Difference between Selenite and Selenate in the Regulation of Growth and Physiological Parameters of Nickel-Exposed Lettuce
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Growth Conditions and Experimental Treatments
2.2. Determination of Growth Parameters and Concentration of Photosynthetic Pigments
2.3. Measurement of Lipid Peroxidation
2.4. Visualization of Root Viability with the TTC Method
2.5. Determination of Ni and Se Concentrations
2.6. Statistical Analyses
3. Results
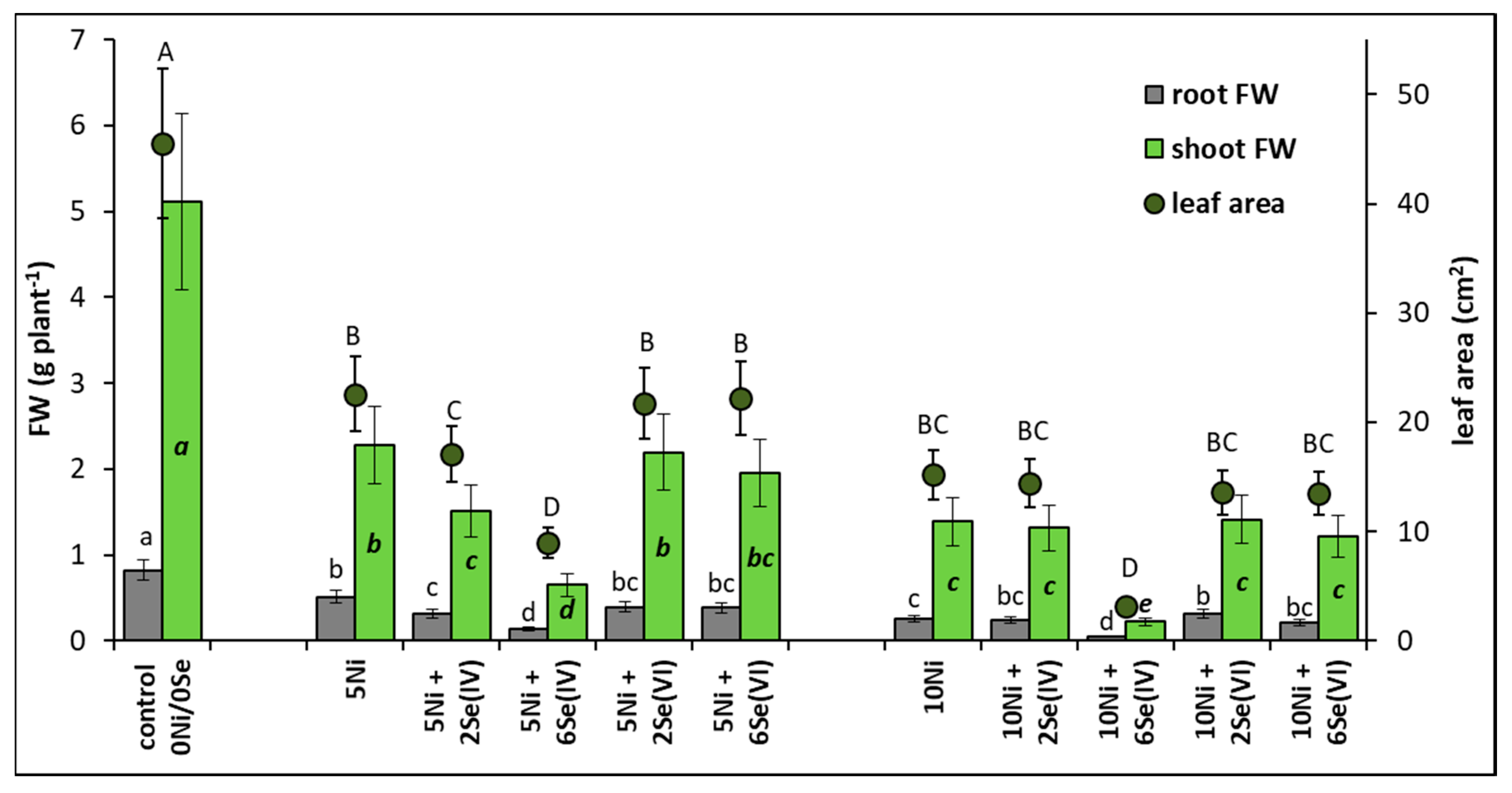
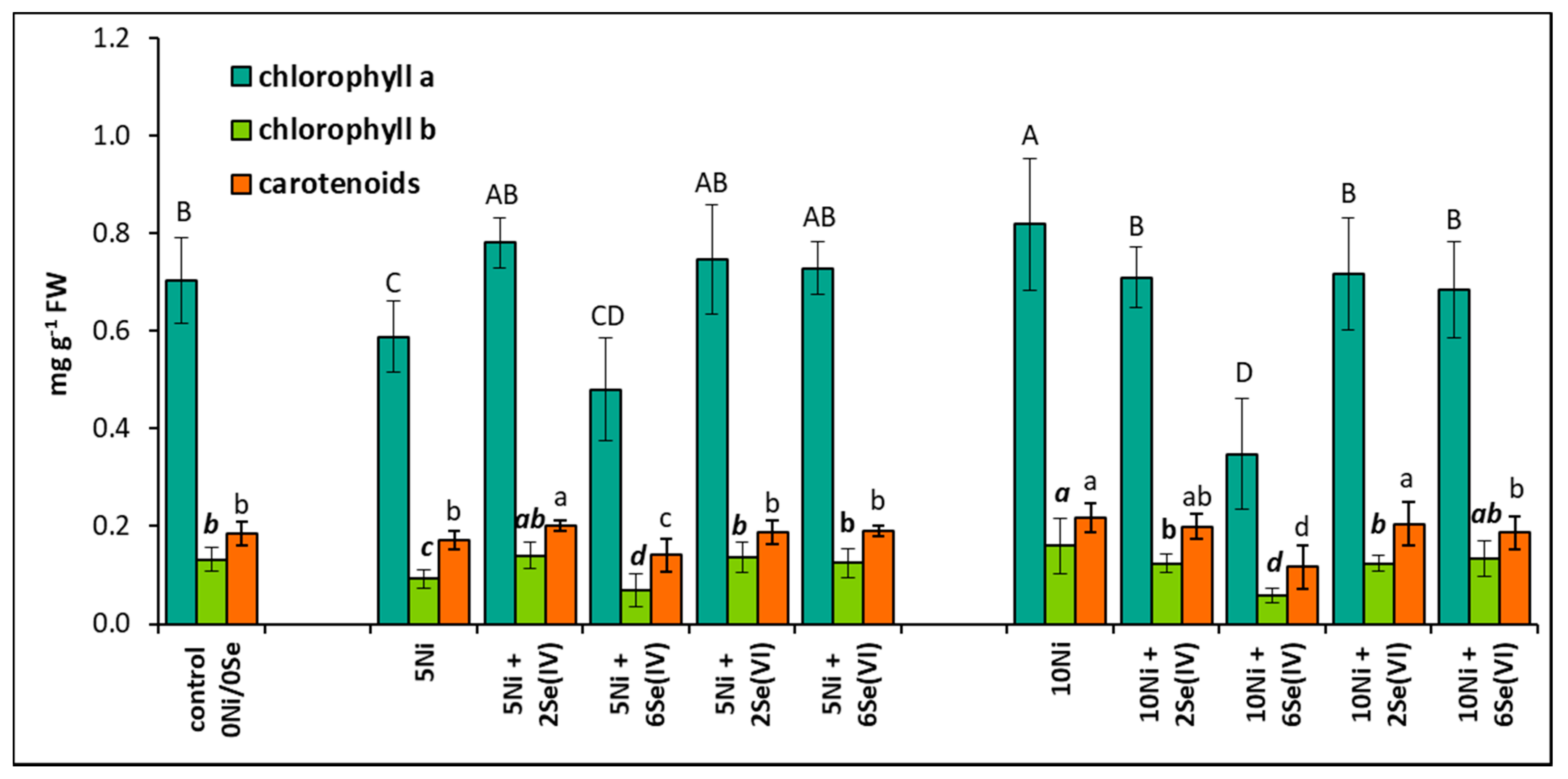
3.1. Effect of Se(IV) and Se(VI) on the Growth and Content of Photosynthetic Pigments in Ni-Exposed Lettuce
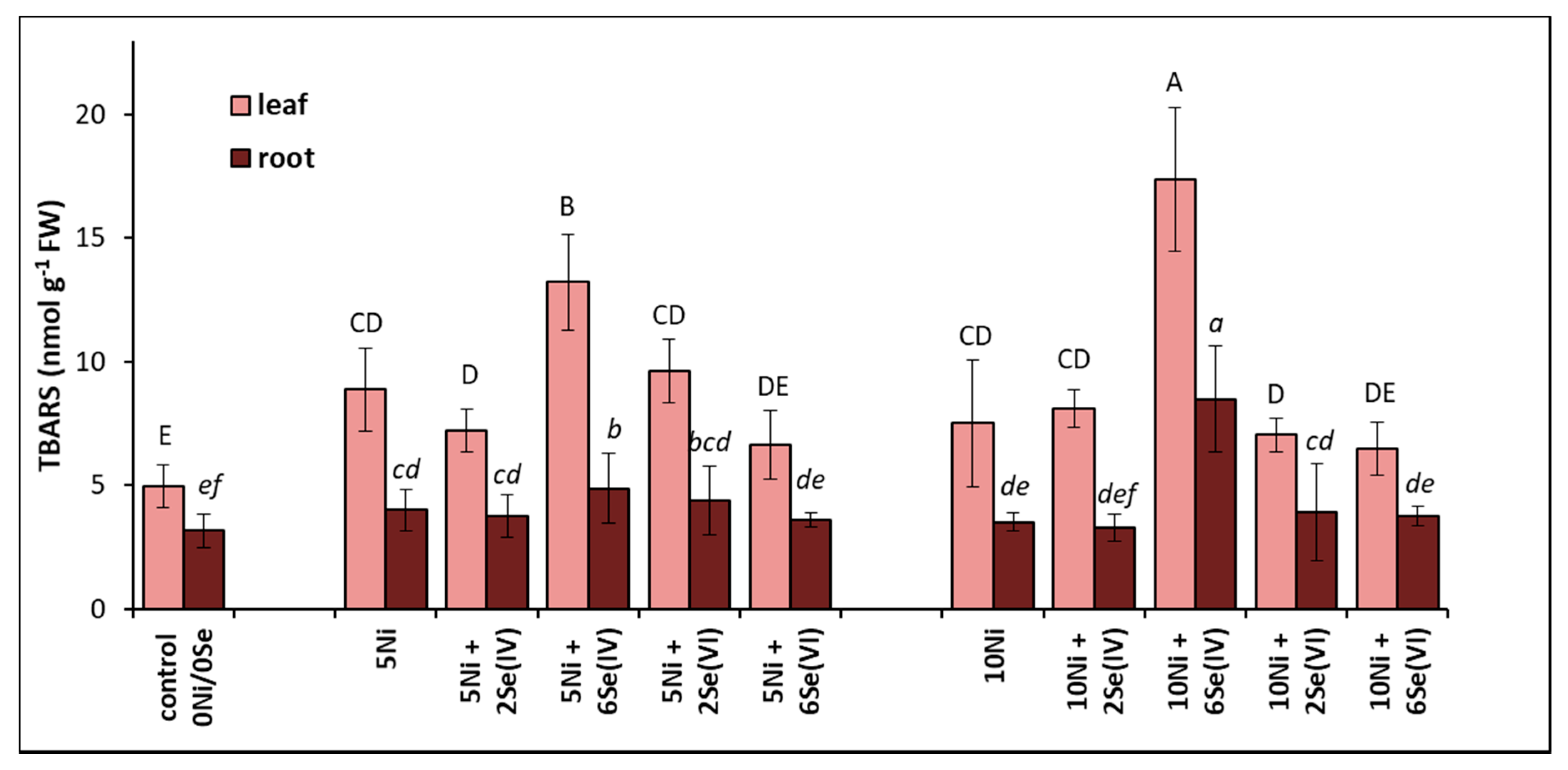
3.2. Effect of Se(IV) and Se(VI) on the Level of Lipid Peroxidation in Ni-Exposed Lettuce
3.3. Effect of Se(IV) and Se(VI) on the Viability of Root Tips in Ni-Exposed Lettuce
3.4. Effect of Se(IV) and Se(VI) on the Concentration and Translocation of Ni and Se in Ni-Exposed Lettuce
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, C.; Huang, D.; Liu, J. Functions and toxicity of nickel in plants: Recent advances and future prospects. CLEAN Soil Air Water 2009, 5, 304–313. [Google Scholar] [CrossRef]
- Polacco, J.C.; Mazzafera, P.; Tezotto, T. Opinion–nickel and urease in plants: Still many knowledge gaps. Plant Sci. 2013, 199, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Fabiano, C.; Tezotto, T.; Favarin, J.L.; Polacco, J.C.; Mazzafera, P. Essentiality of nickel in plants: A role in plant stresses. Front. Plant Sci. 2015, 6, 754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matraszek, R.; Hawrylak-Nowak, B.; Chwil, S.; Chwil, M. Macronutrient composition of nickel-treated wheat under different sulfur concentrations in the nutrient solution. Environ. Sci. Pollut. Res. 2016, 23, 5902–5914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matraszek-Gawron, R.; Hawrylak-Nowak, B. Micronutrient status and selected physiological parameters of roots in nickel-exposed Sinapis alba L. affected by different sulphur levels. Plants 2019, 8, 440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cempel, M.; Nikel, G. Nickel: A review of its sources and environmental toxicology. Polish J. Environ. Stud. 2006, 15, 375–382. [Google Scholar]
- Buxton, S.; Garman, E.; Heim, K.E.; Lyons-Darden, T.; Schlekat, C.E.; Taylor, M.D.; Oller, A.R. Concise review of nickel human health toxicology and ecotoxicology. Inorganics 2019, 7, 89. [Google Scholar] [CrossRef] [Green Version]
- Silva, E.B.; Alves, I.S.; Alleoni, L.R.F.; Grazziotti, P.H.; Farnezi, M.M.M.; Santos, L.L.; Prochnow, J.T.; Fontan, I.C.I. Availability and toxic level of cadmium, lead and nickel in contaminated soils. Commun. Soil Sci. Plant Anal. 2020, 51, 1341–1356. [Google Scholar] [CrossRef]
- Prajapati, D.H.; Ausma, T.; de Boer, J.; Hawkesford, M.J.; De Kok, L.J. Nickel toxicity in Brassica rapa seedlings: Impact on sulfur metabolism and mineral nutrient content. J. Kulturpflanzen 2020, 72, 473–478. [Google Scholar]
- Hawrylak-Nowak, B.; Hasanuzzaman, M.; Matraszek-Gawron, R. Mechanisms of selenium-induced enhancement of abiotic stress tolerance in plants. In Plant Nutrients and Abiotic Stress Tolerance; Hasanuzzaman, M., Fujita, M., Oku, H., Nahar, K., Hawrylak-Nowak, B., Eds.; Springer: Singapore, 2018; pp. 269–295. [Google Scholar]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Raza, A.; Hawrylak-Nowak, B.; Matraszek-Gawron, R.; Al Mahmud, J.; Nahar, K.; Fujita, M. Selenium in plants: Boon or bane? Environ. Exp. Bot. 2020, 178, 104170. [Google Scholar] [CrossRef]
- Feng, R.; Wang, L.; Yang, J.; Zhao, P.; Zhu, Y.; Li, Y.; Yu, Y.; Liu, H.; Rensing, C.; Wu, Z.; et al. Underlying mechanisms responsible for restriction of uptake and translocation of heavy metals (metalloids) by selenium via root application in plants. J. Hazard. Mat. 2021, 402, 123570. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hu, C.; Wu, Z.; Liu, X.; Cai, M.; Jia, W.; Zhao, X. Selenium reduces cadmium accumulation in seed by increasing cadmium retention in root of oilseed rape (Brassica napus L.). Environ. Exp. Bot. 2019, 158, 161–170. [Google Scholar] [CrossRef]
- Mozafariyan, M.; Shekari, L.; Hawrylak-Nowak, B.; Kamelmanesh, M.M. Protective role of selenium on pepper exposed to cadmium stress during reproductive stage. Biol. Trace Elem. Res. 2014, 160, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Norton, G.J.; Duan, G.; Huang, Y.; Liu, Y. Effect of selenium fertilization on the accumulation of cadmium and lead in rice plants. Plant Soil 2014, 384, 131–140. [Google Scholar] [CrossRef]
- Wu, C.; Dun, Y.; Zhang, Z.; Li, M.; Wu, G. Foliar application of selenium and zinc to alleviate wheat (Triticum aestivum L.) cadmium toxicity and uptake from cadmium-contaminated soil. Ecotoxicol. Environ. Saf. 2020, 190, 110091. [Google Scholar] [CrossRef]
- Alyemeni, M.N.; Ahanger, M.A.; Wijaya, L.; Alam, P.; Bhardwaj, R.; Ahmad, P. Selenium mitigates cadmium-induced oxidative stress in tomato (Solanum lycopersicum L.) plants by modulating chlorophyll fluorescence, osmolyte accumulation, and antioxidant system. Protoplasma 2018, 255, 459–469. [Google Scholar] [CrossRef]
- Hawrylak-Nowak, B.; Dresler, S.; Wójcik, M. Selenium affects physiological parameters and phytochelatins accumulation in cucumber (Cucumis sativus L.) plants grown under cadmium exposure. Sci. Hortic. 2014, 172, 10–18. [Google Scholar] [CrossRef]
- Hawrylak, B.; Matraszek, R.; Szymańska, M. Response of lettuce (Lactuca sativa L.) to selenium in nutrient solution contaminated with nickel. Veg. Crop. Res. Bull. 2007, 67, 63–70. [Google Scholar] [CrossRef] [Green Version]
- Matraszek, R.; Hawrylak-Nowak, B. Macronutrients accumulation in useable parts of lettuce as affected by nickel and selenium concentrations in nutrient solution. Fresenius Environ. Bull. 2009, 18, 1059–1065. [Google Scholar]
- Bărbieru, O.G.; Dimitriu, L.; Călin, M.; Răut, I.; Constantinescu-Aruxandei, D.; Oancea, F. Plant biostimulants based on selenium nanoparticles biosynthesized by Trichoderma strains. Proceedings 2019, 29, 95. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Fu, P.; Huang, Q.; Zhang, J.; Li, H. Accumulation, subcellular distribution, and oxidative stress of cadmium in Brassica chinensis supplied with selenite and selenate at different growth stages. Chemosphere 2019, 216, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhuang, Z.; Luo, L.Y.; Wang, Y.Q.; Li, H.F. Difference between selenite and selenate in selenium transformation and the regulation of cadmium accumulation in Brassica chinensis. Environ. Sci. Pollut. Res. 2019, 26, 24532–24541. [Google Scholar] [CrossRef] [PubMed]
- Kabata-Pendias, A.; Mukherjee, A.B. Trace Elements from Soil to Human; Springer: New York, NY, USA; Berlin/Heidelberg, Germany, 2007; pp. 237–248. [Google Scholar]
- Ahmad, M.S.; Ashraf, M. Essential roles and hazardous effects of nickel in plants. In Reviews of Environmental Contamination and Toxicology; Whitacre, D.M., Ed.; Springer: New York, NY, USA, 2011; pp. 125–167. [Google Scholar]
- Charles, J.; Sancey, B.; Morin-Crini, N.; Badot, P.M.; Degiorgi, F.; Trunfio, G.; Crini, G. Evaluation of the phytotoxicity of polycontaminated industrial effluents using the lettuce plant (Lactuca sativa) as a bioindicator. Ecotoxicol. Environ. Saf. 2011, 74, 2057–2064. [Google Scholar] [CrossRef] [PubMed]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Calif. Agric. Exp. Sta. Circ. 1950, 347, 1–32. [Google Scholar]
- Hawrylak-Nowak, B. Comparative effects of selenite and selenate on growth and selenium accumulation in lettuce plants under hydroponic conditions. Plant Growth Regul. 2013, 70, 149–157. [Google Scholar] [CrossRef] [Green Version]
- Lichtenthaler, H.K.; Wellburn, A.R. Determination of total carotenoids and chlorophyll a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 603, 591–592. [Google Scholar] [CrossRef] [Green Version]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 89–198. [Google Scholar] [CrossRef]
- Kurzbaum, E.; Kirzhner, F.; Armon, R. A simple method for dehydrogenase activity visualization of intact plant roots grown in soilless culture using tetrazolium violet. Plant Root 2010, 4, 12–16. [Google Scholar] [CrossRef] [Green Version]
- Singh, G.; Agnihotri, R.K.; Reshma, R.S.; Ahmad, M. Effect of lead and nikel toxicity on chlorophyll and proline content of Urd (Vigna mungo L.) seedlings. Int. J. Plant Physiol. Biochem. 2012, 4, 136–141. [Google Scholar]
- Selvaraj, K. Effect of nickel chloride on the growth and biochemical characteristics of Phaseolus mungo. JOJ Sci. 2018, 1, 555556. [Google Scholar]
- Hawrylak-Nowak, B. Selenite is more efficient than selenate in alleviation of salt stress in lettuce plants. Acta Biol. Cracov. Bot. 2015, 57, 49–54. [Google Scholar] [CrossRef]
- Filek, M.; Gzyl-Malcher, B.; Zembala, M.; Bednarska, E.; Laggner, P.; Kriechbaum, M. Effect of selenium on characteristics of rape chloroplasts modified by cadmium. J. Plant Physiol. 2010, 167, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Diao, M.; Ma, L.; Wang, J.; Cui, J.; Fu, A.; Liu, H.Y. Selenium promotes the growth and photosynthesis of tomato seedlings under salt stress by enhancing chloroplast antioxidant defense system. J. Plant Growth Regul. 2014, 33, 671–682. [Google Scholar] [CrossRef]
- Parlak, K.U. Effect of nickel on growth and biochemical characteristics of wheat (Triticum aestivum L.) seedlings. NJAS Wagen. J. Life Sci. 2016, 76, 1–5. [Google Scholar] [CrossRef]
- Saidi, I.; Chtourou, Y.; Djebali, W. Selenium alleviates cadmium toxicity by preventing oxidative stress in sunflower (Helianthus annuus) seedlings. J. Plant Physiol. 2014, 171, 85–91. [Google Scholar] [CrossRef]
- Bernat, P.; Gajewska, E.; Bernat, T.; Wielanek, M. Characterisation of the wheat phospholipid fraction in the presence of nickel and/or selenium. Plant Growth Regul. 2014, 72, 163–170. [Google Scholar] [CrossRef] [Green Version]
- Hawrylak-Nowak, B.; Matraszek, R.; Pogorzelec, M. The dual effects of two inorganic selenium forms on the growth, selected physiological parameters and macronutrients accumulation in cucumber plants. Acta Physiol. Plant. 2015, 37, 41. [Google Scholar] [CrossRef] [Green Version]
- Hawrylak-Nowak, B.; Dresler, S.; Matraszek, R. Exogenous malic and acetic acids reduce cadmium phytotoxicity and enhance cadmium accumulation in roots of sunflower plants. Plant Physiol. Biochem. 2015, 94, 225–234. [Google Scholar] [CrossRef]
- Redjala, T.; Sterckeman, T.; Skiker, S.; Echevarria, G. Contribution of apoplast and symplast to short term nickel uptake by maize and Leptoplax emarginata roots. Environ. Exp. Bot. 2010, 68, 99–106. [Google Scholar] [CrossRef]
- Dalir, N.; Khoshgoftarmanesh, A.H. Symplastic and apoplastic uptake and root to shoot translocation of nickel in wheat as affected by exogenous amino acids. J. Plant Physiol. 2014, 171, 531–536. [Google Scholar] [CrossRef]
- Feng, R.; Wei, C.; Tu, S.; Ding, Y.; Song, Z. A dual role of Se on Cd toxicity: Evidences from the uptake of Cd and some essential elements and the growth responses in paddy rice. Biol. Trace Elem. Res. 2013, 151, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Dinh, Q.T.; Qi, M.; Wang, M.; Yang, W.; Zhou, F.; Liang, D. Radicular and foliar uptake, and xylem-and phloem-mediated transport of selenium in maize (Zea mays L.): A comparison of five Se exogenous species. Plant Soil 2020, 446, 111–123. [Google Scholar] [CrossRef]
- Li, H.F.; McGrath, S.P.; Zhao, F.J. Selenium uptake, translocation and speciation in wheat supplied with selenate or selenite. New Phytol. 2008, 178, 92–102. [Google Scholar] [CrossRef] [PubMed]

| Concentration of the Element in the Medium (µM) | Concentration of the Element in the Shoots (mg kg−1 DW) | Concentration of the Element in the Roots (mg kg−1 DW) | Translocation Factor (TF); Shoot/Root Element Content Ratios | ||||
|---|---|---|---|---|---|---|---|
| Ni | Se | Ni | Se | Ni | Se | Ni | Se |
| 0 | 0 | 1.12 ± 0.44 h | 0.37 ± 0.12 e | 1.90 ± 0.12 g | 0.41 ± 0.10 h | 0.589 ± 0.23 a | 0.902 ± 0.11 a |
| 5 | 0 | 12.90 ± 1.65 f | 0.42 ± 0.16 e | 312.9 ± 35.2 d | 0.56 ± 0.12 h | 0.041 ± 0.008 f | 0.750 ± 0.08 bc |
| 5 | 2 Se(IV) | 13.30 ± 1.34 f | 2.97 ± 0.38 d | 268.4 ± 24.8 e | 40.40 ± 4.33 c | 0.050 ± 0.014 ef | 0.074 ± 0.02 f |
| 5 | 6 Se(IV) | 20.80 ± 2.64 e | 31.60 ± 4.88 a | 231.2 ± 20.1 f | 216.60 ± 28.3 a | 0.090 ± 0.012 c | 0.146 ± 0.02 e |
| 5 | 2 Se(VI) | 14.30 ± 1.01 f | 6.23 ± 0.75 c | 241.3± 21.4 f | 8.90 ± 1.36 f | 0.059 ± 0.009 e | 0.700 ± 0.06 cd |
| 5 | 6 Se(VI) | 15.80 ± 2.11 g | 18.90 ± 2.92 b | 263.7± 12.3 e | 28.30 ± 3.16 d | 0.060 ± 0.005 e | 0.668 ± 0.04 d |
| 10 | 0 | 23.70 ± 3.65 d | 0.59 ± 0.21 e | 426.5± 36.8 a | 0.66 ± 0.21 h | 0.056 ± 0.007 e | 0.894 ± 0.11 a |
| 10 | 2 Se(IV) | 23.90 ± 3.11 d | 2.82 ± 0.38 d | 365.5± 22.6 c | 62.90 ± 5.25 b | 0.065 ± 0.004 cd | 0.045 ± 0.02 f |
| 10 | 6 Se(IV) | 32.90 ± 3.58 a | 33.30 ± 5.44 a | 320.5± 24.9 d | 225.0 ± 17.54 a | 0.103 ± 0.013 b | 0.148 ± 0.03 e |
| 10 | 2 Se(VI) | 25.30 ± 2.47 cd | 6.34 ± 0.74 c | 338.5± 22.7 d | 8.50 ± 0.98 f | 0.075 ± 0.005 d | 0.746 ± 0.09 bc |
| 10 | 6 Se(VI) | 27.50 ± 3.25 bc | 19.50 ± 2.36 b | 397.5± 16.6 b | 25.70 ± 3.00 d | 0.069 ± 0.004 d | 0.759 ± 0.04 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hawrylak-Nowak, B.; Matraszek-Gawron, R. Difference between Selenite and Selenate in the Regulation of Growth and Physiological Parameters of Nickel-Exposed Lettuce. Biology 2020, 9, 465. https://doi.org/10.3390/biology9120465
Hawrylak-Nowak B, Matraszek-Gawron R. Difference between Selenite and Selenate in the Regulation of Growth and Physiological Parameters of Nickel-Exposed Lettuce. Biology. 2020; 9(12):465. https://doi.org/10.3390/biology9120465
Chicago/Turabian StyleHawrylak-Nowak, Barbara, and Renata Matraszek-Gawron. 2020. "Difference between Selenite and Selenate in the Regulation of Growth and Physiological Parameters of Nickel-Exposed Lettuce" Biology 9, no. 12: 465. https://doi.org/10.3390/biology9120465





