Cholesterol Homeostasis: An In Silico Investigation into How Aging Disrupts Its Key Hepatic Regulatory Mechanisms
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Network Diagram Construction and Model Assembly
2.2. Model Analysis
2.3. Hypothesis Exploration
3. Results
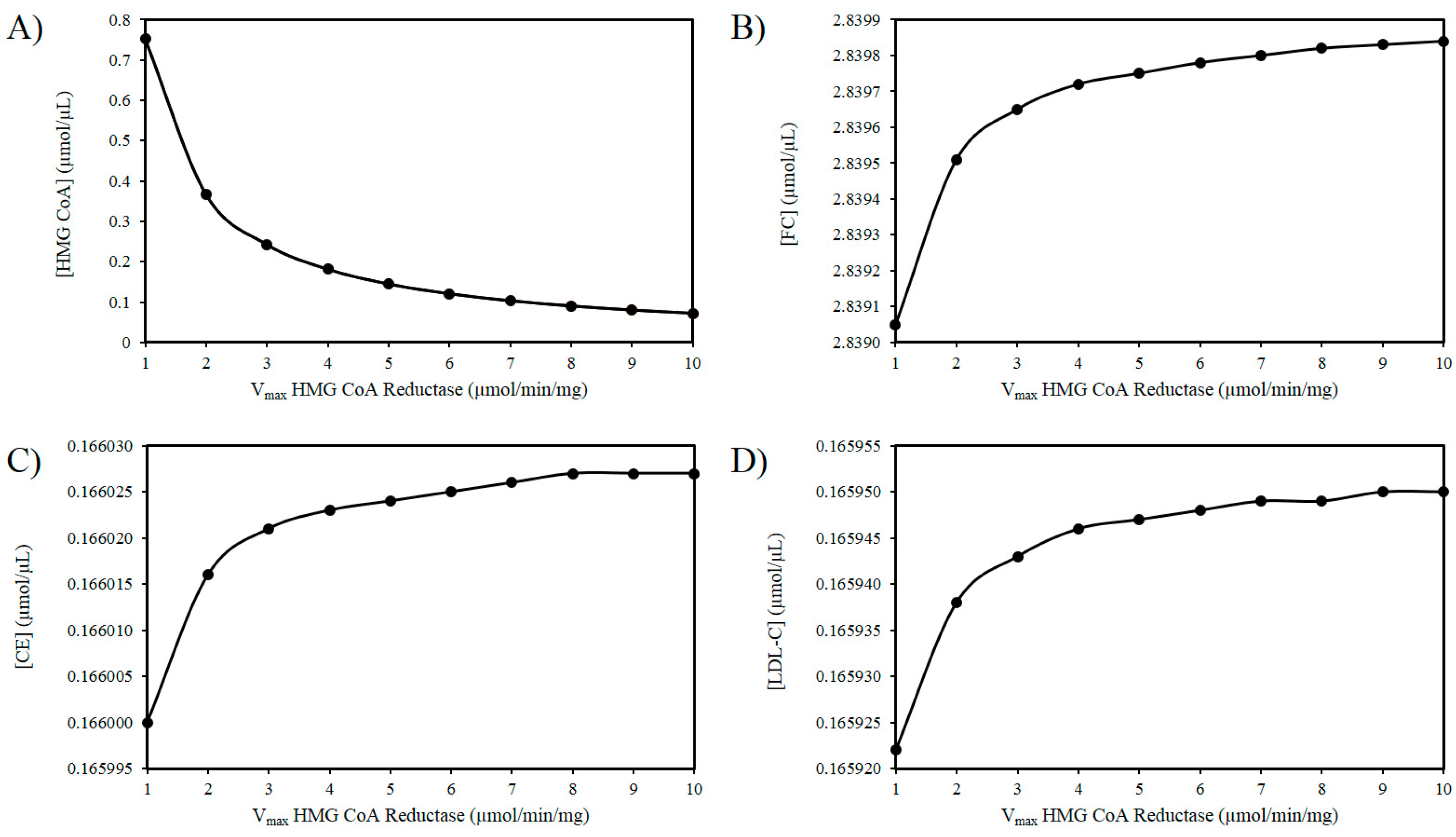
3.1. The ROS and HMG-CoA Reductase Hypothesis
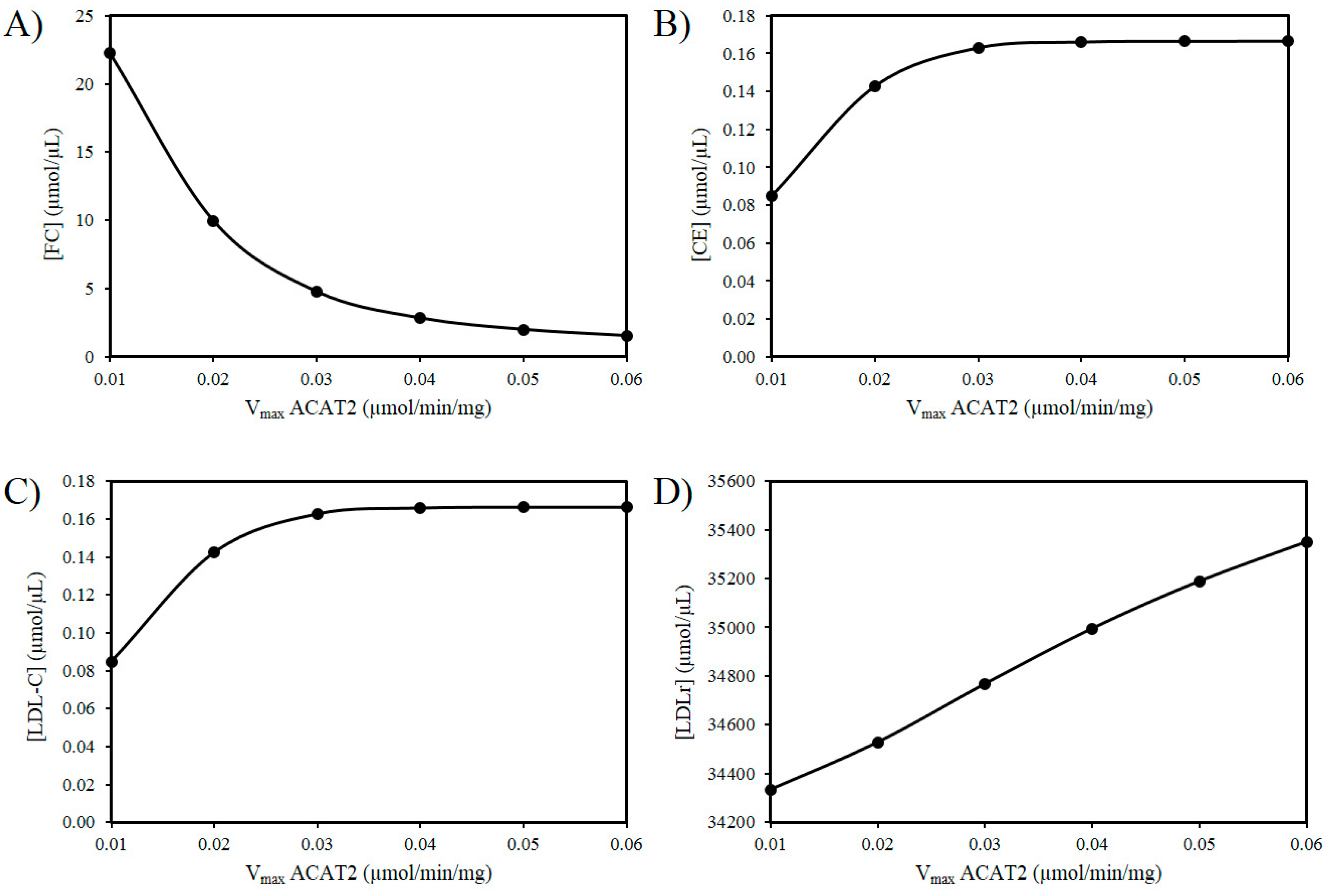
3.2. Exploring the Effects of a Decrease in ACAT2 Activity with Age
3.3. ROS Combined with a Decrease in ACAT2 with Age
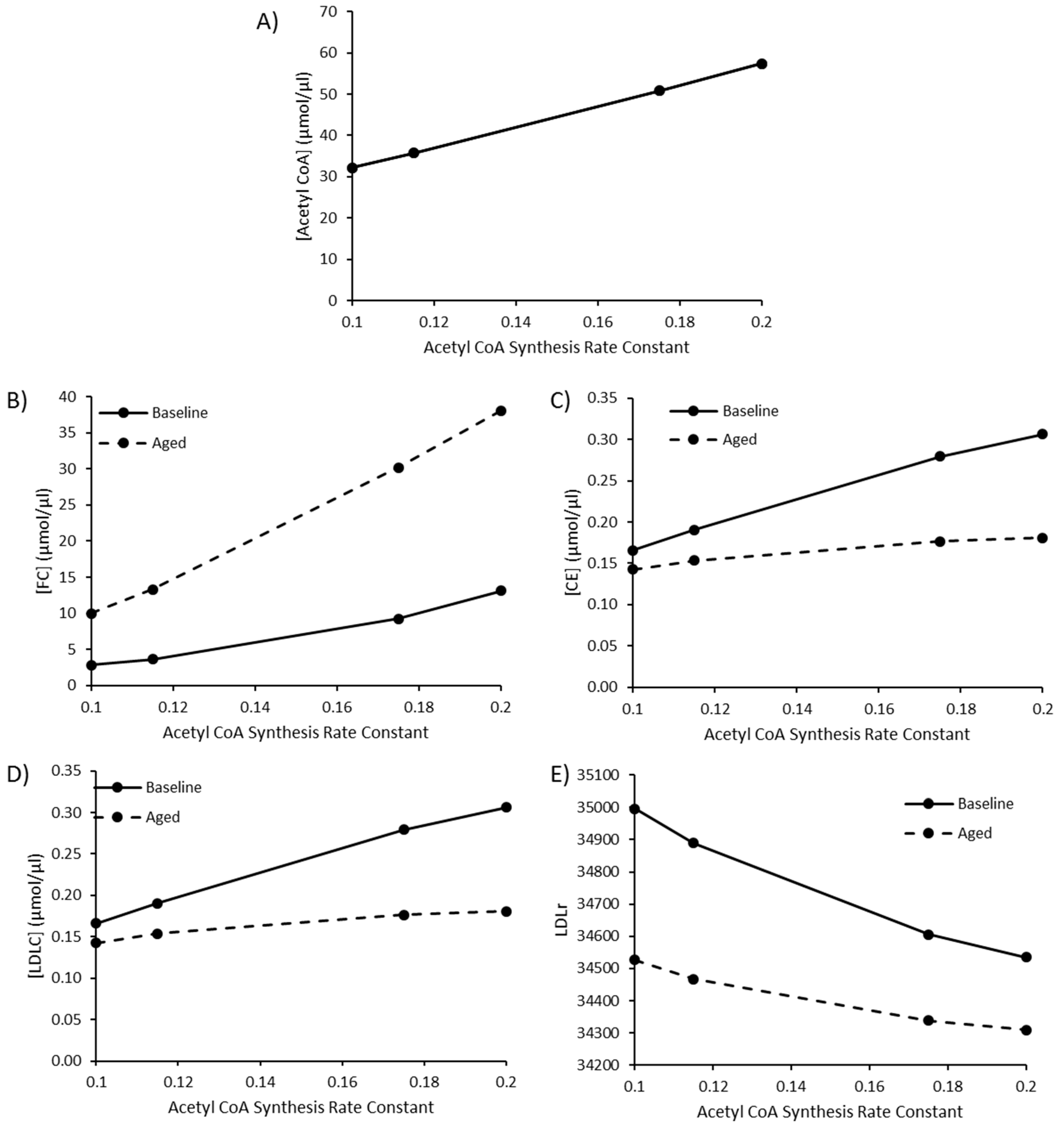
3.4. The Impact of Increasing Acetyl CoA Synthesis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Homan, R.; Krause, B.R. Established and emerging strategies for inhibition of cholesterol absorption. Curr. Pharm. Des. 1997, 3, 29–44. [Google Scholar]
- van der Wulp, M.Y.; Verkade, H.J.; Groen, A.K. Regulation of cholesterol homeostasis. Mol. Cell. Endocrinol. 2013, 368, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Javitt, N.B. Bile acid synthesis from cholesterol: Regulatory and auxiliary pathways. FASEB J. 1994, 8, 1308–1311. [Google Scholar] [CrossRef] [PubMed]
- Payne, A.H.; Hales, D.B. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr. Rev. 2004, 25, 947–970. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Vitamin d metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef] [Green Version]
- Castelli, W.P.; Anderson, K.; Wilson, P.W.; Levy, D. Lipids and risk of coronary heart disease. The framingham study. Ann. Epidemiol. 1992, 2, 23–28. [Google Scholar] [CrossRef]
- O’Donnell, C.J.; Elosua, R. Cardiovascular risk factors. Insights from framingham heart study. Rev. Esp. Cardiol. 2008, 61, 299–310. [Google Scholar]
- Mc Auley, M.T.; Mooney, K.M. Lipid metabolism and hormonal interactions: Impact on cardiovascular disease and healthy aging. Expert Rev. Endocrinol. Metab. 2014, 9, 357–367. [Google Scholar] [CrossRef] [Green Version]
- Mooney, K.M.; Mc Auley, M.T. Cardiovascular disease and healthy ageing. J. Integr. Cardiol. 2016, 1, 76–78. [Google Scholar] [CrossRef]
- Fajemiroye, J.O.; Cunha, L.C.d.; Saavedra-Rodríguez, R.; Rodrigues, K.L.; Naves, L.M.; Mourão, A.A.; Silva, E.F.d.; Williams, N.E.E.; Martins, J.L.R.; Sousa, R.B. Aging-induced biological changes and cardiovascular diseases. Biomed. Res. Int. 2018, 2018, 7156435. [Google Scholar] [CrossRef] [Green Version]
- Austin, M.A.; Breslow, J.L.; Hennekens, C.H.; Buring, J.E.; Willett, W.C.; Krauss, R.M. Low-density lipoprotein subclass patterns and risk of myocardial infarction. JAMA 1988, 260, 1917–1921. [Google Scholar] [CrossRef] [PubMed]
- Ference, B.A.; Ginsberg, H.N.; Graham, I.; Ray, K.K.; Packard, C.J.; Bruckert, E.; Hegele, R.A.; Krauss, R.M.; Raal, F.J.; Schunkert, H. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the european atherosclerosis society consensus panel. Eur. Heart J. 2017, 38, 2459–2472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, A.E.; Mooney, K.M.; Wilkinson, S.J.; Pickles, N.A.; Mc Auley, M.T. Cholesterol metabolism: A review of how ageing disrupts the biological mechanisms responsible for its regulation. Ageing Res. Rev. 2016, 27, 108–124. [Google Scholar] [CrossRef]
- Kreisberg, R.A.; Kasim, S. Cholesterol metabolism and aging. Am. J. Med. 1987, 82, 54–60. [Google Scholar] [CrossRef]
- Berrougui, H.; Khalil, A. Age-associated decrease of high-density lipoprotein-mediated reverse cholesterol transport activity. Rejuvenation Res. 2009, 12, 117–126. [Google Scholar] [CrossRef]
- Holzer, M.; Trieb, M.; Konya, V.; Wadsack, C.; Heinemann, A.; Marsche, G. Aging affects high-density lipoprotein composition and function. Biochim. Biophys. Acta 2013, 1831, 1442–1448. [Google Scholar] [CrossRef] [Green Version]
- Parini, P.; Angelin, B.; Rudling, M. Cholesterol and lipoprotein metabolism in aging: Reversal of hypercholesterolemia by growth hormone treatment in old rats. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 832–839. [Google Scholar] [CrossRef]
- Uchida, K.; Nomura, Y.; Kadowaki, M.; Takase, H.; Takano, K.; Takeuchi, N. Age-related changes in cholesterol and bile acid metabolism in rats. J. Lipid Res. 1978, 19, 544–552. [Google Scholar]
- Liu, H.H.; Li, J.J. Aging and dyslipidemia: A review of potential mechanisms. Ageing Res. Rev. 2015, 19, 43–52. [Google Scholar] [CrossRef]
- Bertolotti, M.; Abate, N.; Bertolotti, S.; Loria, P.; Concari, M.; Messora, R.; Carubbi, F.; Pinetti, A.; Carulli, N. Effect of aging on cholesterol 7 alpha-hydroxylation in humans. J. Lipid Res. 1993, 34, 1001–1007. [Google Scholar]
- Mc Auley, M.; Jones, J.; Wilkinson, D.; Kirkwood, T. Modelling lipid metabolism to improve healthy ageing. BMC Bioinform. 2005, 6, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carroll, M.D.; Lacher, D.A.; Sorlie, P.D.; Cleeman, J.I.; Gordon, D.J.; Wolz, M.; Grundy, S.M.; Johnson, C.L. Trends in serum lipids and lipoproteins of adults, 1960–2002. JAMA 2005, 294, 1773–1781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Hou, X.; Hu, W.; Chen, L.; Chen, S. Serum lipid and lipoprotein levels of middle-aged and elderly chinese men and women in shandong province. Lipids Health Dis. 2019, 18, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farzadfar, F.; Finucane, M.M.; Danaei, G.; Pelizzari, P.M.; Cowan, M.J.; Paciorek, C.J.; Singh, G.M.; Lin, J.K.; Stevens, G.A.; Riley, L.M.; et al. National, regional, and global trends in serum total cholesterol since 1980: Systematic analysis of health examination surveys and epidemiological studies with 321 country-years and 3.0 million participants. Lancet 2011, 377, 578–586. [Google Scholar] [CrossRef]
- Duncan, M.S.; Vasan, R.S.; Xanthakis, V. Trajectories of blood lipid concentrations over the adult life course and risk of cardiovascular disease and all-cause mortality: Observations from the framingham study over 35 years. J. Am. Heart Assoc. 2019, 8, e011433. [Google Scholar] [CrossRef] [Green Version]
- Felix-Redondo, F.J.; Grau, M.; Fernandez-Berges, D. Cholesterol and cardiovascular disease in the elderly. Facts and gaps. Aging Dis. 2013, 4, 154–169. [Google Scholar]
- Ferrara, A.; Barrett-Connor, E.; Shan, J. Total, ldl, and hdl cholesterol decrease with age in older men and women. The rancho bernardo study 1984–1994. Circulation 1997, 96, 37–43. [Google Scholar] [CrossRef]
- Garry, P.J.; Hunt, W.C.; Koehler, K.M.; VanderJagt, D.J.; Vellas, B.J. Longitudinal study of dietary intakes and plasma lipids in healthy elderly men and women. Am. J. Clin. Nutr. 1992, 55, 682–688. [Google Scholar] [CrossRef]
- Postmus, I.; Deelen, J.; Sedaghat, S.; Trompet, S.; de Craen, A.J.; Heijmans, B.T.; Franco, O.H.; Hofman, A.; Dehghan, A.; Slagboom, P.E.; et al. Ldl cholesterol still a problem in old age? A mendelian randomization study. Int. J. Epidemiol. 2015, 44, 604–612. [Google Scholar] [CrossRef] [Green Version]
- Weverling-Rijnsburger, A.W.; Jonkers, I.J.; van Exel, E.; Gussekloo, J.; Westendorp, R.G. High-density vs. low-density lipoprotein cholesterol as the risk factor for coronary artery disease and stroke in old age. Arch. Intern. Med. 2003, 163, 1549–1554. [Google Scholar] [CrossRef] [Green Version]
- Ravnskov, U. High cholesterol may protect against infections and atherosclerosis. QJM 2003, 96, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Han, R. Plasma lipoproteins are important components of the immune system. Microbiol. Immunol. 2010, 54, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Ravnskov, U.; de Lorgeril, M.; Diamond, D.M.; Hama, R.; Hamazaki, T.; Hammarskjold, B.; Hynes, N.; Kendrick, M.; Langsjoen, P.H.; Mascitelli, L.; et al. Ldl-c does not cause cardiovascular disease: A comprehensive review of the current literature. Expert Rev. Clin. Pharmacol. 2018, 11, 959–970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravnskov, U.; Diamond, D.M.; Hama, R.; Hamazaki, T.; Hammarskjöld, B.; Hynes, N.; Kendrick, M.; Langsjoen, P.H.; Malhotra, A.; Mascitelli, L. Lack of an association or an inverse association between low-density-lipoprotein cholesterol and mortality in the elderly: A systematic review. BMJ Open 2016, 6, e010401. [Google Scholar] [CrossRef] [PubMed]
- Mc Auley, M.T.; Mooney, K.M. Ldl-c levels in older people: Cholesterol homeostasis and the free radical theory of ageing converge. Med. Hypotheses 2017, 104, 15–19. [Google Scholar] [CrossRef] [Green Version]
- Mc Auley, M.T. The interplay between cholesterol metabolism and intrinsic ageing. Subcell. Biochem. 2018, 90, 99–118. [Google Scholar]
- Tiwari, S.; Siddiqi, S.A. Intracellular trafficking and secretion of vldl. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1079–1086. [Google Scholar] [CrossRef] [Green Version]
- Goldberg, I.J. Lipoprotein lipase and lipolysis: Central roles in lipoprotein metabolism and atherogenesis. J. Lipid Res. 1996, 37, 693–707. [Google Scholar]
- Goldstein, J.L.; Brown, M.S. The ldl receptor. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 431–438. [Google Scholar] [CrossRef] [Green Version]
- Brown, M.S.; Radhakrishnan, A.; Goldstein, J.L. Retrospective on cholesterol homeostasis: The central role of scap. Annu. Rev. Biochem. 2018, 87, 783–807. [Google Scholar] [CrossRef] [Green Version]
- Sato, R. Sterol metabolism and srebp activation. Arch. Biochem. Biophys. 2010, 501, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Eberlé, D.; Hegarty, B.; Bossard, P.; Ferré, P.; Foufelle, F. Srebp transcription factors: Master regulators of lipid homeostasis. Biochimie 2004, 86, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.J.; Lee, H.-S.; Kim, K.-S.; Kim, Y.-K.; Yoon, D.; Park, S.W. Sterol-dependent regulation of proprotein convertase subtilisin/kexin type 9 expression by sterol-regulatory element binding protein-2. J. Lipid Res. 2008, 49, 399–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lagace, T.A. Pcsk9 and ldlr degradation: Regulatory mechanisms in circulation and in cells. Curr. Opin. Lipidol. 2014, 25, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Reiss, A.B.; Shah, N.; Muhieddine, D.; Zhen, J.; Yudkevich, J.; Kasselman, L.J.; DeLeon, J. Pcsk9 in cholesterol metabolism: From bench to bedside. Clin. Sci. 2018, 132, 1135–1153. [Google Scholar] [CrossRef]
- Chaudhary, R.; Garg, J.; Shah, N.; Sumner, A. Pcsk9 inhibitors: A new era of lipid lowering therapy. World J. Cardiol. 2017, 9, 76–91. [Google Scholar] [CrossRef]
- Parini, P.; Davis, M.; Lada, A.T.; Erickson, S.K.; Wright, T.L.; Gustafsson, U.; Sahlin, S.; Einarsson, C.; Eriksson, M.; Angelin, B.; et al. Acat2 is localized to hepatocytes and is the major cholesterol-esterifying enzyme in human liver. Circulation 2004, 110, 2017–2023. [Google Scholar] [CrossRef] [Green Version]
- Semsei, I.; Rao, G.; Richardson, A. Changes in the expression of superoxide dismutase and catalase as a function of age and dietary restriction. Biochem. Biophys. Res. Commun. 1989, 164, 620–625. [Google Scholar] [CrossRef]
- Ji, L.L. Antioxidant enzyme response to exercise and aging. Med. Sci. Sports Exerc. 1993, 25, 225–231. [Google Scholar] [CrossRef]
- Pallottini, V.; Martini, C.; Bassi, A.M.; Romano, P.; Nanni, G.; Trentalance, A. Rat hmgcoa reductase activation in thioacetamide-induced liver injury is related to an increased reactive oxygen species content. J. Hepatol. 2006, 44, 368–374. [Google Scholar] [CrossRef]
- Pallottini, V.; Martini, C.; Cavallini, G.; Bergamini, E.; Mustard, K.J.; Hardie, D.G.; Trentalance, A. Age-related hmg-coa reductase deregulation depends on ros-induced p38 activation. Mech. Ageing Dev. 2007, 128, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Pallottini, V.; Martini, C.; Pascolini, A.; Cavallini, G.; Gori, Z.; Bergamini, E.; Incerpi, S.; Trentalance, A. 3-hydroxy-3-methylglutaryl coenzyme a reductase deregulation and age-related hypercholesterolemia: A new role for ros. Mech. Ageing Dev. 2005, 126, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Trapani, L.; Pallottini, V. Age-related hypercholesterolemia and hmg-coa reductase dysregulation: Sex does matter (a gender perspective). Curr. Gerontol. Geriatr. Res. 2010, 2010, 420139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trapani, L.; Violo, F.; Pallottini, V. Hypercholesterolemia and 3-hydroxy-3-methylglutaryl coenzyme a reductase regulation in aged female rats. Exp. Gerontol. 2010, 45, 119–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, E.; Kang, H.; Choi, H.; Choi, W.; Jun, H.S. Reactive oxygen species-induced changes in glucose and lipid metabolism contribute to the accumulation of cholesterol in the liver during aging. Aging Cell 2019, 18, e12895. [Google Scholar] [CrossRef] [Green Version]
- Mulas, M.F.; Demuro, G.; Mulas, C.; Putzolu, M.; Cavallini, G.; Donati, A.; Bergamini, E.; Dessi, S. Dietary restriction counteracts age-related changes in cholesterol metabolism in the rat. Mech. Ageing Dev. 2005, 126, 648–654. [Google Scholar] [CrossRef]
- Ståhlberg, D.; Angelin, B.; Einarsson, K. Age-related changes in the metabolism of cholesterol in rat liver microsomes. Lipids 1991, 26, 349–352. [Google Scholar] [CrossRef]
- Shiomi, M.; Ito, T.; Fujioka, T.; Tsujita, Y. Age-associated decrease in plasma cholesterol and changes in cholesterol metabolism in homozygous watanabe heritable hyperlipidemic rabbits. Metabolism 2000, 49, 552–556. [Google Scholar] [CrossRef]
- Ioannou, G.N. The role of cholesterol in the pathogenesis of nash. Trends Endocrinol. Metab. 2016, 27, 84–95. [Google Scholar] [CrossRef]
- Hagstrom, H.; Nasr, P.; Ekstedt, M.; Hammar, U.; Stal, P.; Askling, J.; Hultcrantz, R.; Kechagias, S. Cardiovascular risk factors in non-alcoholic fatty liver disease. Liver Int. 2019, 39, 197–204. [Google Scholar] [CrossRef] [Green Version]
- Morgan, A.E.; Mooney, K.M.; Wilkinson, S.J.; Pickles, N.A.; Mc Auley, M.T. Investigating cholesterol metabolism and ageing using a systems biology approach. Proc. Nutr. Soc. 2017, 76, 378–391. [Google Scholar] [CrossRef] [Green Version]
- Mc Auley, M.T.; Mooney, K.M. Computationally modeling lipid metabolism and aging: A mini-review. Comput. Struct. Biotechnol. J. 2015, 13, 38–46. [Google Scholar] [CrossRef] [Green Version]
- Mc Auley, M.; Mooney, K. Chapter 7—Using computational models to study aging. In Conn’s Handbook of Models for Human Aging, 2nd ed.; Ram, J.L., Conn, P.M., Eds.; Academic Press: London, UK, 2018; pp. 79–91. [Google Scholar]
- Neumann, S.J.; Berceli, S.A.; Sevick, E.M.; Lincoff, A.M.; Warty, V.S.; Brant, A.M.; Herman, I.M.; Borovetz, H.S. Experimental determination and mathematical model of the transient incorporation of cholesterol in the arterial wall. Bull. Math Biol. 1990, 52, 711–732. [Google Scholar] [CrossRef]
- Lu, J.; Hubner, K.; Nanjee, M.N.; Brinton, E.A.; Mazer, N.A. An in-silico model of lipoprotein metabolism and kinetics for the evaluation of targets and biomarkers in the reverse cholesterol transport pathway. PLoS Comput. Biol. 2014, 10, e1003509. [Google Scholar] [CrossRef] [Green Version]
- Mc Auley, M.T.; Kenny, R.A.; Kirkwood, T.B.; Wilkinson, D.J.; Jones, J.J.; Miller, V.M. A mathematical model of aging-related and cortisol induced hippocampal dysfunction. BMC Neurosci. 2009, 10, 26. [Google Scholar]
- Fabregat, A.; Sidiropoulos, K.; Garapati, P.; Gillespie, M.; Hausmann, K.; Haw, R.; Jassal, B.; Jupe, S.; Korninger, F.; McKay, S.; et al. The reactome pathway knowledgebase. Nucleic Acids Res. 2016, 44, D481–D487. [Google Scholar] [CrossRef] [Green Version]
- Kanehisa, M.; Furumichi, M.; Tanabe, M.; Sato, Y.; Morishima, K. Kegg: New perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017, 45, D353–D361. [Google Scholar] [CrossRef] [Green Version]
- Mc Auley, M.T.; Guimera, A.M.; Hodgson, D.; McDonald, N.; Mooney, K.M.; Morgan, A.E.; Proctor, C.J. Modelling the molecular mechanisms of aging. Biosci. Rep. 2017, 37, BSR20160177. [Google Scholar] [CrossRef] [Green Version]
- Mooney, K.M.; Morgan, A.E.; Mc Auley, M.T. Aging and computational systems biology. Wiley interdisciplinary reviews. Syst. Biol. Med. 2016, 8, 123–139. [Google Scholar] [CrossRef]
- Mc Auley, M.T.; Proctor, C.J.; Corfe, B.M.; Cuskelly, G.J.; Mooney, K.M. Nutrition research and the impact of computational systems biology. J. Comput. Sci. Syst. Biol. 2013, 6, 271–285. [Google Scholar] [CrossRef]
- Mc Auley, M.T.; Mooney, K.M.; Angell, P.J.; Wilkinson, S.J. Mathematical modelling of metabolic regulation in aging. Metabolites 2015, 5, 232–251. [Google Scholar] [CrossRef] [Green Version]
- Kilner, J.; Corfe, B.M.; McAuley, M.T.; Wilkinson, S.J. A deterministic oscillatory model of microtubule growth and shrinkage for differential actions of short chain fatty acids. Mol. Biosyst. 2016, 12, 93–101. [Google Scholar] [CrossRef] [Green Version]
- Mc Auley, M.T.; Choi, H.; Mooney, K.; Paul, E.; Miller, V.M. Systems biology and synthetic biology: A new epoch for toxicology research. Adv. Toxicol. 2015, 2015, 575403. [Google Scholar] [CrossRef] [Green Version]
- Saqi, M.; Pellet, J.; Roznovat, I.; Mazein, A.; Ballereau, S.; De Meulder, B.; Auffray, C. Systems medicine: The future of medical genomics, healthcare, and wellness. Methods Mol. Biol. (Clifton N.J.) 2016, 1386, 43–60. [Google Scholar]
- Ostaszewski, M.; Gebel, S.; Kuperstein, I.; Mazein, A.; Zinovyev, A.; Dogrusoz, U.; Hasenauer, J.; Fleming, R.M.T.; Le Novère, N.; Gawron, P.; et al. Community-driven roadmap for integrated disease maps. Brief. Bioinform. 2019, 20, 659–670. [Google Scholar] [CrossRef]
- Mc Auley, M.T.; Mooney, K.M. Computational systems biology for aging research. Interdiscip. Top. Gerontol. 2015, 40, 35–48. [Google Scholar]
- Parton, A.; McGilligan, V.; O’Kane, M.; Baldrick, F.R.; Watterson, S. Computational modelling of atherosclerosis. Brief. Bioinform. 2016, 17, 562–575. [Google Scholar] [CrossRef]
- Mc Auley, M.T.; Wilkinson, D.J.; Jones, J.J.; Kirkwood, T.B. A whole-body mathematical model of cholesterol metabolism and its age-associated dysregulation. BMC Syst. Biol. 2012, 6, 130. [Google Scholar] [CrossRef] [Green Version]
- Pool, F.; Currie, R.; Sweby, P.K.; Salazar, J.D.; Tindall, M.J. A mathematical model of the mevalonate cholesterol biosynthesis pathway. J. Theor. Biol. 2018, 443, 157–176. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharya, B.S.; Sweby, P.K.; Minihane, A.M.; Jackson, K.G.; Tindall, M.J. A mathematical model of the sterol regulatory element binding protein 2 cholesterol biosynthesis pathway. J. Theor. Biol. 2014, 349, 150–162. [Google Scholar] [CrossRef]
- Watterson, S.; Guerriero, M.L.; Blanc, M.; Mazein, A.; Loewe, L.; Robertson, K.A.; Gibbs, H.; Shui, G.; Wenk, M.R.; Hillston, J.; et al. A model of flux regulation in the cholesterol biosynthesis pathway: Immune mediated graduated flux reduction versus statin-like led stepped flux reduction. Biochimie 2013, 95, 613–621. [Google Scholar] [CrossRef] [Green Version]
- Morgan, A.; Mooney, K.M.; Wilkinson, S.J.; Pickles, N.; Mc Auley, M.T. Mathematically modelling the dynamics of cholesterol metabolism and ageing. Biosystems 2016, 145, 19–32. [Google Scholar] [CrossRef] [Green Version]
- Tindall, M.J.; Wattis, J.A.; O’Malley, B.J.; Pickersgill, L.; Jackson, K.G. A continuum receptor model of hepatic lipoprotein metabolism. J. Theor. Biol. 2009, 257, 371–384. [Google Scholar] [CrossRef] [Green Version]
- August, E.; Parker, K.H.; Barahona, M. A dynamical model of lipoprotein metabolism. Bull. Math Biol. 2007, 69, 1233–1254. [Google Scholar] [CrossRef] [Green Version]
- Pool, F.; Sweby, P.; Tindall, M.J.P. An integrated mathematical model of cellular cholesterol biosynthesis and lipoprotein metabolism. Processes 2018, 6, 134. [Google Scholar] [CrossRef] [Green Version]
- Toroghi, M.K.; Cluett, W.R.; Mahadevan, R.J.C.; Engineering, C. A multi-scale model for low-density lipoprotein cholesterol (ldl-c) regulation in the human body: Application to quantitative systems pharmacology. Comput. Chem. Eng. 2019, 130, 106507. [Google Scholar] [CrossRef]
- Kervizic, G.; Corcos, L. Dynamical modeling of the cholesterol regulatory pathway with boolean networks. BMC Syst. Biol. 2008, 2, 99. [Google Scholar] [CrossRef] [Green Version]
- Benson, H.E.; Watterson, S.; Sharman, J.L.; Mpamhanga, C.P.; Parton, A.; Southan, C.; Harmar, A.J.; Ghazal, P. Is systems pharmacology ready to impact upon therapy development? A study on the cholesterol biosynthesis pathway. Br. J. Pharmacol. 2017, 174, 4362–4382. [Google Scholar] [CrossRef]
- Mazein, A.; Watterson, S.; Hsieh, W.Y.; Griffiths, W.J.; Ghazal, P. A comprehensive machine-readable view of the mammalian cholesterol biosynthesis pathway. Biochem. Pharmacol. 2013, 86, 56–66. [Google Scholar] [CrossRef] [Green Version]
- Bourgin, M.; Labarthe, S.; Kriaa, A.; Lhomme, M.; Gérard, P.; Lesnik, P.; Laroche, B.; Maguin, E.; Rhimi, M. Exploring the bacterial impact on cholesterol cycle: A numerical study. Front. Microbiol. 2020, 11, 1121. [Google Scholar] [CrossRef]
- Gomez-Cabrero, D.; Compte, A.; Tegner, J. Workflow for generating competing hypothesis from models with parameter uncertainty. Interface Focus. 2011, 1, 438–449. [Google Scholar] [CrossRef]
- Parton, A.; McGilligan, V.; Chemaly, M.; O’Kane, M.; Watterson, S. New models of atherosclerosis and multi-drug therapeutic interventions. Bioinformatics 2019, 35, 2449–2457. [Google Scholar] [CrossRef] [Green Version]
- Bekkar, A.; Estreicher, A.; Niknejad, A.; Casals-Casas, C.; Bridge, A.; Xenarios, I.; Dorier, J.; Crespo, I. Expert curation for building network-based dynamical models: A case study on atherosclerotic plaque formation. Database 2018, 2018, bay031. [Google Scholar] [CrossRef]
- Le Novere, N.; Hucka, M.; Mi, H.; Moodie, S.; Schreiber, F.; Sorokin, A.; Demir, E.; Wegner, K.; Aladjem, M.I.; Wimalaratne, S.M. The systems biology graphical notation. Nat. Biotechnol. 2009, 27, 735–741. [Google Scholar] [CrossRef]
- Junker, B.H.; Klukas, C.; Schreiber, F. Vanted: A system for advanced data analysis and visualization in the context of biological networks. BMC Bioinform. 2006, 7, 109. [Google Scholar]
- Czauderna, T.; Klukas, C.; Schreiber, F. Editing, validating and translating of sbgn maps. Bioinformatics 2010, 26, 2340–2341. [Google Scholar] [CrossRef] [Green Version]
- Wildermuth, M.C. Metabolic control analysis: Biological applications and insights. Genome Biol. 2000, 1, reviews1031.1–reviews1031.5. [Google Scholar] [CrossRef]
- Mc Auley, M.T. Model analysis in greater depth. In Computer Modelling for Nutritionists; Springer International Publishing: Cham, Switzerland, 2019; pp. 63–78. [Google Scholar]
- Fell, D.; Cornish-Bowden, A. Understanding the Control of Metabolism; Portland Press: London, UK, 1997; Volume 2. [Google Scholar]
- Shi, L.; Tu, B.P. Acetyl-coa and the regulation of metabolism: Mechanisms and consequences. Curr. Opin. Cell Biol. 2015, 33, 125–131. [Google Scholar] [CrossRef] [Green Version]
- Perry, R.J.; Peng, L.; Cline, G.W.; Petersen, K.F.; Shulman, G.I. A non-invasive method to assess hepatic acetyl-coa in vivo. Cell Metab. 2017, 25, 749–756. [Google Scholar] [CrossRef] [Green Version]
- Johnston, T.P.; Palmer, W.K. The effect of pravastatin on hepatic 3-hydroxy-3-methylglutaryl coa reductase obtained from poloxamer 407-induced hyperlipidemic rats. Pharmacotherapy 1997, 17, 342–347. [Google Scholar]
- Pedersen, T.R. The success story of ldl cholesterol lowering. Circ. Res. 2016, 118, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.B.; Yin, Z.X.; Chei, C.L.; Qian, H.Z.; Kraus, V.B.; Zhang, J.; Brasher, M.S.; Shi, X.M.; Matchar, D.B.; Zeng, Y. Low-density lipoprotein cholesterol was inversely associated with 3-year all-cause mortality among chinese oldest old: Data from the chinese longitudinal healthy longevity survey. Atherosclerosis 2015, 239, 137–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weverling-Rijnsburger, A.W.; Blauw, G.J.; Lagaay, A.M.; Knook, D.L.; Meinders, A.E.; Westendorp, R.G. Total cholesterol and risk of mortality in the oldest old. Lancet 1997, 350, 1119–1123. [Google Scholar] [CrossRef]
- Al-Mallah, M.H.; Hatahet, H.; Cavalcante, J.L.; Khanal, S. Low admission ldl-cholesterol is associated with increased 3-year all-cause mortality in patients with non st segment elevation myocardial infarction. Cardiol. J. 2009, 16, 227–233. [Google Scholar]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [Green Version]
- Tirosh, O. Hypoxic signaling and cholesterol lipotoxicity in fatty liver disease progression. Oxid. Med. Cell. Longev. 2018, 2018, 2548154. [Google Scholar] [CrossRef]
- Chang, T.Y.; Li, B.L.; Chang, C.C.; Urano, Y. Acyl-coenzyme a: Cholesterol acyltransferases. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E1–E9. [Google Scholar] [CrossRef] [Green Version]
- Bell, T.A., 3rd; Brown, J.M.; Graham, M.J.; Lemonidis, K.M.; Crooke, R.M.; Rudel, L.L. Liver-specific inhibition of acyl-coenzyme a: Cholesterol acyltransferase 2 with antisense oligonucleotides limits atherosclerosis development in apolipoprotein b100-only low-density lipoprotein receptor-/- mice. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1814–1820. [Google Scholar] [CrossRef] [Green Version]
- Temel, R.E.; Lee, R.G.; Kelley, K.L.; Davis, M.A.; Shah, R.; Sawyer, J.K.; Wilson, M.D.; Rudel, L.L. Intestinal cholesterol absorption is substantially reduced in mice deficient in both abca1 and acat2. J. Lipid Res. 2005, 46, 2423–2431. [Google Scholar] [CrossRef] [Green Version]
- Lebeau, P.F.; Byun, J.H.; Platko, K.; MacDonald, M.E.; Poon, S.V.; Faiyaz, M.; Seidah, N.G.; Austin, R.C. Diet-induced hepatic steatosis abrogates cell-surface ldlr by inducing de novo pcsk9 expression in mice. J. Biol. Chem. 2019, 294, 9037–9047. [Google Scholar] [CrossRef]

| Reaction | Name | Abbreviation |
|---|---|---|
| R1 | Acetyl coenzyme A (CoA) synthesis | CoAS → ACoA |
| R2 | Interconversion of Acetyl CoA and Acetoacetyl CoA | ACoA = AACoA |
| R3 | 3-hydroxy-3-methylglutaryl (HMG)-CoA formation | ACoA + AACoA → HMGCoA |
| R4 | Mevalonate (MV) formation | HMGCoA → MV |
| R5 | Mevalonate5P (MV5P) formation | MV → MV5P |
| R6 | Mevalonate5PP (MV5PP) formation | MV5P = MV5PP |
| R7 | Isopentenyl-PP (IPP) formation | MV5PP → IPP |
| R8 | Dimethylallyl-PP (DMAPP) interconversion | IPP = DMAPP |
| R9 | GeranylPP (GPP) formation | DMAPP + IPP → GPP |
| R10 | FarnesylPP (FPP) formation | GPP + IPP → FPP |
| R11 | Squalene formation | FPP → SQ |
| R12 | Squalene epoxide formation | SQ → SQE |
| R13 | Lanosterol formation | SQE → LAN |
| R14 | Free cholesterol (FC) formation | LAN → FC |
| R15 | Conversion of FC to cholesteryl esters (CE) | FC → CE |
| R16 | Conversion of CE to FC | CE → FC |
| R17 | Cholesterol esters flux to low-density lipoprotein cholesterol (LDL-C) | CE → LDLC |
| R18 | LDL-C sink | LDLC → LDLCs |
| R19 | LDL receptor (LDLr) synthesis | sLDLR → LDLR |
| R20 | LDLr degradation | LDLR → dLDLR |
| R21 | Reuptake of LDL-C | LDLC → FC |
| R22 | SREBP synthesis | sSRBP2 → SRBP2 |
| R23 | SREBP degradation | SRBP2 → dSRBP2 |
| R24 | Antioxidant production | sAOX → AOX |
| R25 | Reactive oxygen species (ROS) production | sROS → ROS |
| R26 | ROS degradation | AOX+ROS → ROSsink |
| R27 | HMGCoA reductase synthesis | sHMGCoAR → HMGCoAR |
| R28 | HMGCoA reductase degradation | HMGCoAR → dHMGCoAR |
| R29 | Acetyl-CoA acetyltransferase 2 (ACAT2) synthesis | sACAT2 → ACAT2 |
| R30 | ACAT2 Degradation | ACAT2 → dACAT2 |
| Vmax R15 Conversion of FC to CE (µMoles/min) | Vmax R4 Mevalonate Formation (µMoles/min) | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| FC | |||||
| 0.01 | 22.1333 | 22.2205 | 22.2485 | 22.2623 | 22.2705 |
| 0.02 | 9.93711 | 9.96287 | 9.97106 | 9.97509 | 9.97749 |
| 0.03 | 4.75843 | 4.76165 | 4.76266 | 4.76315 | 4.76345 |
| 0.04 | 2.83914 | 2.8396 | 2.83975 | 2.83981 | 2.83985 |
| 0.05 | 1.99568 | 1.99583 | 1.99587 | 1.99589 | 1.9959 |
| CE | |||||
| 0.01 | 0.084617 | 0.084669 | 0.084685 | 0.084693 | 0.084698 |
| 0.02 | 0.14247 | 0.142576 | 0.14261 | 0.142627 | 0.142637 |
| 0.03 | 0.162901 | 0.162953 | 0.162969 | 0.162976 | 0.162981 |
| 0.04 | 0.166003 | 0.166019 | 0.166024 | 0.166026 | 0.166028 |
| 0.05 | 0.166386 | 0.166394 | 0.166397 | 0.166398 | 0.166399 |
| LDL-C | |||||
| 0.01 | 0.08454 | 0.084591 | 0.084608 | 0.084616 | 0.084621 |
| 0.02 | 0.14232 | 0.142428 | 0.142462 | 0.142479 | 0.142489 |
| 0.03 | 0.162786 | 0.162839 | 0.162855 | 0.162863 | 0.162867 |
| 0.04 | 0.165925 | 0.165941 | 0.165947 | 0.165949 | 0.16595 |
| 0.05 | 0.166317 | 0.166326 | 0.166328 | 0.166329 | 0.16633 |
| LDLr | |||||
| 0.01 | 34,335.9 | 34,334 | 34,333.4 | 34,333.1 | 34,332.9 |
| 0.02 | 34,530.3 | 34,527 | 34,526 | 34,525.5 | 34,525.2 |
| 0.03 | 34,770.5 | 34,766.7 | 34,765.4 | 34,764.8 | 34,764.4 |
| 0.04 | 34,998.3 | 34,994.4 | 34,993.2 | 34,992.5 | 34,992.2 |
| 0.05 | 35,192 | 35,188.2 | 35,187 | 35,186.4 | 35,186 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morgan, A.E.; Mc Auley, M.T. Cholesterol Homeostasis: An In Silico Investigation into How Aging Disrupts Its Key Hepatic Regulatory Mechanisms. Biology 2020, 9, 314. https://doi.org/10.3390/biology9100314
Morgan AE, Mc Auley MT. Cholesterol Homeostasis: An In Silico Investigation into How Aging Disrupts Its Key Hepatic Regulatory Mechanisms. Biology. 2020; 9(10):314. https://doi.org/10.3390/biology9100314
Chicago/Turabian StyleMorgan, Amy Elizabeth, and Mark Tomás Mc Auley. 2020. "Cholesterol Homeostasis: An In Silico Investigation into How Aging Disrupts Its Key Hepatic Regulatory Mechanisms" Biology 9, no. 10: 314. https://doi.org/10.3390/biology9100314




