Histomorphological and Redox Delineations in the Testis and Epididymis of Albino Rats Fed with Green-Synthesized Cellulose
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of GSC
2.3. Animal Model and Experimental Design
2.4. Biochemical Assays
2.5. Histological Studies
2.6. Statistics
3. Results
3.1. Effect of GSC on Testicular and Epididymal Absolute Weights, Organosomatic Indices and Protein Levels
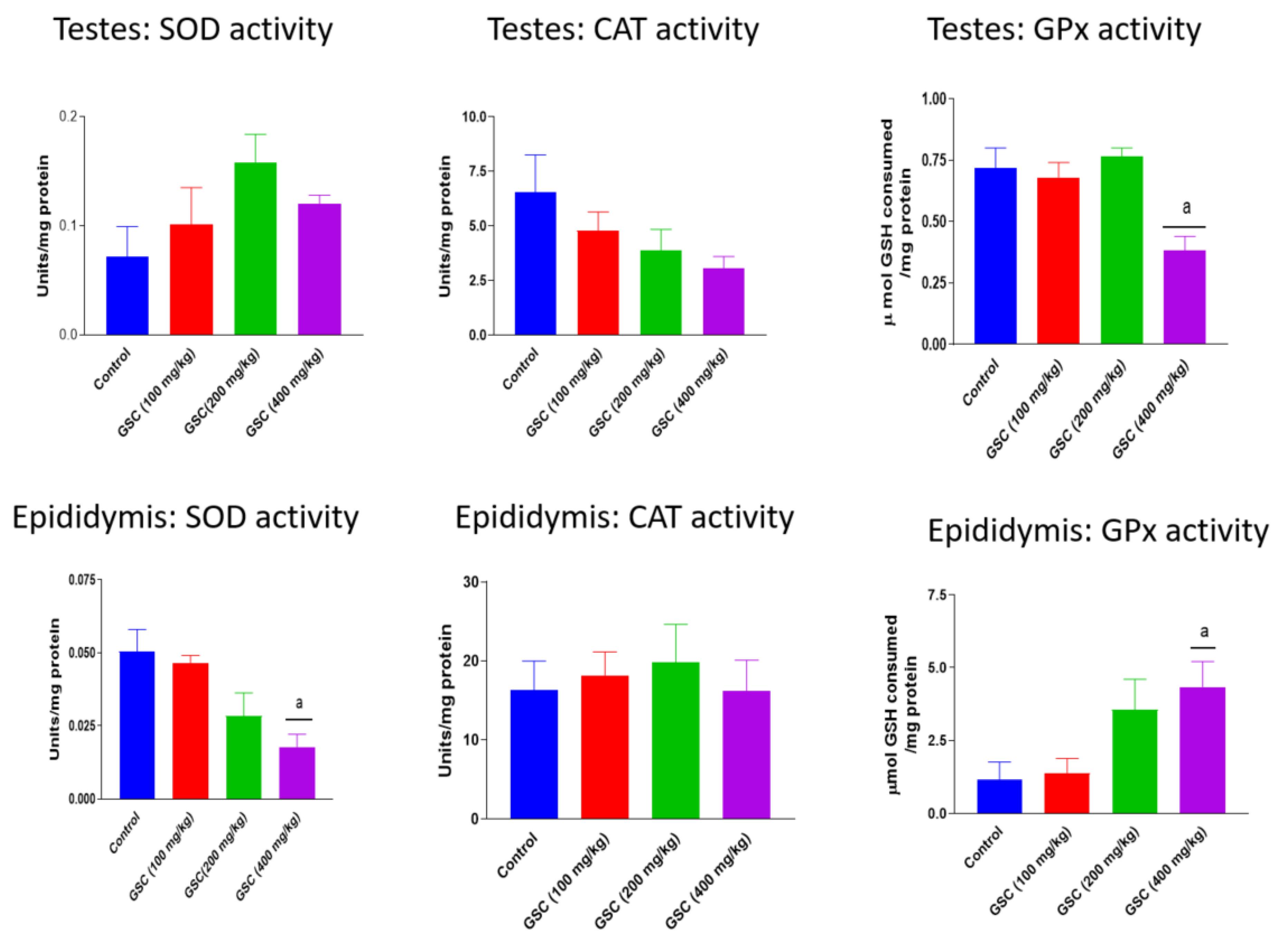
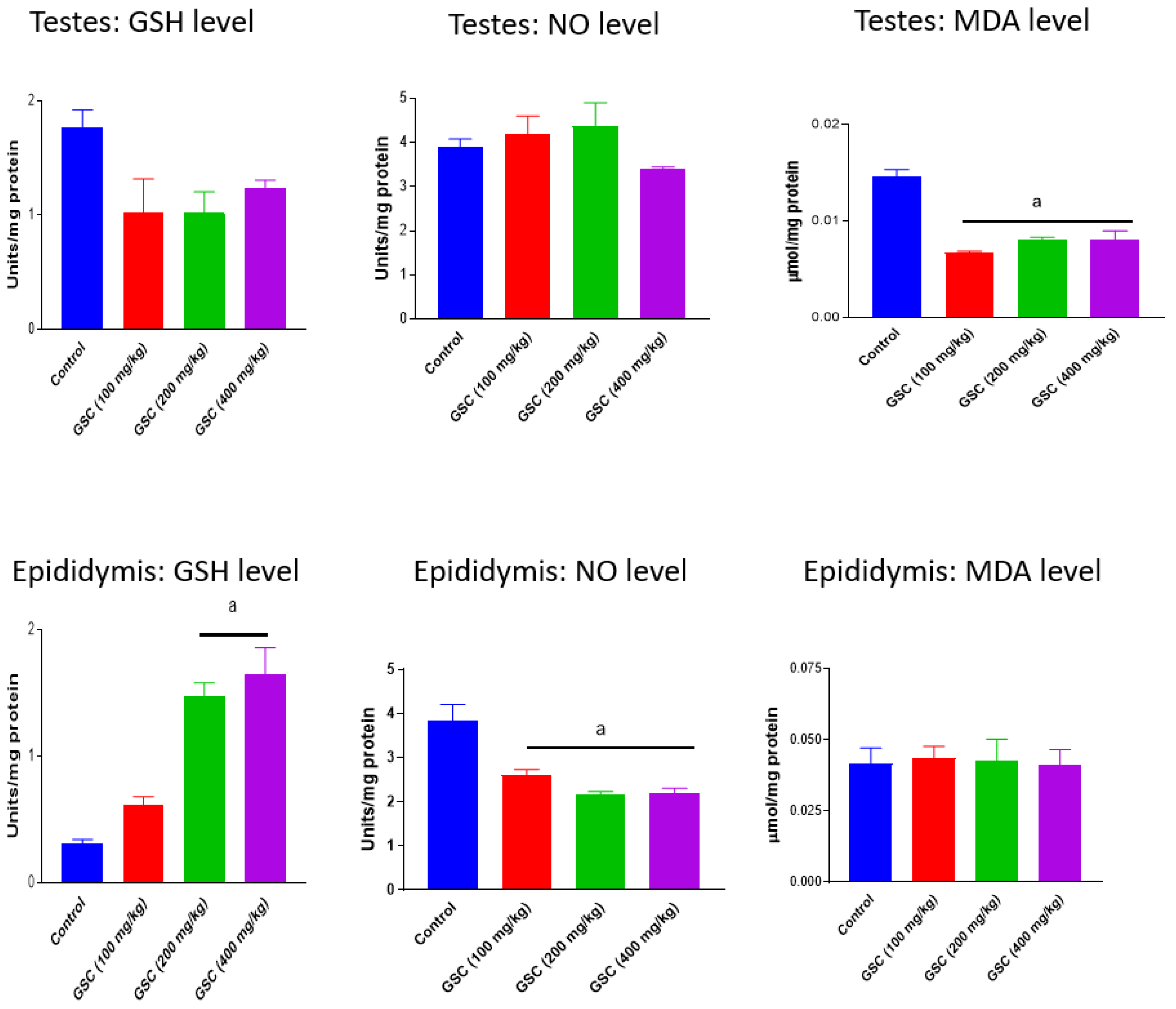
3.2. Effect of GSC on Testicular and Epididymal Redox Status
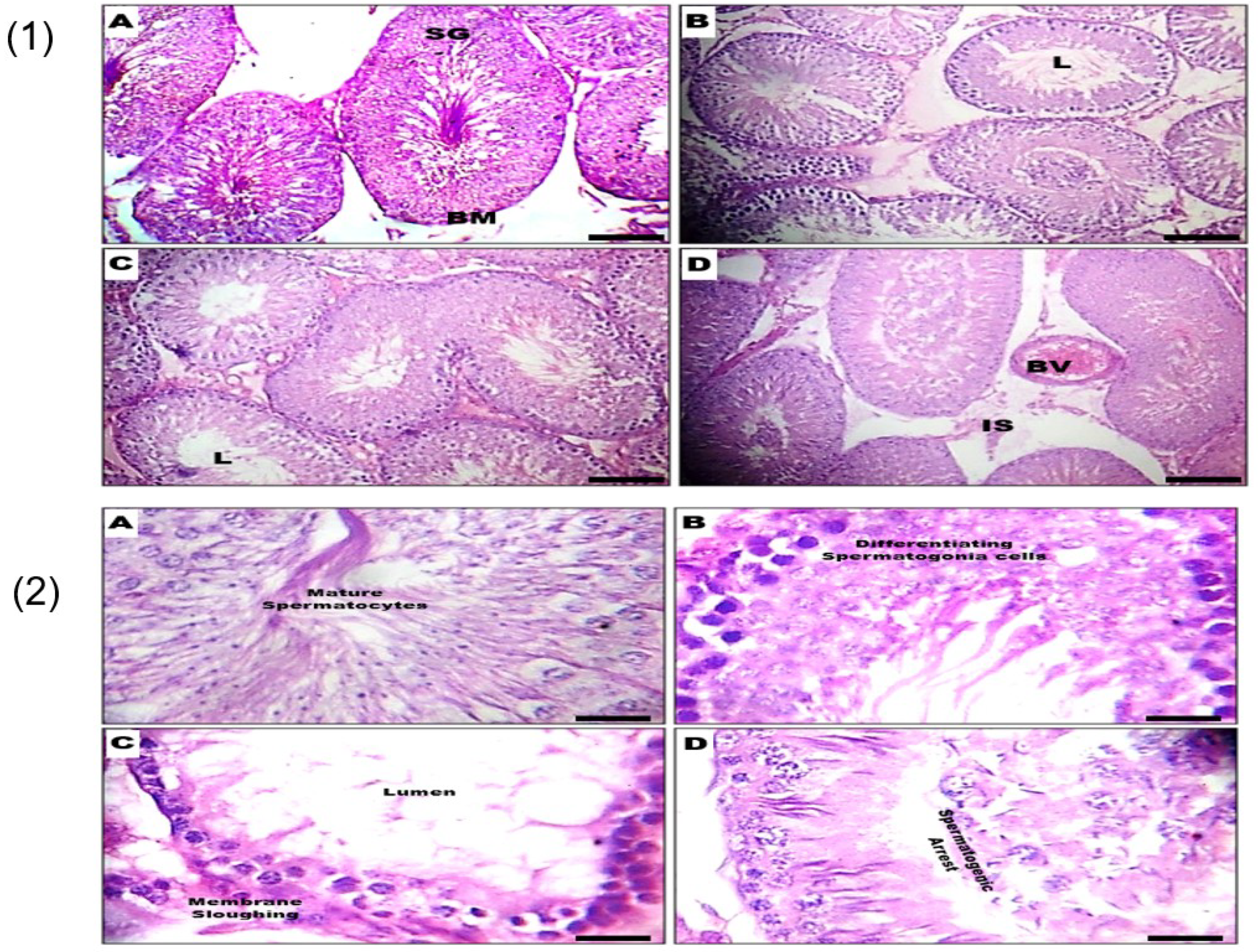
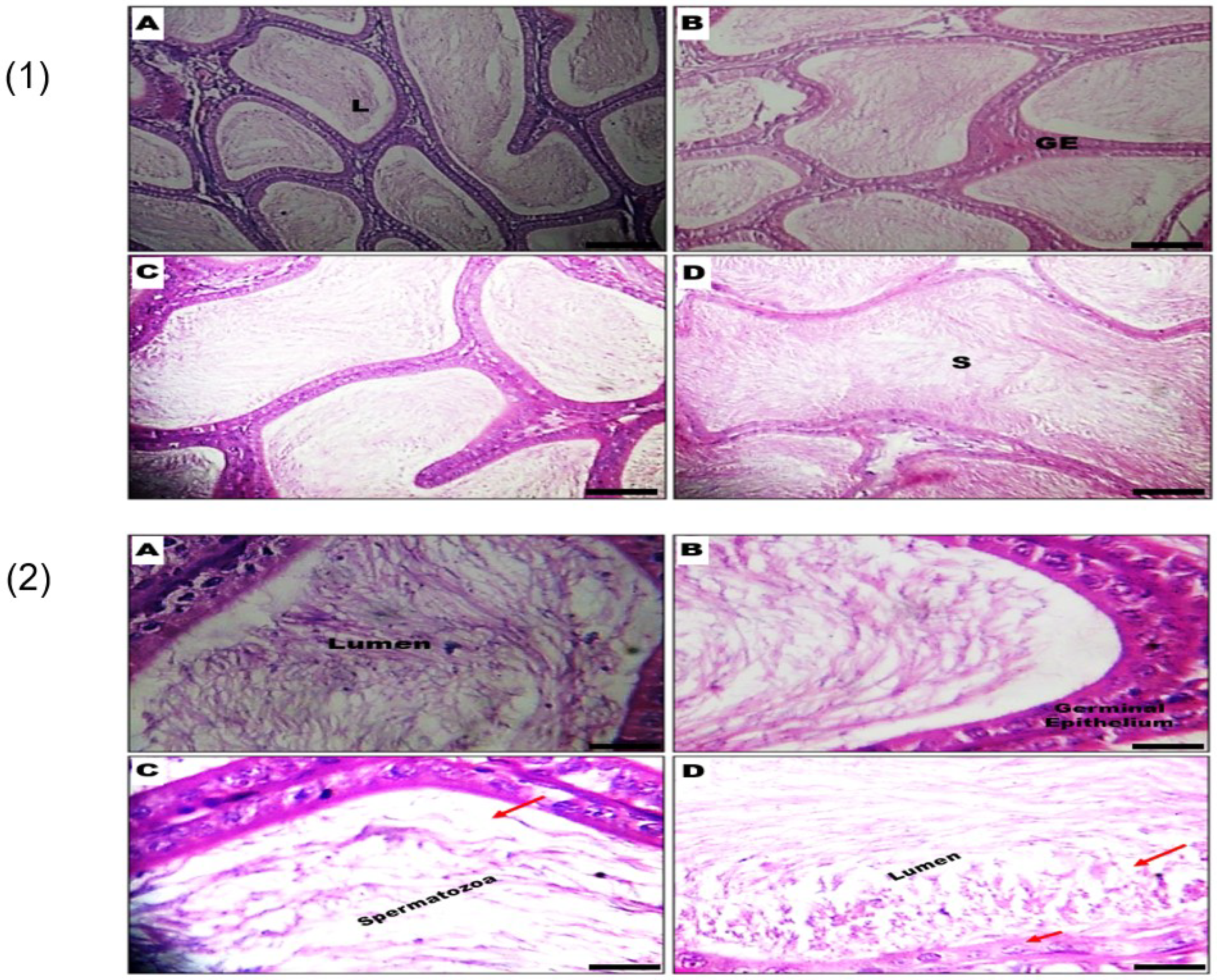
3.3. Effect of GSC on Histomorphology of the Testes and Epididymis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FDA. 2018. Available online: https://www.fda.gov/media/113659/download (accessed on 15 May 2020).
- Gupta, P.K.; Raghunath, S.S.; Prasanna, D.V.; Venkat, P.; Shree, V.; Chithananthan, C.; Choudhary, S.; Surender, K.; Geetha, K. An Update on Overview of Cellulose, Its Structure and Applications. Cellulose 2019. [Google Scholar] [CrossRef] [Green Version]
- Adewuyi, A.; Pereira, F.V. Surface modification of cellulose isolated from Sesamun indicum underutilized seed: A means of enhancing cellulose hydrophobicity. J. Sci. Adv. Mater. Devices 2017, 2, 326–332. [Google Scholar] [CrossRef]
- Menon, M.P.; Selvakumar, R.; Kumar, P.S.; Ramakrishna, S. Extraction and modification of cellulose nanofibers derived from biomass for environmental application. RSC Adv. 2017, 7, 42750–42773. [Google Scholar] [CrossRef] [Green Version]
- Adewuyi, A.; Otuechere, C.A.; Adebayo, O.L.; Anazodo, C.; Pereira, F.V. Renal toxicological evaluations of sulphonated nanocellulose from Khaya sengalensis seed in Wistar rats. Chem. Interact. 2018, 284, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Otuechere, C.A.; Adewuyi, A.; Adebayo, O.L.; Ebigwei, I.A. In vivo hepatotoxicity of chemically modified nanocellulose in rats. Hum. Exp. Toxicol. 2019, 39, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Otuechere, C.A.; Adewuyi, A.; Oluwabayo, T.; Afolayan, F.; Avwioroko, O.; Abazuh, U. Salubrious effects of a vermiculite–cellulose-based bionanocomposite on oxidative stress indices and histomorphology of male Wistar rats. Andrologia 2019, 52, e13426. [Google Scholar] [CrossRef]
- Tristantini, D.; Sandra, C. Synthesis of cellulose acetate from palm oil bunches and dried jackfruit leaves. E3S Web Conf. 2018, 67, 04035. [Google Scholar] [CrossRef]
- Khenblouche, A.; Bechki, D.; Gouamid, M.; Charradi, K.; Segni, L.; Hadjadj, M.; Boughali, S. Extraction and characterization of cellulose microfibers from Retama raetam stems. Polímeros Ciência Tecnol. 2019, 29, 2019011. [Google Scholar] [CrossRef]
- Adewuyi, A.; Otuechere, C.A.; Adebayo, O.L.; Ajisodun, I. Synthesis and toxicity profiling of sebacic acid-modified cellulose from unexploited watermelon exocarp. Polym. Bull. 2020, 1–25. [Google Scholar] [CrossRef]
- Burdock, G.A. Safety assessment of hydroxypropyl methylcellulose as a food ingredient. Food Chem. Toxicol. 2007, 45, 2341–2351. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS); Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gundert-Remy, U.; et al. Safety of low-substituted hydroxypropyl cellulose (L-HPC) to be used as a food additive in food supplements in tablet form. EFSA J. 2018, 16, 05062. [Google Scholar] [CrossRef] [Green Version]
- Farcas, M.T.; Kisin, E.R.; Menas, A.L.; Gutkin, D.W.; Star, A.; Reiner, R.S.; Yanamala, N.; Savolainen, K.; Shvedova, A.A. Pulmonary exposure to cellulose nanocrystals caused deleterious effects to reproductive system in male mice. J. Toxicol. Environ. Health Part A 2016, 79, 984–997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adeniran, A.; Adeyemo, O.K.; Emikpe, B.O.; Alarape, S.A. Organosomatic indices, haematological and histological assessment as biomarkers of health status in feral and cultured Clarias gariepinus. Afr. J. Biomed. Res. 2017, 20, 189–194. [Google Scholar]
- Mead, R. The Design of Experiments; Cambridge University Press: Cambridge, UK, 1988. [Google Scholar]
- Gornall, A.G.; Bardawill, C.J.; David, M.M. Determination of serum proteins by means of the biuret reaction. J. Boil. Chem. 1949, 177, 751–766. [Google Scholar]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the autooxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [PubMed]
- Sinha, A.K. Colorimetric assay of catalase. Anal. Biochem. 1972, 47, 389–394. [Google Scholar] [CrossRef]
- Rotruck, J.T.; Pope, A.L.; Ganther, H.E.; Swanson, A.B.; Hafeman, D.G.; Hoekstra, W.G. Selenium: Biochemical Role as a Component of Glutathione Peroxidase. Science 1973, 179, 588–590. [Google Scholar] [CrossRef] [PubMed]
- Beutler, E.; Duron, O.; Kelly, B.M. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963, 61, 882–888. [Google Scholar]
- Bryan, N.S.; Grisham, M.B. Methods to detect nitric oxide and its metabolites in biological samples. Free. Radic. Boil. Med. 2007, 43, 645–657. [Google Scholar] [CrossRef] [Green Version]
- Varshney, R.; Kale, R. Effects of Calmodulin Antagonists on Radiation-induced Lipid Peroxidation in Microsomes. Int. J. Radiat. Boil. 1990, 58, 733–743. [Google Scholar] [CrossRef]
- Adedara, I.A.; Abolaji, A.O.; Odion, B.E.; Omoloja, A.A.; Okwudi, I.J.; Farombi, E.O. Redox status of the testes and sperm of rats following exposure to 2,5-hexanedione. Redox Rep. 2016, 21, 239–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Björkgren, I.; Sipilä, P. The impact of epididymal proteins on sperm function. Reprod. 2019, 158, R155–R167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bisht, S.; Faiq, M.; Tolahunase, M.; Dada, R. Oxidative stress and male infertility. Nat. Rev. Urol. 2017, 14, 470–485. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Roman, S.D. Antioxidant systems and oxidative stress in the testes. In Madame Curie Bioscience Database; Landes Bioscience: Austin, TX, USA, 2013. Available online: https://www.ncbi.nlm.nih.gov/books/NBK6370 (accessed on 15 May 2020).
- Kuchakulla, M.; Masterson, T.; Arora, H.; Kulandavelu, S.; Ramasamy, R. Effect of nitroso-redox imbalance on male reproduction. Transl. Androl. Urol. 2018, 7, 968–977. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.A.; Kuchakulla, M.; Arora, H.; Kulandavelu, S.; Gonzalez, E.; Masterson, T.A.; Hare, J.M.; Kaiser, U.B.; Ramasamy, R. Age Induced Nitroso-Redox Imbalance Leads to Subclinical Hypogonadism in Male Mice. Front. Endocrinol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Asadi, N.; Bahmani, M.; Kheradmand, A.; Rafieian-Kopaei, M. The Impact of Oxidative Stress on Testicular Function and the Role of Antioxidants in improving it: A Review. J. Clin. Diagn. Res. 2017, 11, IE01–IE05. [Google Scholar] [CrossRef] [PubMed]
- O’Flaherty, C. Orchestrating the antioxidant defenses in the epididymis. Andrology 2019, 7, 662–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Flaherty, C. The Enzymatic Antioxidant System of Human Spermatozoa. Adv. Androl. 2014, 2014, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Wu, P.Y.; Scarlata, E.; O’Flaherty, C. Long-Term Adverse Effects of Oxidative Stress on Rat Epididymis and Spermatozoa. Antioxidants 2020, 9, 170. [Google Scholar] [CrossRef] [Green Version]
- Radi, R. Oxygen radicals, nitric oxide, and peroxynitrite: Redox pathways in molecular medicine. Proc. Natl. Acad. Sci. USA 2018, 115, 5839–5848. [Google Scholar] [CrossRef] [Green Version]
- Castillo, J.; Knol, J.C.; Korver, C.M.; Piersma, S.R.; Pham, T.V.; Van Pelt, A.M.; Jimenez, C.R.; Jansen, B.J.; Haas, R.R.D.G.-D. Human Testis Phosphoproteome Reveals Kinases as Potential Targets in Spermatogenesis and Testicular Cancer. Mol. Cell. Proteom. 2019, 18, S132–S144. [Google Scholar] [CrossRef] [PubMed]
| Control | GSC (100 mg/kg) | GSC (200 mg/kg) | GSC (400 mg/kg) | |
|---|---|---|---|---|
| Testes (g) | 2.08 + 0.19 | 2.37 + 0.11 | 1.96 + 0.17 | 2.37 + 0.60 |
| Organosomatic index (Testes) | 1.22 + 0.06 | 1.17 + 0.04 | 1.12 + 0.08 | 1.14 + 0.20 |
| Epididymis (g) | 0.90 + 0.11 | 0.90 + 0.11 | 0.87 + 0.07 | 0.99 + 0.07 |
| Organosomatic index (Epididymis) | 0.48 + 0.05 | 0.46 + 0.01 | 0.50 + 0.03 | 0.48 + 0.01 |
| Testicular total protein (g/dL) | 3.33 + 0.14 | 3.63 + 0.37 | 3.61 + 0.51 | 4.09 + 0.38 |
| Epididymal total protein (g/dL) | 2.38 + 0.01 | 2.81 + 0.24 | 3.17 + 0.62 | 3.23 + 0.48 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otuechere, C.A.; Adewuyi, A.; Adebayo, O.L.; Yawson, E.; Kabiawu, O.; Al-Rashed, S.; Okubio, B.; Beshbishy, A.M.; Batiha, G.E.-S. Histomorphological and Redox Delineations in the Testis and Epididymis of Albino Rats Fed with Green-Synthesized Cellulose. Biology 2020, 9, 246. https://doi.org/10.3390/biology9090246
Otuechere CA, Adewuyi A, Adebayo OL, Yawson E, Kabiawu O, Al-Rashed S, Okubio B, Beshbishy AM, Batiha GE-S. Histomorphological and Redox Delineations in the Testis and Epididymis of Albino Rats Fed with Green-Synthesized Cellulose. Biology. 2020; 9(9):246. https://doi.org/10.3390/biology9090246
Chicago/Turabian StyleOtuechere, Chiagoziem A., Adewale Adewuyi, Olusegun L. Adebayo, Emmanuel Yawson, Omolara Kabiawu, Sarah Al-Rashed, Blessing Okubio, Amany M. Beshbishy, and Gaber El-Saber Batiha. 2020. "Histomorphological and Redox Delineations in the Testis and Epididymis of Albino Rats Fed with Green-Synthesized Cellulose" Biology 9, no. 9: 246. https://doi.org/10.3390/biology9090246






