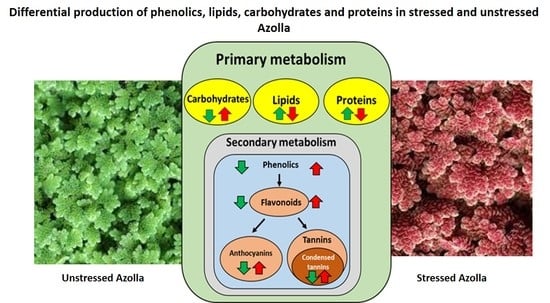
Differential Production of Phenolics, Lipids, Carbohydrates and Proteins in Stressed and Unstressed Aquatic Plants, Azolla filiculoides and Azolla pinnata
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Growth Conditions
2.2. Biochemical Analysis
2.2.1. Lipid Extraction of Fatty Acid Methyl Esters
2.2.2. Total Carbohydrates
2.2.3. Total Protein
2.2.4. Total Phenolics
2.2.5. Total Flavonoids
2.2.6. Total Condensed Tannins
2.2.7. DMACA Staining of Condensed Tannins
2.2.8. Anthocyanin Levels
2.3. Statistical Analysis
3. Results and Discussion
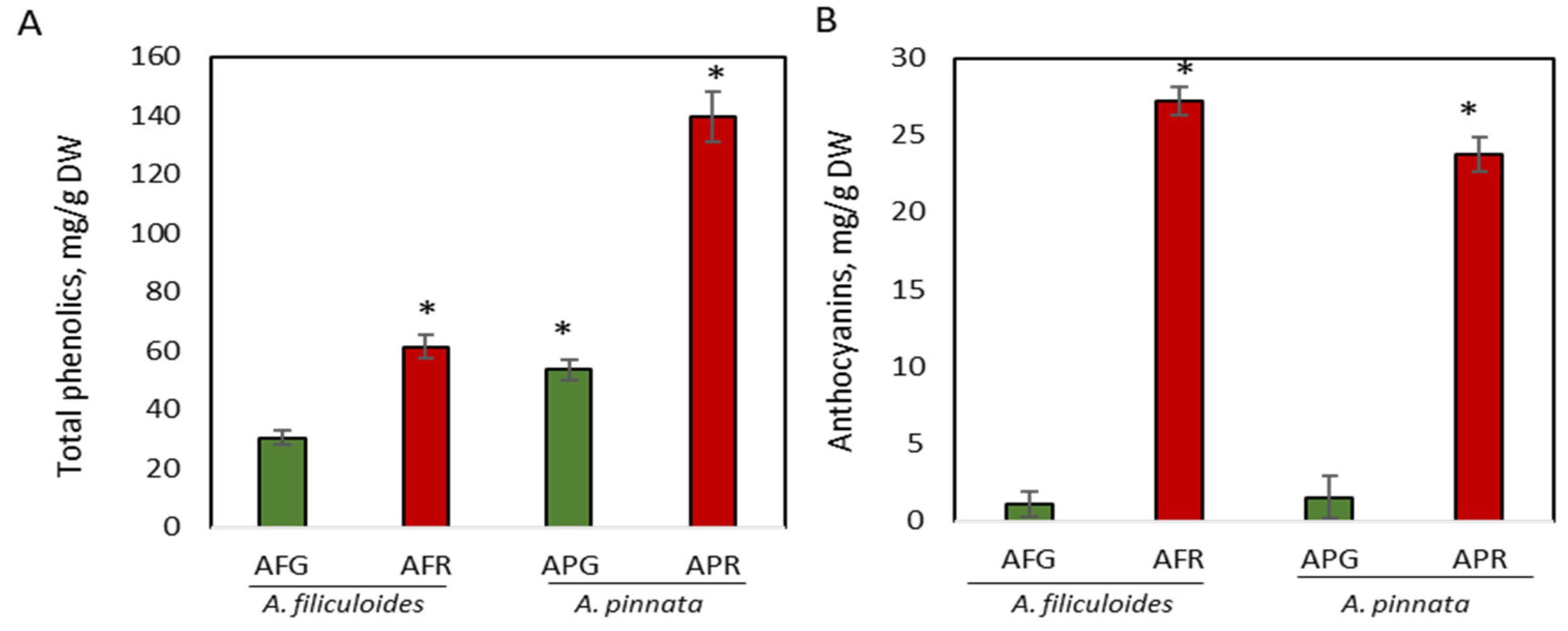
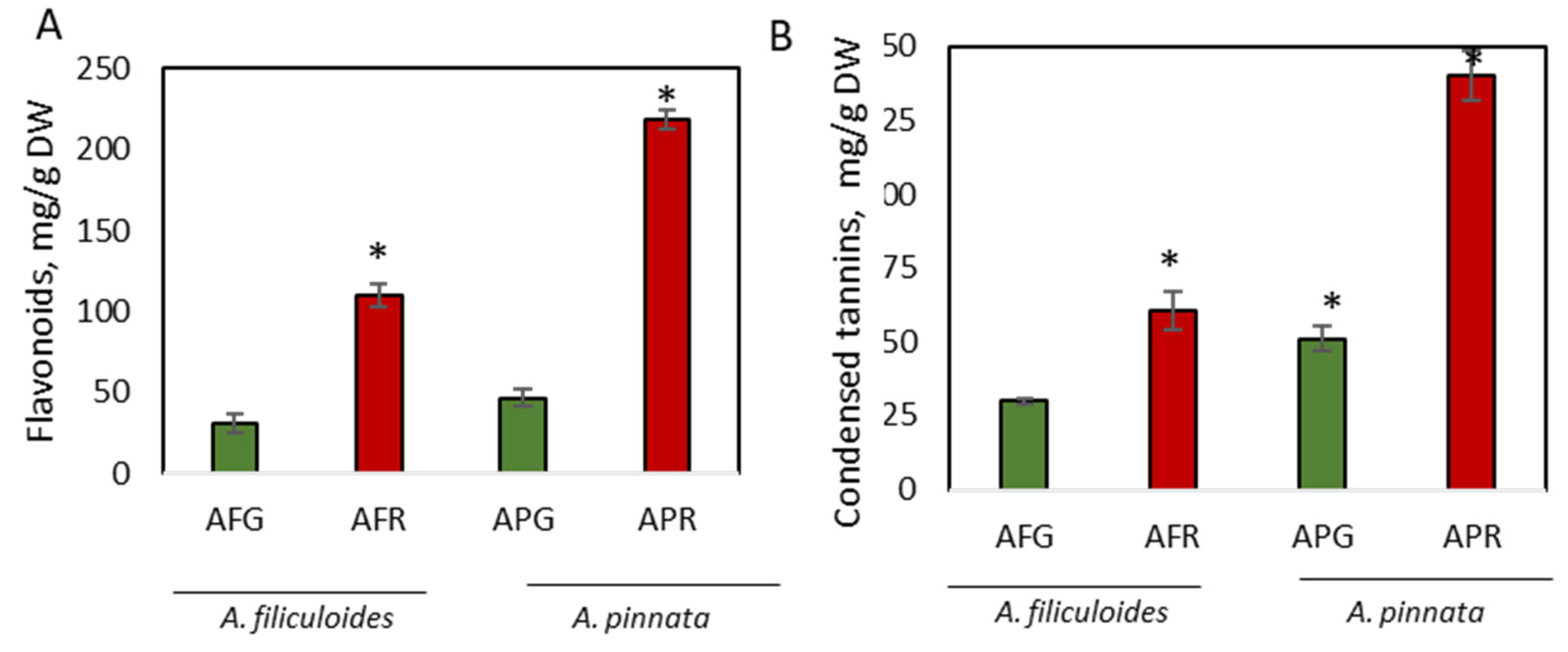
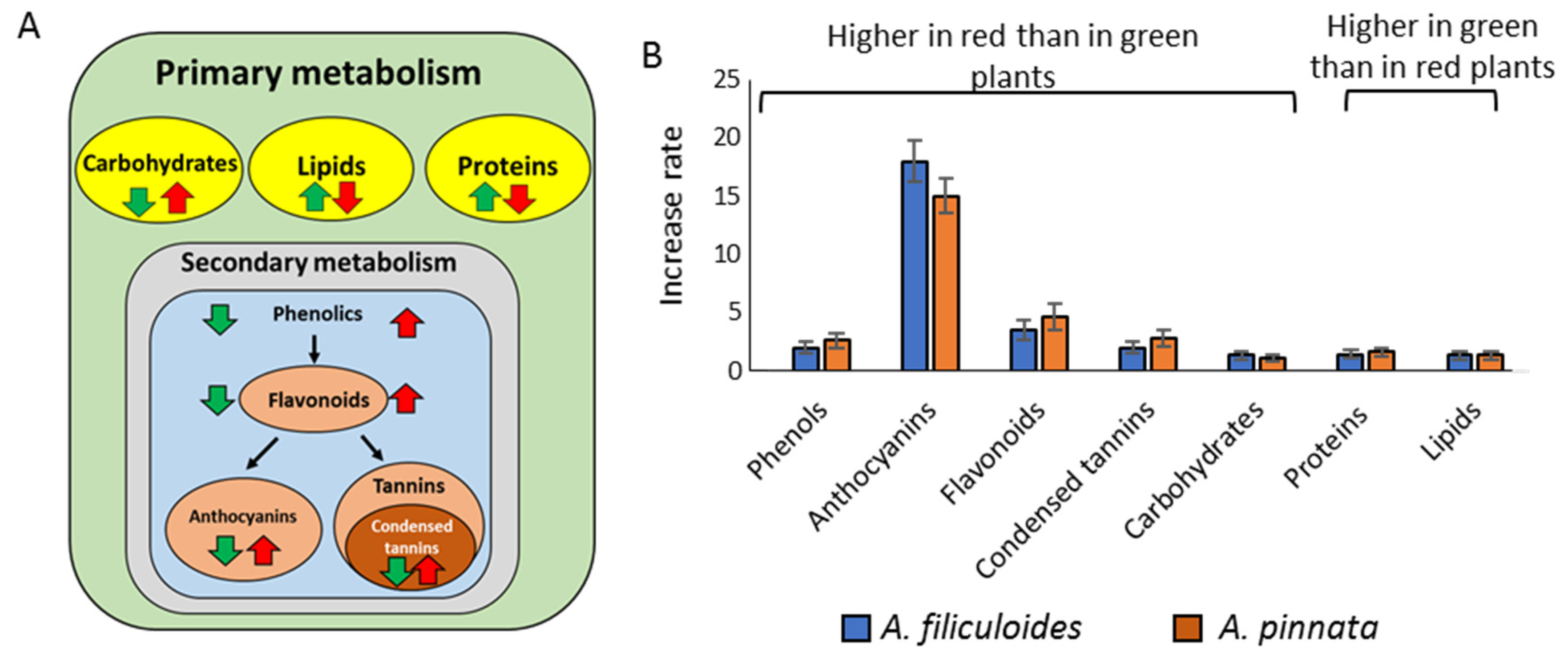
3.1. Accumulation of Phenolic Compounds in Green and Red A. filiculoides and A. pinnata Plants
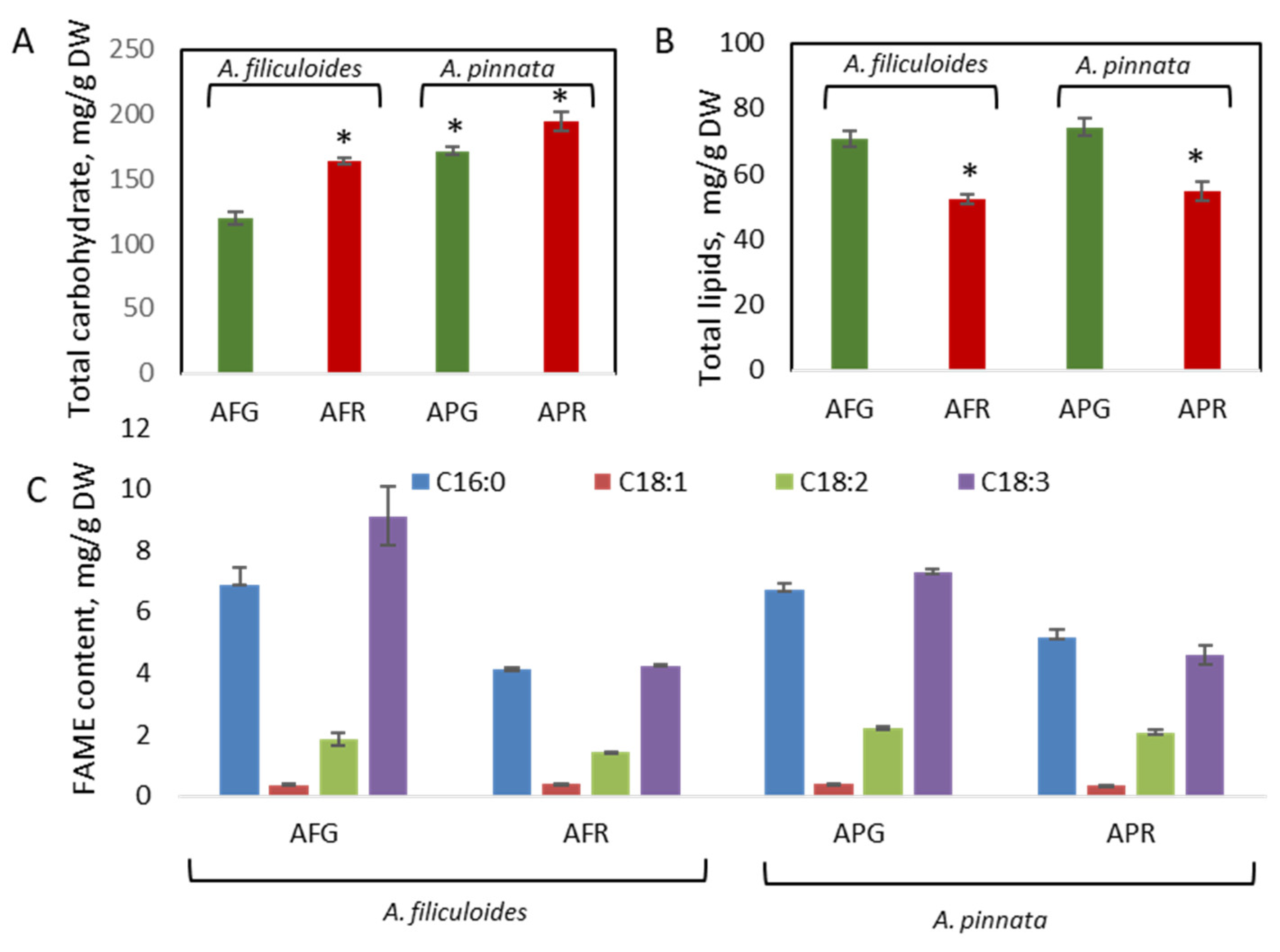
3.2. Accumulation of Proteins, Lipids, and Carbohydrates in Green and Red A. filiculoides and A. pinnata
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ritchie, H.; Roser, M. Environmental impacts of food production. Our World Data. 2020. Published online at OurWorldInData.org. Available online: https://ourworldindata.org/environmental-impacts-of-food (accessed on 5 September 2020).
- Popp, J.; Lakner, Z.; Harangi-Rákos, M.; Fári, M. The effect of bioenergy expansion: Food, energy, and environment. Renew. Sustain. Energy Rev. 2014, 32, 559–578. [Google Scholar] [CrossRef] [Green Version]
- Siddiqui, M.R.; Miranda, A.; Mouradov, A. Microalgae as Bio-Converters of Wastewater into Biofuel and Food. In Water Scarcity and Ways to Reduce the Impact: Management Strategies and Technologies for Zero Liquid Discharge and Future Smart Cities; Pannirselvam, M., Shu, L., Griffin, G., Philip, L., Natarajan, A., Hussain, S., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 75–94. [Google Scholar]
- Miranda, A.F.; Ramkumar, N.; Andriotis, C.; Höltkemeier, T.; Yasmin, A.; Rochfort, S.; Wlodkowic, D.; Morrison, P.; Roddick, F.; Spangenberg, G. Applications of microalgal biofilms for wastewater treatment and bioenergy production. Biotechnol. Biofuels 2017, 10, 120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pulz, O.; Gross, W. Valuable products from biotechnology of microalgae. Appl. Microbio. Biotechnol. 2004, 65, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Borowitzka, M.A.; Moheimani, N.R. Sustainable biofuels from algae. Mitiga. Adapt. Strat. Glob. Chang. 2013, 18, 13–25. [Google Scholar] [CrossRef]
- Muradov, N.; Taha, M.; Miranda, A.F.; Wrede, D.; Kadali, K.; Gujar, A.; Stevenson, T.; Ball, A.S.; Mouradov, A. Fungal-assisted algal flocculation: Application in wastewater treatment and biofuel production. Biotechnol. Biofuels 2015, 8, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, A.L. The Unique Symbiotic System Between a Fern and a Cyanobacterium, Azolla-Anabaena azollae: Their potential as biofertilizer, feed, and remediation. In Symbiosis; IntechOpen: London, UK, 2017. [Google Scholar]
- Costa, M.; Santos, M.; Carrapiço, F.; Pereira, A. Azolla–Anabaena’s behaviour in urban wastewater and artificial media—Influence of combined nitrogen. Water Res. 2009, 43, 3743–3750. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, P.; Nierop, K.G.; Huijgen, W.J.; Schluepmann, H. Aquatic weeds as novel protein sources: Alkaline extraction of tannin-rich Azolla. Biotechnol. Rep. 2019, 24, e00368. [Google Scholar] [CrossRef]
- Brouwer, P.; Schluepmann, H.; Nierop, K.G.; Elderson, J.; Bijl, P.K.; van der Meer, I.; de Visser, W.; Reichart, G.-J.; Smeekens, S.; van der Werf, A. Growing Azolla to produce sustainable protein feed: The effect of differing species and CO2 concentrations on biomass productivity and chemical composition. J. Sci. Food Agric. 2018, 98, 4759–4768. [Google Scholar] [CrossRef] [Green Version]
- Chandran, R.; Nivedhini, V.; Parimelazhagan, T. Nutritional composition and antioxidant properties of Cucumis dipsaceus ehrenb. ex spach leaf. Sci. World J. 2013, 2013. [Google Scholar] [CrossRef] [Green Version]
- Miranda, A.F.; Biswas, B.; Ramkumar, N.; Singh, R.; Kumar, J.; James, A.; Roddick, F.; Lal, B.; Subudhi, S.; Bhaskar, T.; et al. Aquatic plant Azolla as the universal feedstock for biofuel production. Biotechnol. Biofuels 2016, 9, 221. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, I.; Liu, C.C. Improving nitrogen-fixing systems and integrating them into sustainable rice farming. Plant Soil 1992, 141, 57–67. [Google Scholar] [CrossRef]
- van Hove, C.; Lejeune, A. The Azolla: Anabaena Symbiosis. Biol. Environ. Proceed. R. Ir. Acad. 2002, 102B, 23–26. [Google Scholar] [CrossRef]
- Carrapico, F. The Azolla–Anabaena–Bacteria association: A case of symbiotic abduction? In Algal and Cyanobacteria Symbioses; World Scientific: Hackensack, NJ, USA, 2017; pp. 329–345. [Google Scholar]
- Brouwer, P. Turning the Aquatic Weed Azolla Into a Sustainable Crop. Ph.D. Thesis, Universiteit Utrecht, Utrecht, The Netherlands, 7 July 2017. [Google Scholar]
- Dohaei, M.; Karimi, K.; Rahimmalek, M.; Satari, B. Integrated biorefinery of aquatic fern Azolla filiculoides for enhanced extraction of phenolics, protein, and lipid and methane production from the residues. J. Clean. Prod. 2020, 276, 123175. [Google Scholar] [CrossRef]
- Miranda, A.F.; Kumar, N.R.; Spangenberg, G.; Subudhi, S.; Lal, B.; Mouradov, A. Aquatic Plants, Landoltia punctata, and Azolla filiculoides as Bio-Converters of Wastewater to Biofuel. Plants 2020, 9, 437. [Google Scholar] [CrossRef] [Green Version]
- Miranda, A.F.; Liu, Z.; Rochfort, S.; Mouradov, A. Lipid production in aquatic plant Azolla at vegetative and reproductive stages and in response to abiotic stress. Plant Physiol. Biochem. 2018, 124, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Lumpkin, T.A.; Plucknett, D.L. Azolla: Botany, physiology, and use as a green manure. Econ. Bot. 1980, 34, 111–153. [Google Scholar] [CrossRef]
- Anitha, K.C.; Rajeshwari, Y.B.; Prasanna, S.B.; Shilpashree, J. Nutritive evaluation of azolla as livestock feed. J. Exp. Biol. Agric. Sci. 2016, 4, 670–674. [Google Scholar] [CrossRef]
- Nierop, K.G.J.; Speelman, E.N.; de Leeuw, J.W.; Reichart, G.-J. The omnipresent water fern Azolla caroliniana does not contain lignin. Org. Geochem. 2011, 42, 846–850. [Google Scholar] [CrossRef]
- Kadir, A.A.; Abdullah, S.R.S.; Othman, B.A.; Hasan, H.A.; Othman, A.R.; Imron, M.F.; Ismail, N.I.; Kurniawan, S.B. Dual function of Lemna minor and Azolla pinnata as phytoremediator for Palm Oil Mill Effluent and as feedstock. Chemosphere 2020, 259, 127468. [Google Scholar] [CrossRef]
- Murthy, T.; Ashok, M.; Thirumalesh, T.; Umesh, B.; Nataraju, O. Effect of partial replacement of Azolla for concentrate supplement on lactating crossbred cows. Environ. Ecol. 2013, 31, 415–417. [Google Scholar]
- Sujatha, T.; Kundu, A.; Jeyakumar, S.; Kundu, M. Azolla supplementation: Feed cost benefit in duck ration in andaman islands. Tamilnadu J. Vet. Anim. Sci. 2013, 9, 130–136. [Google Scholar]
- Prabina, B.J.; Kumar, K. Dried Azolla as a nutritionally rich cost effective and immuno-modulatory feed supplement for broilers. Asian J. Anim. Sci. 2010, 5, 20–22. [Google Scholar]
- Sithara, K.; Kamalaveni, K. Formulation of low-cost feed using azolla as a protein supplement and its influence on feed utilization in fishes. Curr. Biot. 2008, 2, 212–219. [Google Scholar]
- Becerra, M.; Murgueitio, E.; Reyes, G.; Preston, T.R. Azolla filiculoides as partial replacement for traditional protein supplements in diets for growing-fattening pigs based on sugar cane juice. Livest. Res. Rural Dev. 1990, 2, 15–22. [Google Scholar]
- Leterme, P.; Londoño, A.M.; Ordoñez, D.C.; Rosales, A.; Estrada, F.; Bindelle, J.; Buldgen, A. Nutritional value and intake of aquatic ferns (Azolla filiculoides Lam. and Salvinia molesta Mitchell.) in sows. Anim. Feed Sci. Technol. 2010, 155, 55–64. [Google Scholar] [CrossRef]
- Pugesgaard, S.; Schelde, K.; Larsen, S.U.; Lærke, P.E.; Jørgensen, U. Comparing annual and perennial crops for bioenergy production–influence on nitrate leaching and energy balance. GCB Bioenerg. 2015, 7, 1136–1149. [Google Scholar] [CrossRef]
- Anglade, J.; Billen, G.; Garnier, J. Relationships for estimating N2 fixation in legumes: Incidence for N balance of legume-based cropping systems in Europe. Ecosphere 2015, 6, 1–24. [Google Scholar] [CrossRef]
- Olguín, E.J.; Galicia, S.; Mercado, G.; Pérez, T. Annual productivity of Spirulina (Arthrospira) and nutrient removal in a pig wastewater recycling process under tropical conditions. J. Appl. Phycol. 2003, 15, 249–257. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Sood, P.; Citovsky, V. The roles of plant phenolics in defence and communication during Agrobacterium and Rhizobium infection. Mol. Plant. Pathol. 2010, 11, 705–719. [Google Scholar] [CrossRef]
- Mouradov, A.; Spangenberg, G. Flavonoids: A metabolic network mediating plants adaptation to their real estate. Front. Plant Sci. 2014, 5, 620. [Google Scholar] [CrossRef]
- Rupasinghe, H.V.; Arumuggam, N. Health Benefits of Anthocyanins. In Anthocyanins from Natural Sources; Royal Society of Chemistry: Cambridge, UK, 2019; pp. 121–158. [Google Scholar]
- Ballard, C.R.; Junior, M.R.M. Health Benefits of Flavonoids. In Bioactive Compounds; Elsevier: Amsterdam, The Netherlands, 2019; pp. 185–201. [Google Scholar]
- Perez-Vizcaino, F.; Fraga, C.G. Research trends in flavonoids and health. Arch. Biochem. Biophys. 2018, 646, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Iahtisham Ul, H.; Patel, S.; Pan, X.; Naz, S.; Sanches Silva, A.; Saeed, F.; Rasul Suleria, H.A. Proanthocyanidins: A comprehensive review. Biomed. Pharm. 2019, 116, 108999. [Google Scholar] [CrossRef] [PubMed]
- Basak, B.; Pramanik, M.A.H.; Rahman, M.S.; Tarafdar, S.U.; Roy, B.C. Azolla (Azolla pinnata) as a feed ingredient in broiler ration. Int. J. Poult. Sci. 2002, 1, 29–34. [Google Scholar]
- Naghshi, H.; Khojasteh, S.; Jafari, M. Investigation the Effect of Different Levels of Azolla (Azolla pinnata) on Performance and Carcass Characteristics of Cobb Broiler Chicks. Int. J. Farm. Allied Sci. 2014, 1, 45–49. [Google Scholar]
- Alalade, O.A.; Iyayi, E.A. Chemical composition and the feeding value of Azolla (Azolla pinnata) meal for egg-type chicks. Int. J. Poult. Sci. 2006, 2, 137–141. [Google Scholar]
- Güngör, E.; Brouwer, P.; Dijkhuizen, L.W.; Shaffar, D.C.; Nierop, K.G.J.; de Vos, R.C.H.; Sastre Toraño, J.; van der Meer, I.M.; Schluepmann, H. Azolla ferns testify: Seed plants and ferns share a common ancestor for leucoanthocyanidin reductase enzymes. New Phytol. 2020. [Google Scholar] [CrossRef]
- Rivero, R.M.; Ruiz, J.M.; García, P.C.; López-Lefebre, L.R.; Sánchez, E.; Romero, L. Resistance to cold and heat stress: Accumulation of phenolic compounds in tomato and watermelon plants. Plant Sci. 2001, 160, 315–321. [Google Scholar] [CrossRef]
- Isah, T. Stress and defense responses in plant secondary metabolites production. Biol. Res. 2019, 52, 39. [Google Scholar] [CrossRef] [Green Version]
- Pérez-López, U.; Sgherri, C.; Miranda-Apodaca, J.; Micaelli, F.; Lacuesta, M.; Mena-Petite, A.; Quartacci, M.F.; Muñoz-Rueda, A. Concentration of phenolic compounds is increased in lettuce grown under high light intensity and elevated CO2. Plant Physiol. Biochem. 2018, 123, 233–241. [Google Scholar] [CrossRef]
- Muradov, N.; Taha, M.; Miranda, A.F.; Kadali, K.; Gujar, A.; Rochfort, S.; Stevenson, T.; Ball, A.S.; Mouradov, A. Dual application of duckweed and azolla plants for wastewater treatment and renewable fuels and petrochemicals production. Biotechnol. Biofuels 2014, 7, 30. [Google Scholar] [CrossRef] [Green Version]
- Moretti, A.; Siniscalco Gigliano, G. Influence of light and pH on growth and nitrogenase activity on temperate-grown Azolla. Biol. Fertil. Soils 1988, 6, 131–136. [Google Scholar] [CrossRef]
- Schenk, R.U.; Hildebrandt, A. Medium and techniques for induction and growth of monocotyledonous and dicotyledonous plant cell cultures. Can. J. Bot. 1972, 50, 199–204. [Google Scholar] [CrossRef]
- Podpora, B.; Świderski, F.; Sadowska, A.; Rakowska, R.; Wasiak-Zys, G. Spent brewer’s yeast extracts as a new component of functional food. Czech J. Food Sci. 2016, 34, 554–563. [Google Scholar] [CrossRef] [Green Version]
- Makkar, H.P. Quantification of Tannins in Tree and Shrub Foliage: A Laboratory Manual; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Ray, H.; Yu, M.; Auser, P.; Blahut-Beatty, L.; McKersie, B.; Bowley, S.; Westcott, N.; Coulman, B.; Lloyd, A.; Gruber, M.Y. Expression of anthocyanins and proanthocyanidins after transformation of alfalfa with maize Lc. Plant Physiol. 2003, 132, 1448–1463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abeynayake, S.W.; Panter, S.; Mouradov, A.; Spangenberg, G. A high-resolution method for the localization of proanthocyanidins in plant tissues. Plant Meth. 2011, 7, 13. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.G.; Tanner, G.; Larkin, P. The DMACA–HCl protocol and the threshold proanthocyanidin content for bloat safety in forage legumes. J. Sci. Food Agric. 1996, 70, 89–101. [Google Scholar] [CrossRef]
- Neff, M.M.; Chory, J. Genetic interactions between phytochrome A, phytochrome B, and cryptochrome 1 during Arabidopsis development. Plant Physiol. 1998, 118, 27–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forni, C.; Braglia, R.; Harren, F.; Cristescu, S. Stress responses of duckweed (Lemna minor L.) and water velvet (Azolla filiculoides Lam.) to anionic surfactant sodium-dodecyl-sulphate (SDS). Aquat. Toxicol. 2012, 110, 107–113. [Google Scholar] [CrossRef]
- Sánchez-Viveros, G.; Ferrera-Cerrato, R.; Alarcón, A. Short-term effects of arsenate-induced toxicity on growth, chlorophyll and carotenoid contents, and total content of phenolic compounds of Azolla filiculoides. Water Air Soil Pollut. 2011, 217, 455–462. [Google Scholar] [CrossRef]
- Sánchez-Viveros, G.; Gonzalez-Mendoza, D.; Alarcon, A.; Ferrera-Cerrato, R. Copper effects on photosynthetic activity and membrane leakage of Azolla filiculoides and A. caroliniana. Int. J. Agric. Biol. 2010, 12, 365–368. [Google Scholar]
- Dai, L.P.; Xiong, Z.T.; Huang, Y.; Li, M.J. Cadmium-induced changes in pigments, total phenolics, and phenylalanine ammonia-lyase activity in fronds of Azolla imbricata. Environ. Toxicol. 2006, 21, 505–512. [Google Scholar] [CrossRef]
- Saigo, T.; Wang, T.; Watanabe, M.; Tohge, T. Diversity of anthocyanin and proanthocyanin biosynthesis in land plants. Curr. Opin. Plant Biol. 2020, 55, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Tohge, T.; Fernie, A.R. Leveraging Natural Variance towards Enhanced Understanding of Phytochemical Sunscreens. Trends Plant Sci. 2017, 22, 308–315. [Google Scholar] [CrossRef]
- Ishikura, N. 3-Desoxyanthocyanin and other phenolics in the water fernAzolla. Bot. Mag. Shokubutsu–Gaku–Zasshi 1982, 95, 303–308. [Google Scholar] [CrossRef]
- Cohen, M.F.; Sakihama, Y.; Takagi, Y.C.; Ichiba, T.; Yamasaki, H. Synergistic effect of deoxyanthocyanins from symbiotic fern Azolla spp. on hrmA gene induction in the cyanobacterium Nostoc punctiforme. Mol. Plant Microbe Interact. 2002, 15, 875–882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, M.F.; Meziane, T.; Tsuchiya, M.; Yamasaki, H. Feeding deterrence of Azolla in relation to deoxyanthocyanin and fatty acid composition. Aquat. Bot. 2002, 74, 181–187. [Google Scholar] [CrossRef]
- Kunjiappan, S.; Chowdhury, R.; Bhattacharjee, C. Isolation and structural elucidation of flavonoids from aquatic fern Azolla microphylla and evaluation of free radical scavenging activity. Int. J. Pharm. Pharm. Sci. 2013, 5, 743–749. [Google Scholar]
- Tao, X.; Fang, Y.; Huang, M.J.; Xiao, Y.; Liu, Y.; Ma, X.R.; Zhao, H. High flavonoid accompanied with high starch accumulation triggered by nutrient starvation in bioenergy crop duckweed (Landoltia punctata). BMC Genom. 2017, 18, 166. [Google Scholar] [CrossRef] [Green Version]
- Pereira, A.L.; Carrapiço, F. Histochemistry of simple hairs from the foliar cavities of Azolla filiculoides. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2007, 141, 323–328. [Google Scholar] [CrossRef]
- Abeynayake, S.W.; Panter, S.; Chapman, R.; Webster, T.; Rochfort, S.; Mouradov, A.; Spangenberg, G. Biosynthesis of proanthocyanidins in white clover flowers: Cross talk within the flavonoid pathway. Plant Physiol. 2012, 158, 666–678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becker, M.; Ladha, J.K.; Simpson, I.C.; Ottow, J.C.G. Parameters Affecting Residue Nitrogen Mineralization in Flooded Soils. Soil Sci. Soc. Am. J. 1994, 58, 1666–1671. [Google Scholar] [CrossRef]
- Costa, M.L.; Santos, M.C.; Carrapiço, F. Biomass characterization of Azolla filiculoides grown in natural ecosystems and wastewater. Hydrobiologia 1999, 415, 323–327. [Google Scholar] [CrossRef]
- Wang, Y.; Douglas, G.; Waghorn, G.; Barry, T.; Foote, A. Effect of condensed tannins in Lotus corniculatus upon lactation performance in ewes. J. Agric. Sci. 1996, 126, 353–362. [Google Scholar] [CrossRef]
- Mueller-Harvey, I.; Bee, G.; Dohme-Meier, F.; Hoste, H.; Karonen, M.; Kölliker, R.; Lüscher, A.; Niderkorn, V.; Pellikaan, W.F.; Salminen, J.-P.; et al. Benefits of Condensed Tannins in Forage Legumes Fed to Ruminants: Importance of Structure, Concentration, and Diet Composition. Crop Sci. 2019, 59, 861–885. [Google Scholar] [CrossRef] [Green Version]
- Barbehenn, R.V.; Peter Constabel, C. Tannins in plant-herbivore interactions. Phytochemistry 2011, 72, 1551–1565. [Google Scholar] [CrossRef]
- Lourenço, S.O.; Barbarino, E.; Lavín, P.L.; Lanfer Marquez, U.M.; Aidar, E. Distribution of intracellular nitrogen in marine microalgae: Calculation of new nitrogen-to-protein conversion factors. Eur. J. Phycol. 2004, 39, 17–32. [Google Scholar] [CrossRef]
- Mariotti, F.; Tomé, D.; Mirand, P.P. Converting nitrogen into protein—Beyond 6.25 and Jones’ factors. Crit. Rev. Food Sci. Nutr. 2008, 48, 177–184. [Google Scholar] [CrossRef]
- Shi, H.; Ye, T.; Chen, F.; Cheng, Z.; Wang, Y.; Yang, P.; Zhang, Y.; Chan, Z. Manipulation of arginase expression modulates abiotic stress tolerance in Arabidopsis: Effect on arginine metabolism and ROS accumulation. J. Exp. Bot. 2013, 64, 1367–1379. [Google Scholar] [CrossRef] [Green Version]
- Winter, G.; Todd, C.D.; Trovato, M.; Forlani, G.; Funck, D. Physiological implications of arginine metabolism in plants. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef] [Green Version]
- Joshi, V.; Joung, J.-G.; Fei, Z.; Jander, G. Interdependence of threonine, methionine and isoleucine metabolism in plants: Accumulation and transcriptional regulation under abiotic stress. Amino Acids 2010, 39, 933–947. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, A.; Savka, M.A.; Hudson, A.O. The Synthesis and Role of β-Alanine in Plants. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, N.; Yuan, S.; Li, Z.; Zhou, M.; Wu, P.; Hu, Q.; Mendu, V.; Wang, L.; Luo, H. STRESS INDUCED FACTOR 2, a leucine-rich repeat kinase regulates basal plant pathogen defense. Plant Physiol. 2018, 176, 3062–3080. [Google Scholar] [CrossRef] [Green Version]
- Qiu, X.-M.; Sun, Y.-Y.; Ye, X.-Y.; Li, Z.-G. Signaling role of glutamate in plants. Front. Plant. Sci. 2019, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Total N mg/g DW | Total Protein *, mg/g DW | Total Protein **, mg/g DW | Total Protein ***, mg/g DW | Total AA, mg/g | N-Prot Factor | |
|---|---|---|---|---|---|---|
| AFG | 38.1 ± 2.5 # | 237.5 ± 22.2 | 174.42 ± 12.1 | 190 ± 9.1 | 205.3 ± 8.1 # | 5.40 |
| AFR | 26.4 ± 3.2 # | 165 ± 8.3 | 121.176 ± 10.1 | 132 ± 10.8 | 140.9 ± 7.4 # | 5.34 |
| APG | 46.0 ± 4.1 # | 287.5 ± 15.4 | 211.14 ± 14.4 | 230 ± 18.5 | 265.0 ± 10.4 # | 5.76 |
| AFR | 27.5 ± 2.3 # | 171.8 ± 10.0 | 126.225 ± 8.8 | 137.5 ± 6.6 | 134.0 ± 4.3 # | 4.87 |
| AA | AA Content, % DW | AA Content, mg/g | AA Content, % in Total AA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AFG | AFR | APG | APR | AFG | AFR | APG | APR | AFG | AFR | APG | APR | |
| Ala | 0.46 ± 0.01 | 0.44 ± 0.1 | 0.62 ± 0.1 | 0.27 ± 0.01 | 4.6 ± 0.1 | 4.35 ± 0.1 | 6.22 ± 0.02 | 2.705 ± 0.1 | 2.26 ± 0.02 | 3.07 ± 0.02 | 2.32 ± 0.01 | 2.32 ± 0.02 |
| Arg | 2.3 ± 0.05 | 2.28 ± 0.07 | 3.1 ± 0.02 | 1.59 ± 0.08 | 22.97 ± 0.5 | 22.81 ± 0.65 | 31.03 ± 0.19 | 15.93 ± 0.8 | 11.21 ± 0.01 | 16.12 ± 0.02 | 11.55 ± 0.03 | 11.51 ± 0.1 |
| Asp | 2.08 ± 0.07 | 1.55 ± 0.1 | 2.47 ± 0.1 | 1.45 ± 0.06 | 20.8 ± 0.66 | 15.46 ± 0.47 | 24.5 ± 0.11 | 14.46 ± 0.6 | 10.15 ± 0.11 | 10.92 ± 0.12 | 9.19 ± 0.06 | 10.45 ± 0.02 |
| Cys | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Glu | 4.51 ± 0.09 | 2.09 ± 0.06 | 6.94 ± 0.04 | 4.29 ± 0.02 | 45.1 ± 0.9 | 20.89 ± 0.64 | 69.37 ± 0.36 | 38.92 ± 2.1 | 22.00 ± 0.05 | 14.76 ± 0.10 | 25.83 ± 0.04 | 31.3 ± 0.16 |
| Gly | 1.27 ± 0.02 | 0.75 ± 0.02 | 1.56 ± 0.02 | 0.85 ± 0.03 | 12.67 ± 0.16 | 7.50 ± 0.20 | 15.55 ± 0.16 | 8.50 ± 0.3 | 6.18 ± 0.06 | 5.30 ± 0.06 | 5.79 ± 0.04 | 5.79 ± 0.02 |
| His * | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Ile * | 1.06 ± 0.03 | 0.76 ± 0.02 | 1.29 ± 0.02 | 0.59 ± 0.02 | 10.57 ± 0.25 | 7.59 ± 0.21 | 12.89 ± 0.03 | 5.87 ± 0.27 | 5.16 ± 0.01 | 5.37 ± 0.02 | 4.80 ± 0.02 | 4.25 ± 0.02 |
| Leu * | 1.8 ± 0.03 | 1.34 ± 0.03 | 2.26 ± 0.02 | 1.31 ± 0.04 | 17.95 ± 0.32 | 13.35 ± 0.37 | 22.61 ± 0.017 | 11.5 ± 0.49 | 8.76 ± 0.04 | 9.43 ± 0.04 | 8.42 ± 0.03 | 7.99 ± 0.06 |
| Lys * | 1.43 ± 0.02 | 0.96 ± 0.03 | 1.64 ± 0.02 | 0.53 ± 0.03 | 14.34 ± 0.2 | 9.59 ± 0.32 | 16.38 ± 0.21 | 5.34 ± 0.32 | 7.00 ± 0.11 | 6.78 ± 0.13 | 6.10 ± 0.06 | 6.03 ± 0.1 |
| Met * | 1.22 ± 0.04 | 0.62 ± 0.02 | 1.58 ± 0.02 | 0.37 ± 0.01 | 12.25 ± 0.35 | 6.18 ± 0.20 | 15.75 ± 0.21 | 4.85 ± 0.19 | 5.96 ± 0.05 | 4.37 ± 0.03 | 5.87 ± 0.08 | 3.51 ± 0.08 |
| Phe * | 0.92 ± 0.02 | 0.62 ± 0.02 | 1.14 ± 0.00 | 0.54 ± 0.002 | 9.15 ± 0.22 | 6.24 ± 0.18 | 11.36 ± 0.03 | 5.35 ± 0.24 | 4.47 ± 0.01 | 4.41 ± 0.02 | 4.23 ± 0.02 | 3.87 ± 0.01 |
| Pro | 0.09 ± 0.02 | 0.06 ± 0.01 | 0.11 ± 0.02 | 0.06 ± 0.01 | 0.91 ± 0.16 | 0.61 ± 0.11 | 1.09 ± 0.18 | ND | 0.44 ± 0.07 | 0.43 ± 0.08 | 0.41 ± 0.07 | ND |
| Ser | 0.94 ± 0.02 | 0.65 ± 0.02 | 1.15 ± 0.01 | 0.6 ± 0.02 | 9.4 ± 0.16 | 6.49 ± 0.17 | 11.54 ± 0.08 | 6.05 ± 0.2 | 4.6 ± 0.03 | 4.59 ± 0.03 | 4.3 ± 0.02 | 4.37 ± 0.00 |
| Thr * | 0.9 ± 0.02 | 0.73 ± 0.02 | 1.05 ± 0.01 | 0.44 ± 0.02 | 9.01 ± 0.24 | 7.25 ± 0.20 | 10.52 ± 0.06 | 4.45 ± 0.22 | 4.40 ± 0.02 | 5.12 ± 0.02 | 3.92 ± 0.04 | 3.22 ± 0.01 |
| Tyr | 0.33 ± 0.01 | 0.33 ± 0.01 | 0.51 ± 0.00 | 0.12 ± 0.02 | 3.2 ± 0.05 | 3.29 ± 0.08 | 5.12 ± 0.04 | 1.17 ± 0.06 | 1.59 ± 0.02 | 2.33 ± 0.02 | 1.91 ± 0.01 | 1.91 ± 0.02 |
| Val * | 1.23 ± 0.03 | 0.93 ± 0.03 | 1.48 ± 0.00 | 0.72 ± 0.01 | 12.26 ± 0.34 | 9.35 ± 0.27 | 14.84 ± 0.04 | 7.19 ± 0.35 | 5.98 ± 0.03 | 6.60 ± 0.05 | 5.53 ± 0.03 | 5.20 ± 0.01 |
| Total | 20.5 ± 0.4 | 14.1 ± 0.2 | 26.5 ± 0.4 | 14.0 ± 0.5 | 205.3 ± 8.01 | 140.9 ± 7.4 | 265.9 ± 10.4 | 134 ± 4.3 | 100.1 ± 0.9 | 99.6 ± 0.8 | 100.1 ± 0.6 | 99.3 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, T.L.N.; Miranda, A.F.; Abeynayake, S.W.; Mouradov, A. Differential Production of Phenolics, Lipids, Carbohydrates and Proteins in Stressed and Unstressed Aquatic Plants, Azolla filiculoides and Azolla pinnata. Biology 2020, 9, 342. https://doi.org/10.3390/biology9100342
Tran TLN, Miranda AF, Abeynayake SW, Mouradov A. Differential Production of Phenolics, Lipids, Carbohydrates and Proteins in Stressed and Unstressed Aquatic Plants, Azolla filiculoides and Azolla pinnata. Biology. 2020; 9(10):342. https://doi.org/10.3390/biology9100342
Chicago/Turabian StyleTran, Thi Linh Nham, Ana F. Miranda, Shamila Weerakoon Abeynayake, and Aidyn Mouradov. 2020. "Differential Production of Phenolics, Lipids, Carbohydrates and Proteins in Stressed and Unstressed Aquatic Plants, Azolla filiculoides and Azolla pinnata" Biology 9, no. 10: 342. https://doi.org/10.3390/biology9100342