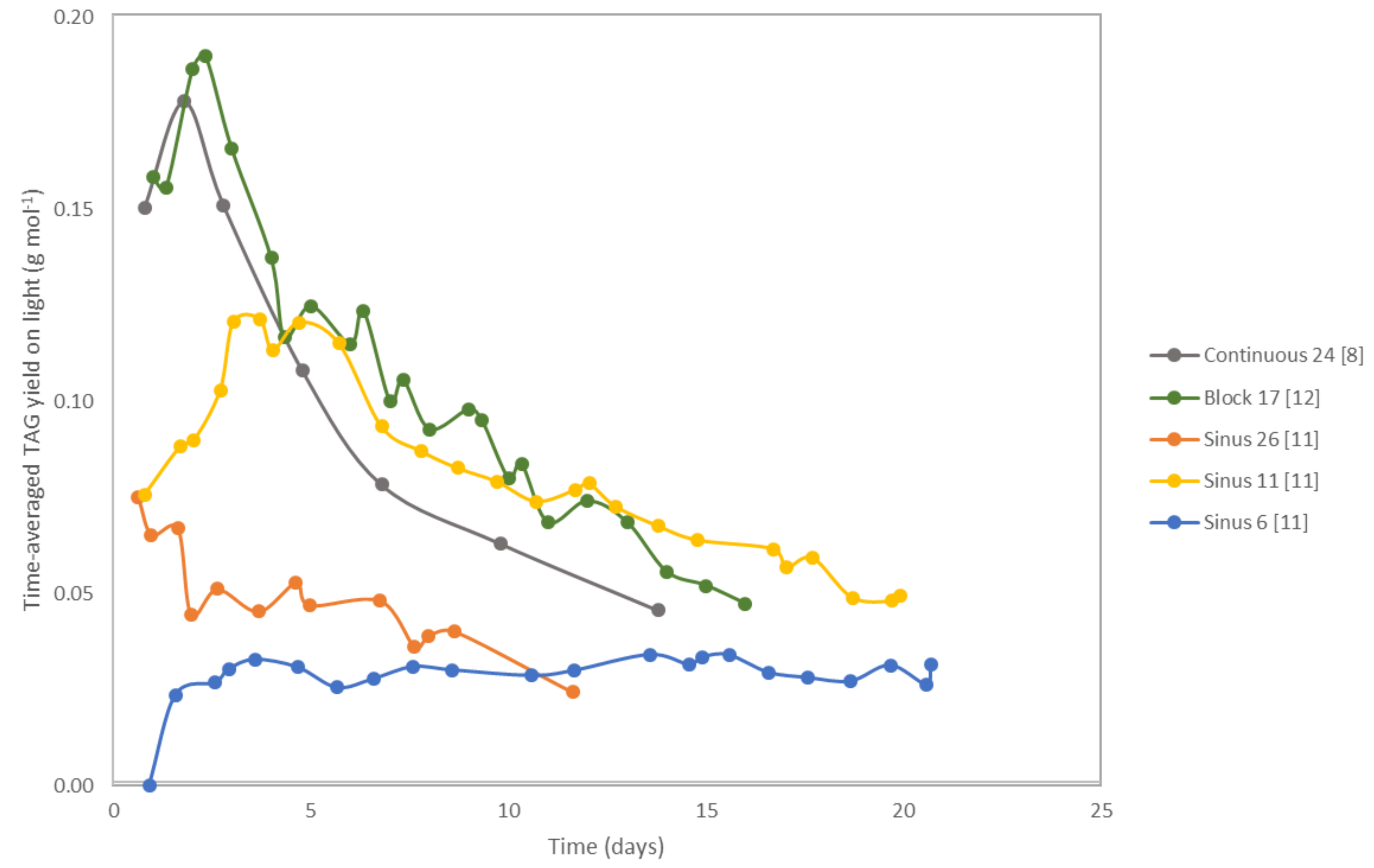
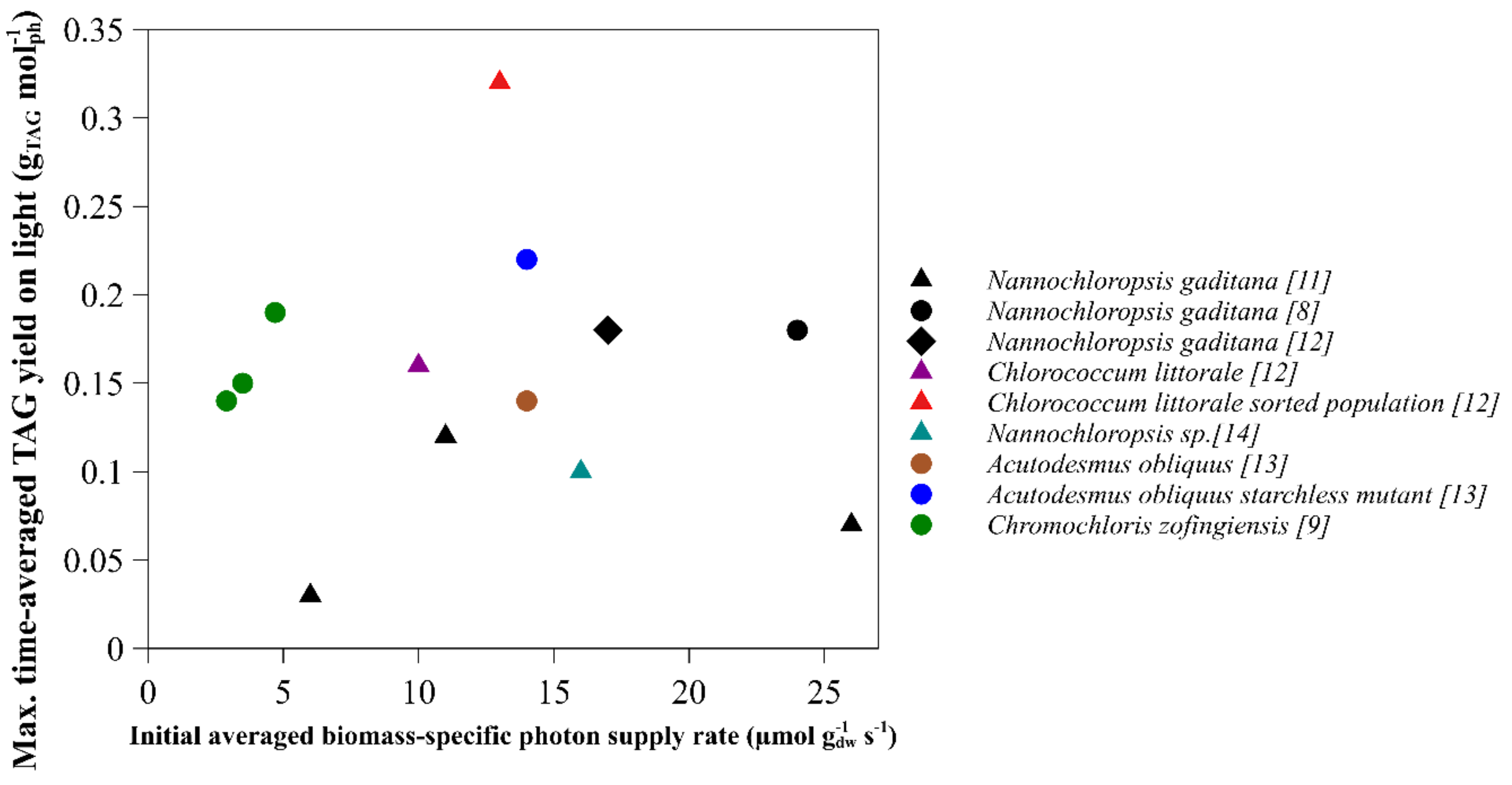
2.1. Time-Averaged TAG Yield on Light
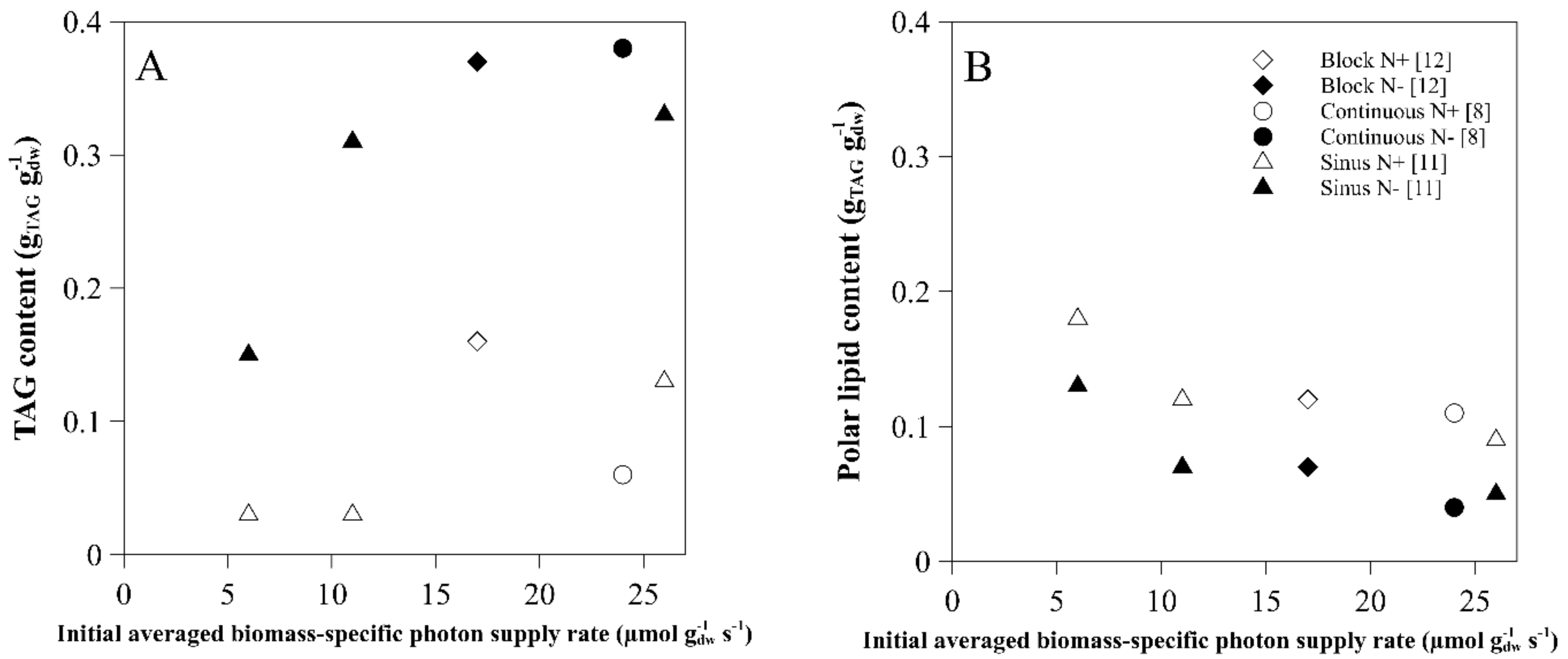
2.2. TAG Content
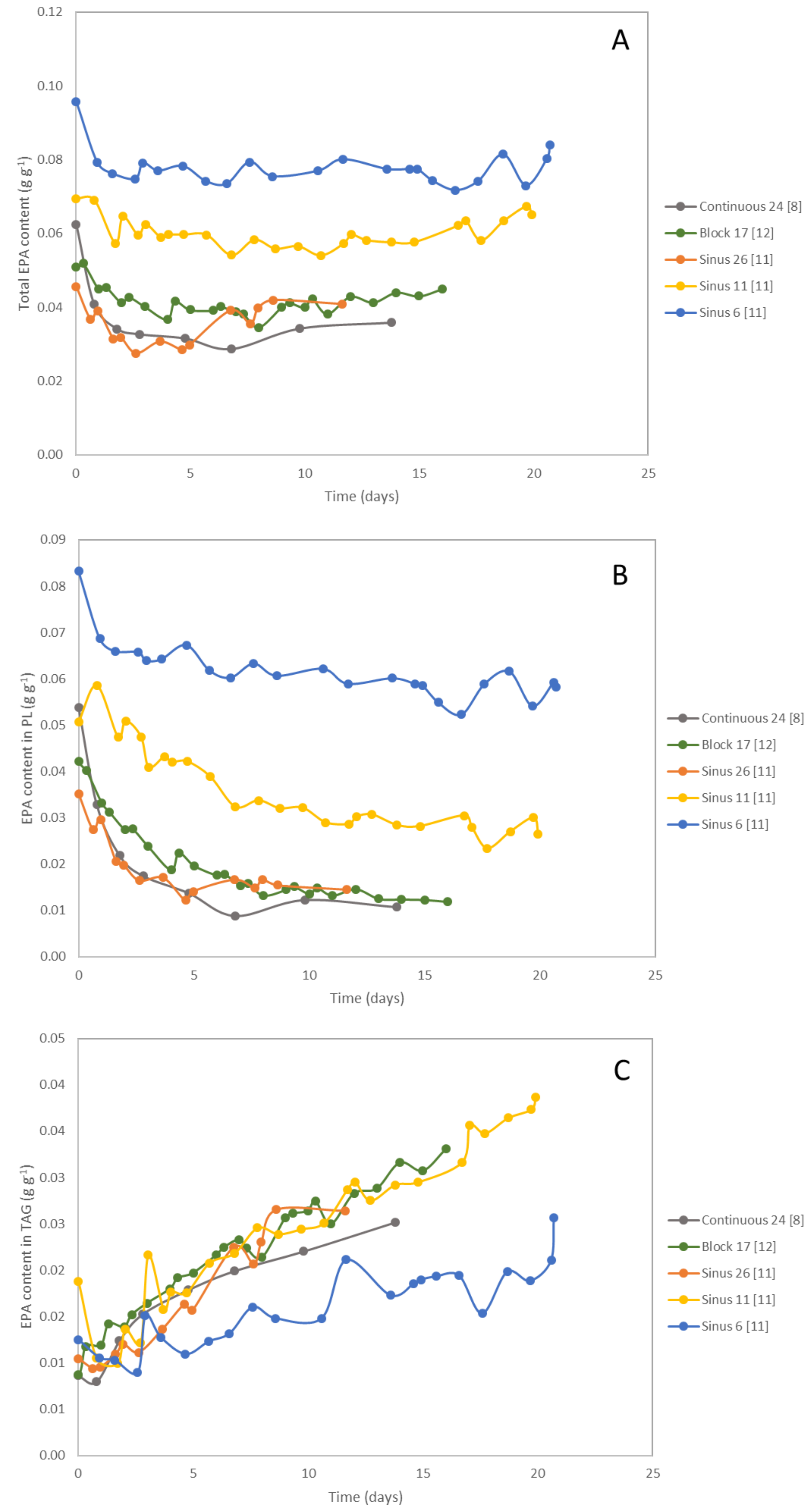
3. Polar Lipids
4. Eicosapentaenoic Acid (EPA)
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Species | Light Regime | Initial Biomass Concentration (g L−1) | Average Light Intensity per day (µmol m−2 s−1) | Initial Average Biomass-Specific Photon Supply Rate (µmol g−1 s−1) | TAG Yield on Light (g mol−1) | Reference | Remarks |
|---|---|---|---|---|---|---|---|
| Nannochloropsis gaditana | Continuous | 1.3 | 636 | 24 | 0.18 | [8] | |
| Nannochloropsis gaditana | Block | 1.2 | 424 | 17 | 0.19 | [12] | Runout estimated by nitrogen content |
| Nannochloropsis gaditana | Sinus | 1.2 | 636 | 26 | 0.07 | [11] | |
| Nannochloropsis gaditana | Sinus | 2.9 | 636 | 11 | 0.12 | [11] | |
| Nannochloropsis gaditana | Sinus | 5.4 | 636 | 6 | 0.03 | [11] | |
| Nannochloropsis sp. | Sinus | 1.9 | 636 | 16 | 0.1 | [15] | |
| Chlorococcum littorale | Sinus | 3.1 | 636 | 10 | 0.16 | [13] | |
| Chlorococcum littorale (sorted) | Sinus | 2.4 | 636 | 13 | 0.32 | [13] | |
| Chromochloris zofingiensis | Continuous | 2.5 | 245 | 4.7 | 0.19 | [10] | Yield absorbed light |
| Chromochloris zofingiensis | Continuous | 3.4 | 245 | 3.5 | 0.15 | [10] | |
| Chromochloris zofingiensis | Continuous | 4.1 | 245 | 2.9 | 0.14 | [10] | |
| Acutodesmus obliquus10 | Continuous | 1.7 | 500 | 14 | 0.14 | [14] | Runout between 1.5–2 g/L averaged |
| Acutodesmus obliquus (starchless mutant) | Continuous | 1.7 | 500 | 14 | 0.22 | [14] | Runout between 1.5–2 g/L averaged |

References
- Ma, X.N.; Chen, T.-P.; Yang, B.; Liu, J.; Chen, F. Lipid production from Nannochloropsis. Mar. Drugs 2016, 14. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Sommerfeld, M.; Jarvis, E.; Ghirardi, M.; Posewitz, M.; Seibert, M.; Darzins, A. Microalgal triacylglycerols as feedstocks for biofuel production: Perspectives and advances. Plant J. 2008, 54, 621–639. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Benning, C. Lipid metabolism in microalgae distinguishes itself. Curr. Opin. Biotechnol. 2013, 24, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Mühlroth, A.; Li, K.; Røkke, G.; Winge, P.; Olsen, Y.; Hohmann-Marriott, M.F.; Vadstein, O.; Bones, A.M. Pathways of lipid metabolism in marine algae, co-expression network, bottlenecks and candidate genes for enhanced production of EPA and DHA in species of chromista. Mar. Drugs 2013, 11, 4662–4697. [Google Scholar] [CrossRef]
- Remmers, I.M.; Martens, D.E.; Wijffels, R.H.; Lamers, P.P. Dynamics of triacylglycerol and EPA production in Phaeodactylum tricornutum under nitrogen starvation at different light intensities. PLoS ONE 2017, 12, e0175630. [Google Scholar] [CrossRef]
- Tonon, T.; Harvey, D.; Larson, T.R.; Graham, I.A. Long chain polyunsaturated fatty acid production and partitioning to triacylglycerols in four microalgae. Phytochemistry 2002, 61, 15–24. [Google Scholar] [CrossRef]
- Khozin-Goldberg, I.; Cohen, Z. Unraveling algal lipid metabolism: Recent advances in gene identification. Biochimie 2011, 93, 91–100. [Google Scholar] [CrossRef]
- Janssen, J.H.; Lamers, P.P.; de Vos, R.C.H.; Wijffels, R.H.; Barbosa, M.J. Translocation and de novo synthesis of eicosapentaenoic acid (EPA) during nitrogen starvation in Nannochloropsis gaditana. Algal Res. 2019, 37, 138–144. [Google Scholar] [CrossRef]
- Schüler, L.M.; Schulze, P.S.C.; Pereira, H.; Barreira, L.; León, R.; Varela, J. Trends and strategies to enhance triacylglycerols and high-value compounds in microalgae. Algal Res. 2017, 25, 263–273. [Google Scholar] [CrossRef]
- Mulders, K.J.M.; Janssen, J.H.; Martens, D.E.; Wijffels, R.H.; Lamers, P.P. Effect of biomass concentration on secondary carotenoids and triacylglycerol (TAG) accumulation in nitrogen-depleted Chlorella zofingiensis. Algal Res. 2014, 6, 8–16. [Google Scholar] [CrossRef]
- Janssen, J.H.; Driessen, J.L.S.P.; Lamers, P.P.; Wijffels, R.H.; Barbosa, M.J. Effect of initial biomass-specific photon supply rate on fatty acid accumulation in nitrogen depleted Nannochloropsis gaditana under simulated outdoor light conditions. Algal Res. 2018, 35, 595–601. [Google Scholar] [CrossRef]
- Janssen, J.H.; Kastenhofer, J.; de Hoop, J.A.; Lamers, P.P.; Wijffels, R.H.; Barbosa, M.J. Effect of nitrogen addition on lipid productivity of nitrogen starved Nannochloropsis gaditana. Algal Res. 2018, 33, 125–132. [Google Scholar] [CrossRef]
- Cabanelas, I.T.D.; van der Zwart, M.; Kleinegris, D.M.M.; Wijffels, R.H.; Barbosa, M.J. Sorting cells of the microalga Chlorococcum littorale with increased triacylglycerol productivity. Biotechnol. Biofuels 2016, 9, 1–12. [Google Scholar] [CrossRef]
- Breuer, G.; de Jaeger, L.; Artus, V.P.G.; Martens, D.E.; Springer, J.; Draaisma, R.B.; Eggink, G.; Wijffels, R.H.; Lamers, P.P. Superior triacylglycerol (TAG) accumulation in starchless mutants of Scenedesmus obliquus: (II) evaluation of TAG yield and productivity in controlled photobioreactor. Biotechnol. Biofuels 2014, 7. [Google Scholar] [CrossRef]
- Benvenuti, G.; Bosma, R.; Ji, F.; Lamers, P.; Barbosa, M.J.; Wijffels, R.H. Batch and semi-continuous microalgal TAG production in lab-scale and outdoor photobioreactors. J. Appl. Phycol. 2016, 28, 3167–3177. [Google Scholar] [CrossRef] [Green Version]
- Sukenik, A.; Zmora, O.; Carmeli, Y. Biochemical quality of marine unicellular algae with special emphasis on lipid composition. II. Nannochloropsis sp. Aquaculture 1993, 117, 313–326. [Google Scholar] [CrossRef]
- Sukenik, A.; Carmeli, Y.; Berner, T. Regulation of fatty acid synthesis by irradiance level in the eustigmatophyte Nannochloropsis sp. J. Phycol. 1989, 25, 686–692. [Google Scholar] [CrossRef]
- Ajjawi, I.; Verruto, J.; Aqui, M.; Soriaga, L.B.; Coppersmith, J.; Kwok, K.; Peach, L.; Orchard, E.; Kalb, R.; Xu, W.; et al. Lipid production in Nannochloropsis gaditana is doubled by decreasing expression of a single transcriptional regulator. Nat. Biotechnol. 2017, 35, 647–652. [Google Scholar] [CrossRef]
- Khozin-Goldberg, I.; Shrestha, P.; Cohen, Z. Mobilization of arachidonyl moieties from triacylglycerols into chloroplastic lipids following recovery from nitrogen starvation of the microalga Parietochloris incisa. Biochim. Biophys. Acta 2005, 1738, 63–71. [Google Scholar] [CrossRef]
- Řezanka, T.; Lukavský, J.; Nedbalová, L.; Sigler, K. Effect of nitrogen and phosphorus starvation on the polyunsaturated triacylglycerol composition, including positional isomer distribution, in the alga Trachydiscus minutus. Phytochemistry 2011, 72, 2342–2351. [Google Scholar] [CrossRef]
- Yoon, K.; Han, D.; Li, Y.; Sommerfeld, M.; Hu, Q. Phospholipid:Diacylglycerol Acyltransferase Is a Multifunctional Enzyme Involved in Membrane Lipid Turnover and Degradation While Synthesizing Triacylglycerol in the Unicellular Green Microalga Chlamydomonas reinhardtii. Plant Cell 2012, 24, 3708–3724. [Google Scholar] [CrossRef] [PubMed]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janssen, J.H.; Wijffels, R.H.; Barbosa, M.J. Lipid Production in Nannochloropsis gaditana during Nitrogen Starvation. Biology 2019, 8, 5. https://doi.org/10.3390/biology8010005
Janssen JH, Wijffels RH, Barbosa MJ. Lipid Production in Nannochloropsis gaditana during Nitrogen Starvation. Biology. 2019; 8(1):5. https://doi.org/10.3390/biology8010005
Chicago/Turabian StyleJanssen, Jorijn H., René H. Wijffels, and Maria J. Barbosa. 2019. "Lipid Production in Nannochloropsis gaditana during Nitrogen Starvation" Biology 8, no. 1: 5. https://doi.org/10.3390/biology8010005
APA StyleJanssen, J. H., Wijffels, R. H., & Barbosa, M. J. (2019). Lipid Production in Nannochloropsis gaditana during Nitrogen Starvation. Biology, 8(1), 5. https://doi.org/10.3390/biology8010005





