Measurable Cytokine Concentrations in Pig Seminal Plasma Are Modified by Semen Handling and Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals, Ejaculates and Seminal Plasma Collection
2.2. Cytokine Measurements in Seminal Plasma Samples
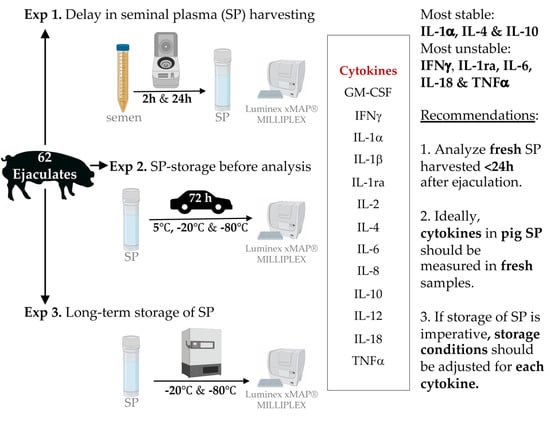
2.3. Experimental Design
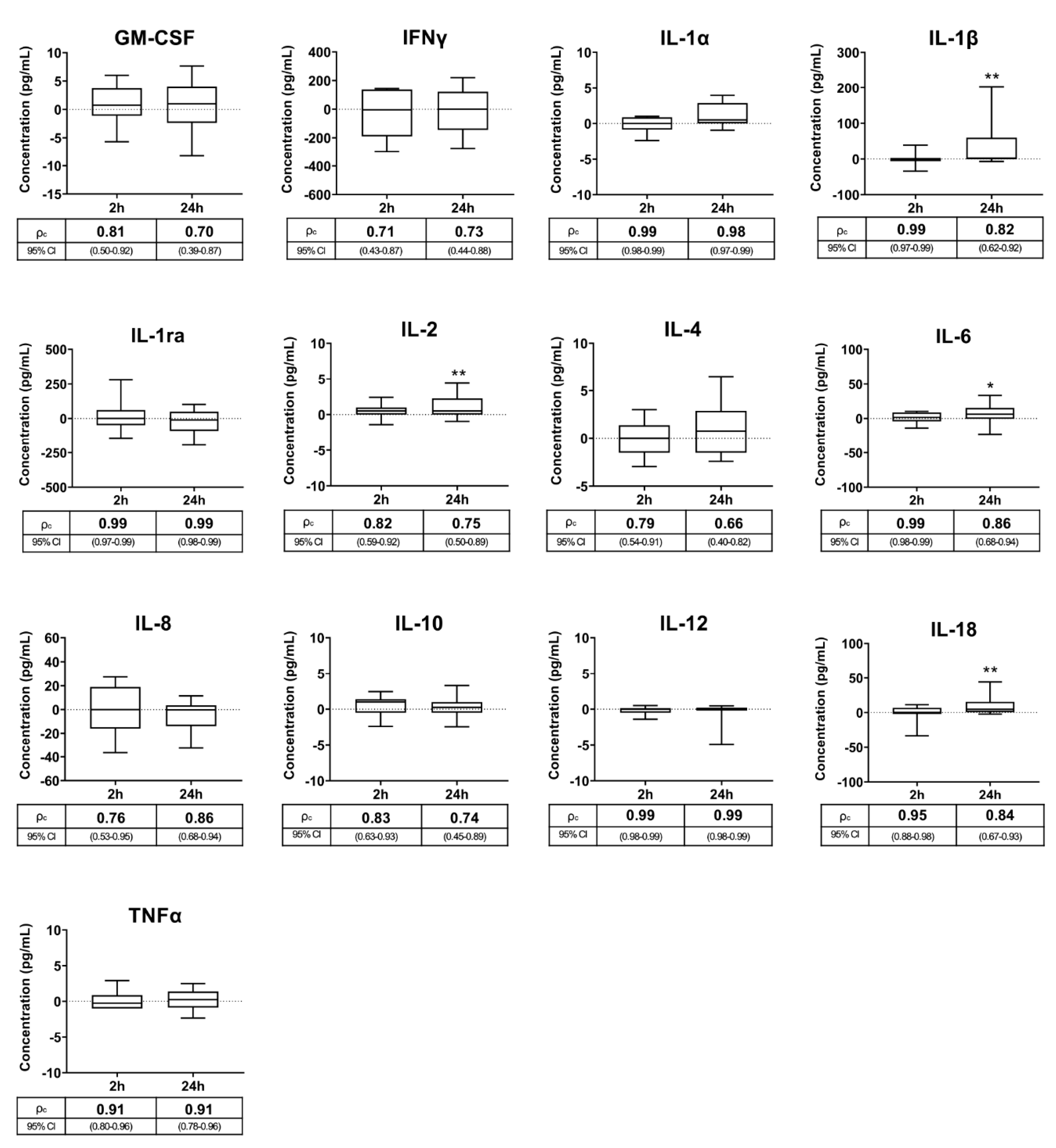
2.3.1. Experiment 1: Interval Ejaculation–Seminal Plasma Harvesting
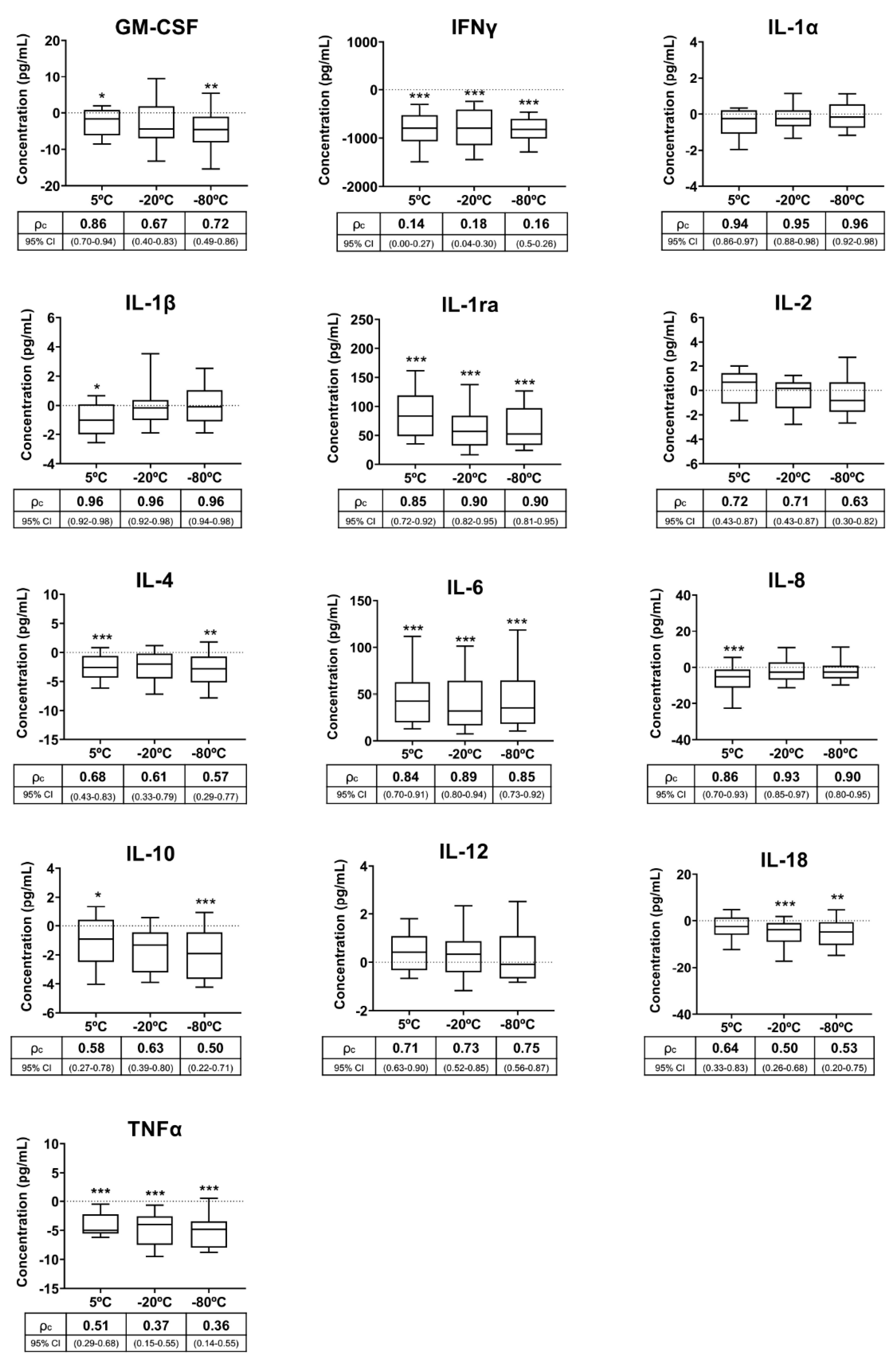
2.3.2. Experiment 2: Storage and Transport Conditions for Seminal Plasma before Analysis
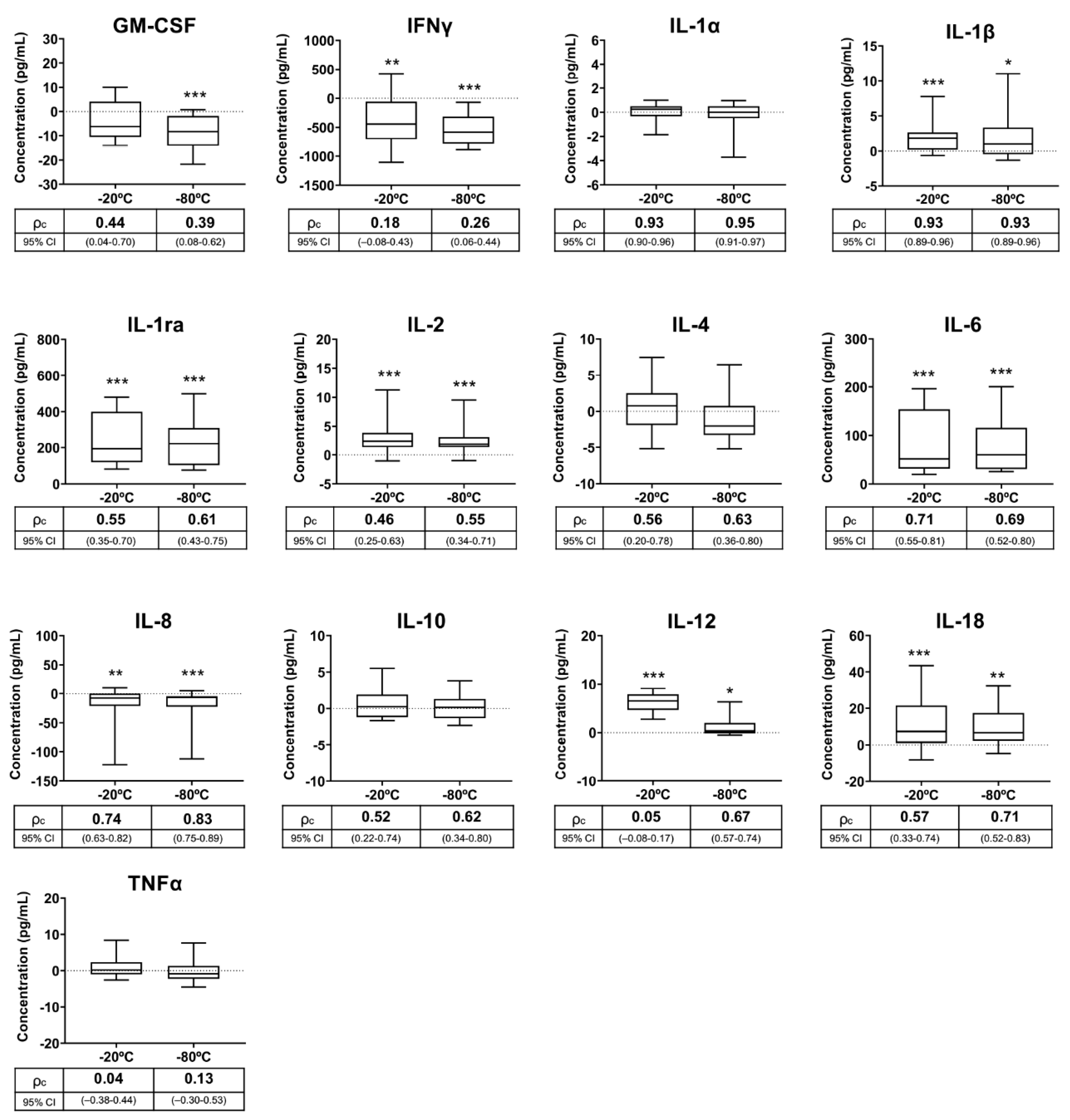
2.3.3. Experiment 3: Effects of Frozen Storage Time on Seminal Plasma Cytokine Contents
2.4. Statistical Analysis
3. Results
3.1. Experiment 1: Interval Ejaculation–Seminal Plasma Harvesting
3.2. Experiment 2: Storage and Transport Conditions for Seminal Plasma before Analysis
3.3. Experiment 3: Effects of Frozen Storage Time on Seminal Plasma Cytokine Contents
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bromfield:, J.J. A role for seminal plasma in modulating pregnancy outcomes in domestic species. Reproduction 2016, 152, R223–R232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, H.L.; Watkins, A.J. The influence of seminal plasma on offspring development and health. Semin. Cell Dev. Biol. 2020, 97, 131–137. [Google Scholar] [CrossRef] [PubMed]
- López Rodríguez, A.; Rijsselaere, T.; Beek, J.; Vyt, P.; Van Soom, A.; Maes, D. Boar seminal plasma components and their relation with semen quality. Syst. Biol. Reprod. Med. 2013, 59, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.; Sá, R.; Barros, A.; Sousa, M. Major regulatory mechanisms involved in sperm motility. Asian J. Androl. 2017, 19, 5–14. [Google Scholar] [CrossRef]
- Waberski, D.; Schäfer, J.; Bölling, A.; Scheld, M.; Henning, H.; Hambruch, N.; Schuberth, H.J.; Pfarrer, C.; Wrenzycki, C.; Hunter, R.H.F. Seminal plasma modulates the immune-cytokine network in the porcine uterine tissue and pre-ovulatory follicles. PLoS ONE 2018, 13, e0202654. [Google Scholar] [CrossRef] [Green Version]
- Loveland, K.L.; Klein, B.; Pueschl, D.; Indumathy, S.; Bergmann, M.; Loveland, B.E.; Hedger, M.P.; Schuppe, H.C. Cytokines in male fertility and reproductive pathologies: Immunoregulation and beyond. Front. Endocrinol. 2017, 8, 307. [Google Scholar] [CrossRef]
- Samanta, L.; Parida, R.; Dias, T.R.; Agarwal, A. The enigmatic seminal plasma: A proteomics insight from ejaculation to fertilization. Reprod. Biol. Endocrinol. 2018, 16, 41. [Google Scholar] [CrossRef] [Green Version]
- Ramani, T.; Auletta, C.S.; Weinstock, D.; Mounho-Zamora, B.; Ryan, P.C.; Salcedo, T.W.; Bannish, G. Cytokines: The Good, the Bad, and the Deadly. Int. J. Toxicol. 2015, 34, 355–365. [Google Scholar] [CrossRef]
- Altan-Bonnet, G.; Mukherjee, R. Cytokine-mediated communication: A quantitative appraisal of immune complexity. Nat. Rev. Immunol. 2019, 19, 205–217. [Google Scholar] [CrossRef]
- Ouyang, W.; O’Garra, A. IL-10 Family Cytokines IL-10 and IL-22: From Basic Science to Clinical Translation. Immunity 2019, 50, 871–891. [Google Scholar] [CrossRef]
- Fraczek, M.; Kurpisz, M. Cytokines in the male reproductive tract and their role in infertility disorders. J. Reprod. Immunol. 2015, 108, 98–104. [Google Scholar] [CrossRef]
- Archana, S.S.; Selvaraju, S.; Binsila, B.K.; Arangasamy, A.; Krawetz, S.A. Immune regulatory molecules as modifiers of semen and fertility: A review. Mol. Reprod. Dev. 2019, 86, 1485–1504. [Google Scholar] [CrossRef]
- Grande, G.; Milardi, D.; Baroni, S.; Luca, G.; Pontecorvi, A. Identification of seminal markers of male accessory gland inflammation: From molecules to proteome. Am. J. Reprod. Immunol. 2018, 80, e12992. [Google Scholar] [CrossRef]
- Syriou, V.; Papanikolaou, D.; Kozyraki, A.; Goulis, D.G. Cytokines and male infertility. Eur. Cytokine Netw. 2018, 29, 73–82. [Google Scholar] [CrossRef]
- Zeinali, M.; Hadian Amree, A.; Khorramdelazad, H.; Karami, H.; Abedinzadeh, M. Inflammatory and anti-inflammatory cytokines in the seminal plasma of infertile men suffering from varicocele. Andrologia 2017, 49, e12685. [Google Scholar] [CrossRef]
- Penna, G.; Mondaini, N.; Amuchastegui, S.; Degli Innocenti, S.; Carini, M.; Giubilei, G.; Fibbi, B.; Colli, E.; Maggi, M.; Adorini, L. Seminal plasma cytokines and chemokines in prostate inflammation: Interleukin 8 as a predictive biomarker in chronic prostatitis/chronic pelvic pain syndrome and benign prostatic hyperplasia. Eur. Urol. 2007, 51, 524–533. [Google Scholar] [CrossRef]
- Lotti, F.; Maggi, M. Interleukin 8 and the male genital tract. J. Reprod. Immunol. 2013, 100, 54–65. [Google Scholar] [CrossRef]
- Havrylyuk, A.; Chopyak, V.; Boyko, Y.; Kril, I.; Kurpisz, M. Cytokines in the blood and semen of infertile patients. Cent. J. Immunol. 2015, 40, 337–344. [Google Scholar] [CrossRef]
- Bromfield, J.J. Review: The potential of seminal fluid mediated paternal-maternal communication to optimise pregnancy success. Animal 2018, 12, s104–s109. [Google Scholar] [CrossRef]
- Schjenken, J.E.; Robertson, S.A. The Female Response to Seminal Fluid. Physiol. Rev. 2020, 100, 1077–1117. [Google Scholar] [CrossRef]
- de Jager, W.; Bourcier, K.; Rijkers, G.T.; Prakken, B.J.; Seyfert-Margolis, V. Prerequisites for cytokine measurements in clinical trials with multiplex immunoassays. BMC Immunol. 2009, 10, 52. [Google Scholar] [CrossRef] [Green Version]
- Keustermans, G.C.E.; Hoeks, S.B.E.; Meerding, J.M.; Prakken, B.J.; de Jager, W. Cytokine assays: An assessment of the preparation and treatment of blood and tissue samples. Methods 2013, 61, 10–17. [Google Scholar] [CrossRef]
- Cohen, L.; Fiore-Gartland, A.; Randolph, A.G.; Panoskaltsis-Mortari, A.; Wong, S.-S.; Ralston, J.; Wood, T.; Seeds, R.; Huang, Q.S.; Webby, R.J.; et al. A Modular Cytokine Analysis Method Reveals Novel Associations With Clinical Phenotypes and Identifies Sets of Co-signaling Cytokines Across Influenza Natural Infection Cohorts and Healthy Controls. Front. Immunol. 2019, 10, 1338. [Google Scholar] [CrossRef]
- Binnington, B.; Sakac, D.; Yi, Q.; Tong, T.N.; Parmar, N.; Duong, T.T.; Yeung, R.S.M.; Pendergrast, J.; Branch, D.R. Stability of 40 cytokines/chemokines in chronically ill patients under different storage conditions. Cytokine 2020, 130, 155057. [Google Scholar] [CrossRef]
- Food and Agricultural Organization of the United Nations. Biannual Report of Global Food Markets; Food and Agricultural Organization of the United Nations: Rome, Italy, 2019; ISBN 9789251319321. [Google Scholar]
- Bassols, A.; Costa, C.; Eckersall, P.D.; Osada, J.; Sabrià, J.; Tibau, J. The pig as an animal model for human pathologies: A proteomics perspective. Proteomics Clin. Appl. 2014, 8, 715–731. [Google Scholar] [CrossRef]
- Whitelaw, C.B.A.; Sheets, T.P.; Lillico, S.G.; Telugu, B.P. Engineering large animal models of human disease. J. Pathol. 2016, 238, 247–256. [Google Scholar] [CrossRef]
- Kuster, C.E.; Althouse, G.C. The fecundity of porcine semen stored for 2 to 6 days in Androhep and X-CELL extenders. Theriogenology 1999, 52, 365–376. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [Green Version]
- Sharkey, D.J.; Tremellen, K.P.; Briggs, N.E.; Dekker, G.A.; Robertson, S.A. Seminal plasma pro-inflammatory cytokines interferon-γ (IFNG) and C-X-C motif chemokine ligand 8 (CXCL8) fluctuate over time within men. Hum. Reprod. 2017, 32, 1373–1381. [Google Scholar] [CrossRef]
- Sharkey, D.J.; Tremellen, K.P.; Briggs, N.E.; Dekker, G.A.; Robertson, S.A. Seminal plasma transforming growth factor-β, activin A and follistatin fluctuate within men over time. Hum. Reprod. 2016, 31, 2183–2191. [Google Scholar] [CrossRef]
- Lin, L.I. A concordance correlation coefficient to evaluate reproducibility. Biometrics 1989, 45, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [Green Version]
- Petrie, A.; Watson, P. Linear Correlation and Regression. In Statistics for Veterinary and Animal Science, 3rd ed.; Wiley-Blackwell, Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2013; pp. 126–142. ISBN 978-0-470-67075-0. [Google Scholar]
- Khan, S.S.; Smith, M.S.; Reda, D.; Suffredini, A.F.; McCoy, J.P.J. Multiplex bead array assays for detection of soluble cytokines: Comparisons of sensitivity and quantitative values among kits from multiple manufacturers. Cytom. B Clin. Cytom. 2004, 61, 35–39. [Google Scholar] [CrossRef]
- Christopher-Hennings, J.; Araujo, K.P.C.; Souza, C.J.H.; Fang, Y.; Lawson, S.; Nelson, E.A.; Clement, T.; Dunn, M.; Lunney, J.K. Opportunities for bead-based multiplex assays in veterinary diagnostic laboratories. J. Vet. Diagn. Investig. 2013, 25, 671–691. [Google Scholar] [CrossRef] [Green Version]
- Butterfield, L.H.; Potter, D.M.; Kirkwood, J.M. Multiplex serum biomarker assessments: Technical and biostatistical issues. J. Transl. Med. 2011, 9, 173. [Google Scholar] [CrossRef] [Green Version]
- Barranco, I.; Rubér, M.; Perez-Patiño, C.; Atikuzzaman, M.; Martinez, E.A.; Roca, J.; Rodriguez-Martinez, H. The Seminal Plasma of the Boar is Rich in Cytokines, with Significant Individual and Intra-Ejaculate Variation. Am. J. Reprod. Immunol. 2015, 74, 523–532. [Google Scholar] [CrossRef] [Green Version]
- Barranco, I.; Padilla, L.; Pérez-Patiño, C.; Vazquez, J.M.; Martínez, E.A.; Rodríguez-Martínez, H.; Roca, J.; Parrilla, I. Seminal Plasma Cytokines Are Predictive of the Outcome of Boar Sperm Preservation. Front. Vet. Sci. 2019, 6, 436. [Google Scholar] [CrossRef]
- Padilla, L.; Lucas, X.; Parrilla, I.; Perez-Patiño, C.; Rodriguez-Martinez, H.; Roca, J.; Barranco, I. Period of Boar Ejaculate Collection Contributes to the Yearly Intra-Male Variability of Seminal Plasma Cytokines. Biology 2020, 9, 105. [Google Scholar] [CrossRef]
- Riches, P.; Gooding, R.; Millar, B.C.; Rowbottom, A.W. Influence of collection and separation of blood samples on plasma IL-1, IL-6 and TNF-alpha concentrations. J. Immunol. Methods 1992, 153, 125–131. [Google Scholar] [CrossRef]
- Guo, G.-H.; Dong, J.; Yuan, X.-H.; Dong, Z.-N.; Tian, Y.-P. Clinical evaluation of the levels of 12 cytokines in serum/plasma under various storage conditions using evidence biochip arrays. Mol. Med. Rep. 2013, 7, 775–780. [Google Scholar] [CrossRef] [Green Version]
- Ford, W.C.L. Regulation of sperm function by reactive oxygen species. Hum. Reprod. Update 2004, 10, 387–399. [Google Scholar] [CrossRef]
- Padilla, L.; Martinez, J.; Barranco, I.; Pastor, L.M.; Rodriguez-Martinez, H.; Roca, J.; Parrilla, I. Granulocyte-Macrophage Colony Stimulating Factor (GM-CSF) is fully expressed in the genital tract, seminal plasma and spermatozoa of male pigs. Sci. Rep. 2020, 10, 13360. [Google Scholar] [CrossRef]
- Guthrie, H.D.; Welch, G.R. Impact of storage prior to cryopreservation on plasma membrane function and fertility of boar sperm. Theriogenology 2005, 63, 396–410. [Google Scholar] [CrossRef]
- Roca, J.; Parrilla, I.; Gil, M.A.; Cuello, C.; Martinez, E.A.; Rodriguez-Martinez, H. Non-viable sperm in the ejaculate: Lethal escorts for contemporary viable sperm. Anim. Reprod. Sci. 2016, 169, 24–31. [Google Scholar] [CrossRef] [Green Version]
- Simpson, S.; Kaislasuo, J.; Guller, S.; Pal, L. Thermal stability of cytokines: A review. Cytokine 2020, 125, 154829. [Google Scholar] [CrossRef]
- Hennø, L.T.; Storjord, E.; Christiansen, D.; Bergseth, G.; Ludviksen, J.K.; Fure, H.; Barene, S.; Waage-Nielsen, E.; Mollnes, T.E.; Brekke, O.L. Effect of the anticoagulant, storage time and temperature of blood samples on the concentrations of 27 multiplex assayed cytokines—Consequences for defining reference values in healthy humans. Cytokine 2017, 97, 86–95. [Google Scholar] [CrossRef] [Green Version]
- Zander, J.; Bruegel, M.; Kleinhempel, A.; Becker, S.; Petros, S.; Kortz, L.; Dorow, J.; Kratzsch, J.; Baber, R.; Ceglarek, U.; et al. Effect of biobanking conditions on short-term stability of biomarkers in human serum and plasma. Clin. Chem. Lab. Med. 2014, 52, 629–639. [Google Scholar] [CrossRef]
- Aziz, N.; Detels, R.; Quint, J.J.; Li, Q.; Gjertson, D.; Butch, A.W. Stability of cytokines, chemokines and soluble activation markers in unprocessed blood stored under different conditions. Cytokine 2016, 84, 17–24. [Google Scholar] [CrossRef] [Green Version]
- Gulevsky, A.K.; Relina, L.I. Molecular and genetic aspects of protein cold denaturation. CryoLetters 2013, 34, 62–82. [Google Scholar]
- Pucci, F.; Rooman, M. Physical and molecular bases of protein thermal stability and cold adaptation. Curr. Opin. Struct. Biol. 2017, 42, 117–128. [Google Scholar] [CrossRef]
- Grankvist, K.; Gomez, R.; Nybo, M.; Lima-Oliveira, G.; von Meyer, A. Preanalytical aspects on short- and long-term storage of serum and plasma. Diagnosis 2019, 6, 51–56. [Google Scholar] [CrossRef]
| Cytokine (pg/mL) | Median (25th, 75th Percentiles) | Range |
|---|---|---|
| GM-CSF | 31.00 (27.50, 34.88) | 27.50 |
| IFNγ | 1317.00 (1073, 1496) | 667.50 |
| IL-1α | 10.75 (4.00, 25.38) | 37.50 |
| IL-1β | 18.75 (15.63, 99.50) | 480.50 |
| IL-1ra | 626.30 (293.90, 1763.00) | 3621.00 |
| IL-2 | 5.25 (3.62, 9.00) | 11.00 |
| IL-4 | 21.75 (18.63, 23.50) | 9.50 |
| IL-6 | 83.25 (64.25, 151.90) | 347.50 |
| IL-8 | 32.75 (22.50, 54.88) | 99.80 |
| IL-10 | 9.50 (8.62, 13.63) | 10.50 |
| IL-12 | 3.00 (3.00, 4.25) | 68.20 |
| IL-18 | 20.50 (4.25, 77.63) | 195.00 |
| TNFα | 18.25 (13.75, 23.50) | 19.00 |
| Cytokine | Spearman Correlation (p-Value) | ||||||
|---|---|---|---|---|---|---|---|
| Experiment 1 | Experiment 2 | Experiment 3 | |||||
| 2 h vs. 0 h | 24 h vs. 0 h | 5 °C vs. Fresh | −20 °C vs. Fresh | −80 °C vs. Fresh | −20 °C vs. Fresh | −80 °C vs. Fresh | |
| GM-CSF | 0.83 (<0.001) | 0.70 (0.001) | 0.89 (<0.001) | 0.74 (<0.001) | 0.81 (<0.001) | 0.48 (0.032) | 0.56 (0.010) |
| IFNγ | 0.62 (0.004) | 0.74 (0.002) | 0.79 (<0.001) | 0.56 (ns) | 0.72 (0.006) | 0.32 (ns) | 0.62 (0.003) |
| IL-1α | 0.98 (<0.001) | 0.99 (<0.00) | 0.90 (<0.001) | 0.90 (<0.001) | 0.88 (<0.001) | 0.81 (<0.001) | 0.85 (<0.001) |
| IL-1β | 0.86 (<0.001) | 0.81 (<0.00) | 0.92 (<0.001) | 0.93 (<0.001) | 0.95 (<0.001) | 0.93 (<0.001) | 0.85 (<0.001) |
| IL-1ra | 0.97 (<0.001) | 0.99 (<0.00) | 0.96 (<0.001) | 0.96 (<0.001) | 0.97 (<0.001) | 0.94 (<0.001) | 0.96 (<0.001) |
| IL-2 | 0.88 (<0.001) | 0.89 (<0.00) | 0.70 (<0.001) | 0.71 (0.006) | 0.70 (0.003) | 0.57 (0.008) | 0.83 (<0.001) |
| IL-4 | 0.79 (<0.001) | 0.76 (<0.00) | 0.81 (<0.001) | 0.73 (0.011) | 0.70 (<0.011) | 0.60 (0.005) | 0.72 (<0.001) |
| IL-6 | 0.95 (<0.001) | 0.91 (<0.00) | 0.96 (<0.001) | 0.97 (<0.001) | 0.96 (<0.001) | 0.98 (<0.001) | 0.97 (<0.001) |
| IL-8 | 0.68 (<0.001) | 0.75 (<0.00) | 0.83 (<0.001) | 0.91 (<0.001) | 0.94 (<0.001) | 0.77 (<0.001) | 0.86 (<0.001) |
| IL-10 | 0.82 (<0.001) | 0.84 (<0.00) | 0.66 (0.003) | 0.84 (0.010) | 0.71 (<0.010) | 0.53 (0.016) | 0.65 (0.002) |
| IL-12 | 0.70 (<0.001) | 0.90 (<0.00) | 0.86 (<0.001) | 0.84 (<0.001) | 0.83 (<0.001) | 0.34 (ns) | 0.84 (<0.001) |
| IL-18 | 0.95 (<0.001) | 0.93 (<0.00) | 0.82 (<0.001) | 0.78 (<0.001) | 0.80 (<0.001) | 0.63 (0.003) | 0.86 (<0.001) |
| TNFα | 0.92 (<0.001) | 0.93 (<0.00) | 0.83 (<0.001) | 0.71 (0.009) | 0.72 (<0.001) | 0.40 (ns) | 0.60 (0.005) |
| Cytokine (pg/mL) | Median (25th, 75th Percentiles) | Range |
|---|---|---|
| GM-CSF | 44.34 (40.17, 54.42) | 34.66 |
| IFNγ | 2457.00 (2227.00, 2699.00) | 1223.00 |
| IL-1α | 4.83 (4.16, 7.08) | 12.00 |
| IL-1β | 16.17 (14.83, 19.58) | 19.67 |
| IL-1ra | 147.80 (57.42, 288,3) | 449.00 |
| IL-2 | 10.33 (7.58, 11.17) | 7.00 |
| IL-4 | 32.67 (29.34, 34.75) | 15.67 |
| IL-6 | 82.17 (47.58, 225.20) | 290.00 |
| IL-8 | 34.00 (29.50, 53.50) | 63.33 |
| IL-10 | 13.67 (12.00, 14.42) | 7.67 |
| IL-12 | 6.50 (4.91, 7.00) | 4.34 |
| IL-18 | 15.34 (9.25, 27.00) | 32.67 |
| TNFα | 23.67 (20.33, 25.00) | 11.33 |
| Cytokine (pg/mL) | Median (25th, 75th Percentiles) | Range |
|---|---|---|
| GM-CSF | 39.67 (36.67, 47.33) | 42.33 |
| IFNγ | 2004.00 (1881.00, 2219.00) | 1097 |
| IL-1α | 4.00 (4.00, 5.75) | 26.67 |
| IL-1β | 17.33 (16.00, 22.00) | 38.33 |
| IL-1ra | 138.00 (51.25, 225.20) | 757.00 |
| IL-2 | 7.67 (6.00, 8.00) | 13.00 |
| IL-4 | 29.33 (27.00, 31.67) | 14.00 |
| IL-6 | 77.17 (40.33, 209.10) | 278.70 |
| IL-8 | 37.17 (25.83, 56.67) | 342.00 |
| IL-10 | 11.67 (10.50, 12.58) | 9.67 |
| IL-12 | 5.00 (4.08, 5.67) | 14.33 |
| IL-18 | 15.33 (10.08, 27.00) | 62.67 |
| TNFα | 20.50 (18.67, 21.59) | 32.34 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padilla, L.; Barranco, I.; Parrilla, I.; Lucas, X.; Rodriguez-Martinez, H.; Roca, J. Measurable Cytokine Concentrations in Pig Seminal Plasma Are Modified by Semen Handling and Storage. Biology 2020, 9, 276. https://doi.org/10.3390/biology9090276
Padilla L, Barranco I, Parrilla I, Lucas X, Rodriguez-Martinez H, Roca J. Measurable Cytokine Concentrations in Pig Seminal Plasma Are Modified by Semen Handling and Storage. Biology. 2020; 9(9):276. https://doi.org/10.3390/biology9090276
Chicago/Turabian StylePadilla, Lorena, Isabel Barranco, Inmaculada Parrilla, Xiomara Lucas, Heriberto Rodriguez-Martinez, and Jordi Roca. 2020. "Measurable Cytokine Concentrations in Pig Seminal Plasma Are Modified by Semen Handling and Storage" Biology 9, no. 9: 276. https://doi.org/10.3390/biology9090276