New Synthesis, Structure and Analgesic Properties of Methyl 1-R-4-Methyl-2,2-Dioxo-1H-2λ6,1-Benzothiazine-3-Carboxylates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemistry
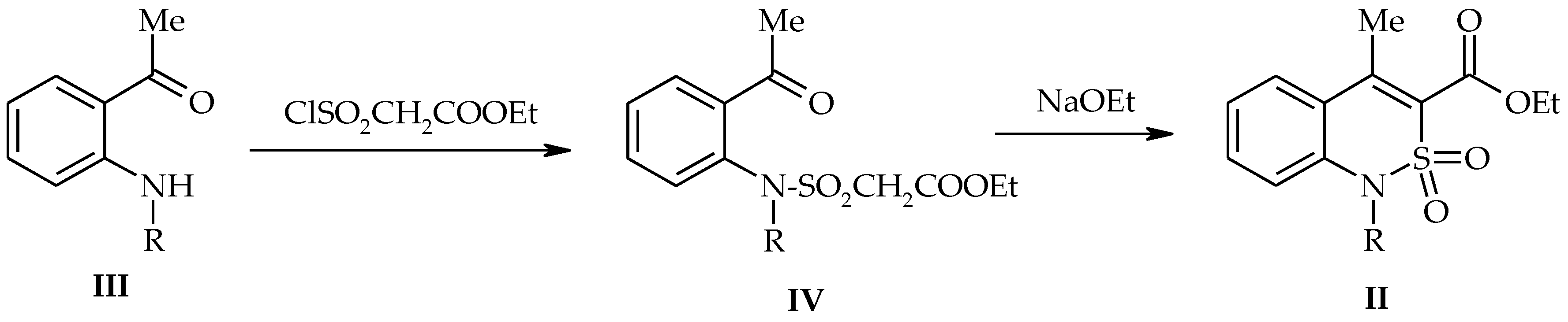
2.2. Methyl 4-Methyl-2,2-Dioxo-1H-2λ6,1-Benzothiazine-3-Carboxylate (3)
2.3. General Procedure for the Synthesis of 1-Alkyl-Substituted Methyl 4-Methyl-2,2-Dioxo-1H-2λ6,1-Benzothiazine-3-Carboxylates (5a–g)
2.4. X-ray Structural Analysis of Methyl 1-Isopropyl-4-Methyl-2,2-Dioxo-1H-2λ6,1-Benzothiazine-3-Carboxylate (5e)
2.5. X-ray Structural Analysis of Methyl 1-Benzyl-4-Methyl-2,2-Dioxo-1H-2λ6,1-Benzothiazine-3-Carboxylate Methanol Monosolvate (6)
2.6. Pharmacology
Analgesic Test
3. Results and Discussion
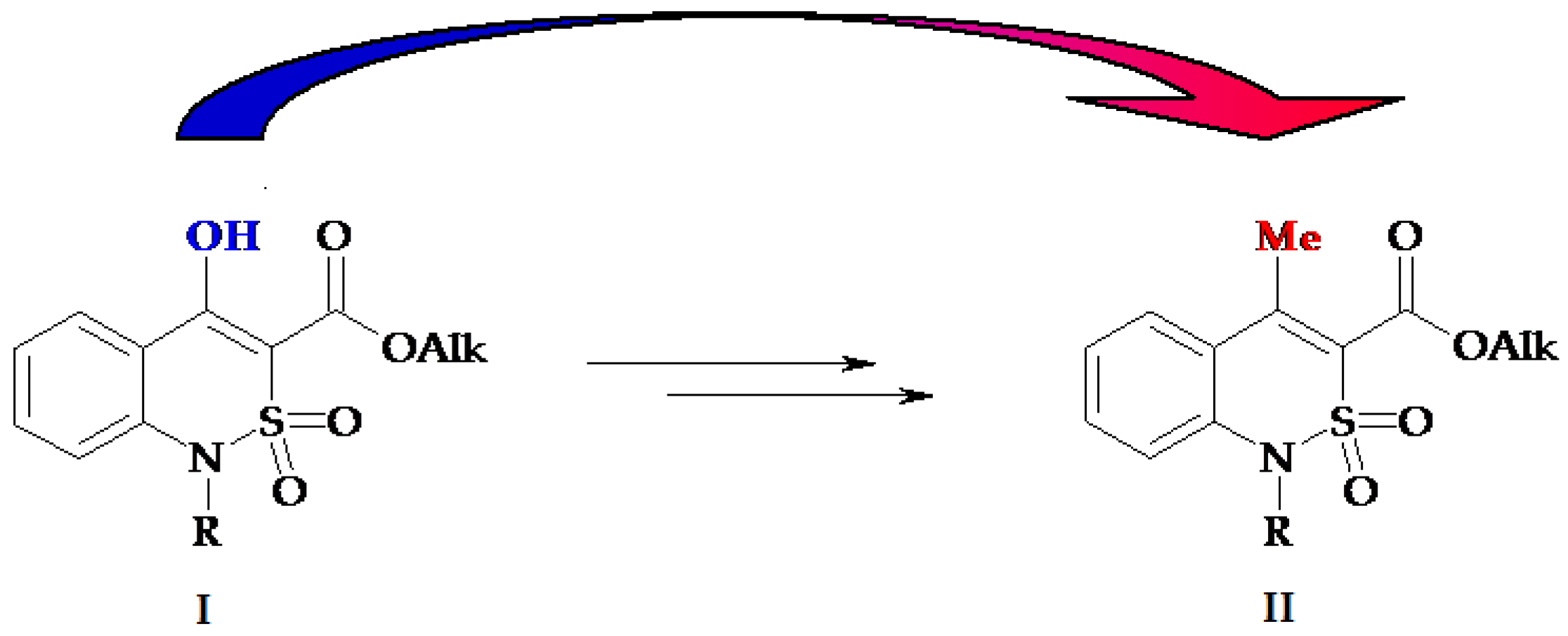
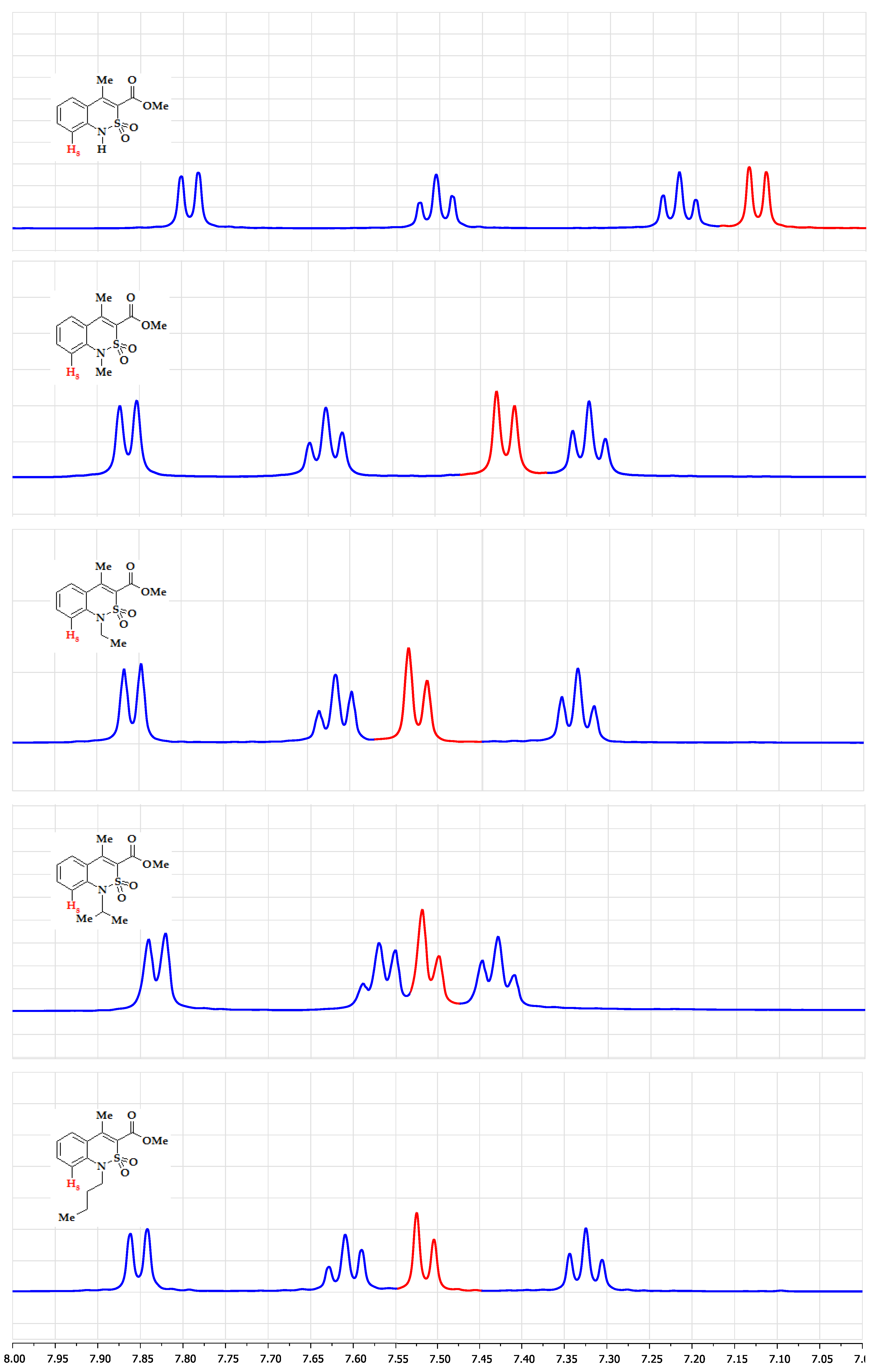
3.1. Chemistry
3.2. Evaluation of the Analgesic Activity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zefirova, O.N.; Zefirov, N.S. On the history emergence and development of the concept bioisosterism. Rep. Mosc. State Univ. Ser. 2 Chem. 2002, 43, 251–256. [Google Scholar]
- Meanwell, N.A. Synopsis of some recent tactical application of bioisosteres in drug design. J. Med. Chem. 2011, 54, 2529–2591. [Google Scholar] [CrossRef] [PubMed]
- Patina, G.A.; LaVoie, E.J. Bioisosterism: A rational approach in drug design. Chem. Rev. 1996, 96, 3147–3176. [Google Scholar] [CrossRef]
- Lima, L.M.; Barreiro, E.J. Bioisosterism: A useful strategy for molecular modification and drug design. Curr. Med. Chem. 2005, 12, 23–49. [Google Scholar] [CrossRef] [PubMed]
- Ukrainets, I.V.; Mospanova, E.V.; Savchenkova, L.V.; Yankovich, S.I. 4-Hydroxy-2-quinolones. 195*. Synthesis of novel, potential analgesics based on 4-(hetarylmethyl)amino-2-oxo-1,2-dihydroquinoline-3-carboxylic acids. Chem. Heterocycl. Compd. 2011, 47, 67–73. [Google Scholar] [CrossRef]
- Tuyishime, M.; Danish, M.; Princiotto, A.; Mankowski, M.K.; Lawrence, R.; Lombart, H.G.; Esikov, K.; Berniac, J.; Liang, K.; Ji, J.; et al. Discovery and optimization of novel small-molecule HIV-1 entry inhibitors using field-based virtual screening and bioisosteric replacement. Bioorg. Med. Chem. Lett. 2014, 24, 5439–5445. [Google Scholar] [CrossRef] [PubMed]
- Rombouts, F.J.; Tovar, F.; Austin, N.; Tresadern, G.; Trabanco, A.A. Benzazaborinines as novel bioisosteric replacements of naphthalene: Propranolol as an example. J. Med. Chem. 2015, 58, 9287–9295. [Google Scholar] [CrossRef] [PubMed]
- Ukrainets, I.V.; Mospanova, E.V.; Davidenko, A.A. Using bioisosteric replacements to enhance the analgesic properties of 4-hydroxy-6,7-dimethoxy-2-oxo-1,2-dihydroquinoline-3-carboxamides. Pharm. Chem. J. 2016, 50, 365–368. [Google Scholar] [CrossRef]
- Silva, F.T.; Franco, C.H.; Favaro, D.C.; Freitas-Junior, L.H.; Moraes, C.B.; Ferreira, E.I. Design, synthesis and antitrypanosomal activity of some nitrofurazone 1,2,4-triazolic bioisosteric analogues. Eur. J. Med. Chem. 2016, 121, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Ukrainets, I.V.; Petrushova, L.A.; Dzyubenko, S.P. 2,1-Benzothiazine 2,2-dioxides. 1. Synthesis, structure, and analgesic activity of 1-R-4-hydroxy-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylic acid esters. Chem. Heterocycl. Compd. 2013, 49, 1378–1383. [Google Scholar] [CrossRef]
- Ukrainets, I.V.; Petrushova, L.A.; Bereznyakova, N.L. Effect of bromination on the pharmacological properties of methyl 1-allyl-4-hydroxy-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylate. Pharm. Chem. J. 2015, 49, 519–522. [Google Scholar] [CrossRef]
- Ukrainets, I.V.; Petrushova, L.A.; Dzyubenko, S.P.; Sim, G.; Grinevich, L.A. The effective synthesis of N-(arylalkyl)-1-R-4-hydroxy-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamides as promising analgesics of a new chemical class. Sci. Pharm. 2015, 83, 549–566. [Google Scholar] [CrossRef] [PubMed]
- Ukrainets, I.V.; Shishkina, S.V.; Baumer, V.N.; Gorokhova, O.V.; Petrushova, L.A.; Sim, G. The structure of two pseudo-enantiomeric forms of N-benzyl-4-hydroxy-1-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamide and their analgesic properties. Acta Crystallogr. Sect. C Struct. Chem. 2016, 72, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Ukrainets, I.V.; Petrushova, L.A.; Shishkina, S.V.; Grinevich, L.A.; Sim, G. Synthesis, spatial structure and analgesic activity of sodium 3-benzylaminocarbonyl-1-methyl-2,2-dioxo-1H-2λ6,1-benzothiazin-4-olate solvates. Sci. Pharm. 2016, 84, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Petrushova, L.A.; Ukrainets, I.V.; Dzyubenko, S.P.; Grinevich, L.A. Synthesis and the biological activity of 4-hydroxy-2,2-dioxo-1H-2λ6,1-benzothiazin-3-carboxylic acids trifluoromethyl-substituted anilides. J. Org. Pharm. Chem. 2015, 13, 44–48. [Google Scholar]
- Ukrainets, I.V.; Petrushova, L.A.; Sidorenko, L.V.; Davidenko, A.A.; Duchenko, M.A. The study of structure—Analgesic activity relationships in a series of 4-hydroxy-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylic acid toluidides and xylidides. Sci. Pharm. 2016, 84, 497–506. [Google Scholar] [CrossRef]
- Ukrainets, I.V.; Petrushova, L.A.; Shishkina, S.V.; Sidorenko, L.V.; Sim, G.; Kryvanych, O.V. The synthesis, structure and analgesic properties of halogen substituted 4-hydroxy-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxanilides. Sci. Pharm. 2016, 84, 523–535. [Google Scholar] [CrossRef]
- Ukrainets, I.V.; Petrushova, L.A.; Dzyubenko, S.P.; Sim, G. 2,1-Benzothiazine 2,2-Dioxides. 3*. 4-Hydroxy-1-Methyl-2,2-Dioxo-N-(1,3-Thiazol-2-yl)-1Н-2λ6,1-Benzothiazine-3-Carboxamides — Aa New Group of Potential Analgetics. Chem. Heterocycl. Compd. 2014, 50, 103–110. [Google Scholar] [CrossRef]
- Ukrainets, I.V.; Petrushova, L.A.; Dzyubenko, S.P.; Liu, Y. 2,1-Benzothiazine 2,2-Dioxides. 4*. Synthesis, Structure, and Analgesic Properties of 4-Hydroxy-1-Methyl-2,2-Dioxo-N-(Pyridin-2-yl)-1H-2λ6,1-Benzothiazine-3-Carboxamides. Chem. Heterocycl. Compd. 2014, 50, 564–572. [Google Scholar] [CrossRef]
- Ukrainets, I.V.; Sidorenko, L.V.; Gorokhova, O.V.; Shishkina, S.V. 4-Hydroxy-2-quinolones. 96. Synthesis and properties of 4-methyl-2-oxo-1,2-dihydroquinoline-3-carboxylic acid. Chem. Heterocycl. Compd. 2006, 42, 776–781. [Google Scholar] [CrossRef]
- Ukrainets, I.V.; Gorokhova, O.V.; Sidorenko, L.V.; Bereznyakova, N.L. 4-Hydroxy-2-quinolones. 111. Simple synthesis of 1-substituted 4-methyl-2-oxo-1,2-dihydroquinoline-3-carboxylic acids. Chem. Heterocycl. Compd. 2007, 43, 58–62. [Google Scholar] [CrossRef]
- Lombardino, J.G.; Kuhla, D.E. 1,2- and 2,1-Benzothiazines and Related Compounds. In Advances in Heterocyclic Chemistry; Katritzky, A.R., Boulton, A.J., Eds.; Academic Press, Inc.: New York, NY, USA, 1981; Volume 28, pp. 73–126. [Google Scholar]
- Hong, X.; Harmata, M. Recent Progress in the Chemistry of 2,1-Benzothiazines. In Progress in Heterocyclic Chemistry; Gribble, G.W., Joule, J.A., Eds.; Elsevier Science Ltd.: Oxford, UK, 2008; Volume 19, pp. 1–43. [Google Scholar]
- Bochkov, A.F.; Smit, V.A. Organic Synthesis. The Aims, Methods, Tactics, Strategy; Nauka: Moscow, Russia, 1987; pp. 242–252. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Cambridge Crystallographic Data Center. Available online: www.ccdc.ac.uk/data_request/cifCCDC1518396 (accessed on 21 November 2016).
- Cambridge Crystallographic Data Center. Available online: www.ccdc.ac.uk/data_request/cifCCDC1518395 (accessed on 21 November 2016).
- Vogel, H.G. Drug Discovery and Evaluation: Pharmacological Assays, 2nd ed.; Springer: Berlin, Germany, 2008; pp. 1014–1016. [Google Scholar]
- Ukrainets, I.V.; Petrushova, L.A.; Shishkina, S.V.; Sim, G. 2,1-Benzothiazine 2,2-Dioxides. 9*. Alkylation of Methyl 4-Hydroxy-1-Methyl-2,2-Dioxo-1Н-2λ6,1-Benzothiazine-3-Carboxylate with Ethyl Iodide. Chem. Heterocycl. Compd. 2014, 50, 1741–1747. [Google Scholar] [CrossRef]
- Günther, H. NMR Spectroscopy: Basic Principles, Concepts and Applications in Chemistry, 3rd ed.; Wiley-VCH: Weinheim, Germany, 2013; pp. 114–116. [Google Scholar]
- Azotla-Cruz, L.; Shishkina, S.; Ukrainets, I.; Lijanova, I.; Likhanova, N. Crystal structure of methyl 1-allyl-4-methyl-1H-benzo[c][1,2]thiazine-3-carboxylate 2,2-dioxide. Acta. Crystallogr. Sect. E Crystallogr. Commun. 2016, E72, 1574–1576. [Google Scholar] [CrossRef] [PubMed]
- Ukrainets, I.V.; Petrushova, L.A.; Bereznyakova, N.L.; Liu, Y.Y. 2,1-Benzothiazine 2,2-Dioxides. 8*. Synthesis and Structure of 2′-Amino-2-Oxo-1,2-Dihydro-6′H-Spiro-[Indole-3,4′-Pyrano[3,2-c][2,1]Benzothiazine]-3′-Carbonitrile 5′,5′-Dioxides. Chem. Heterocycl. Compd. 2014, 50, 1346–1353. [Google Scholar] [CrossRef]
- Zefirov, N.S.; Palyulin, V.A.; Dashevskaya, E.E. Stereochemical studies. XXXIV. Quantitative description of ring puckering via torsional angles. The case of six-membered rings. J. Phys. Org. Chem. 1990, 3, 147–158. [Google Scholar] [CrossRef]
- Zefirov, Y.V. Reduced intermolecular contacts and specific interactions in molecular crystals. Crystallogr. Rep. 1997, 42, 865–886. [Google Scholar]
- Orpen, A.G.; Brammer, L.; Allen, F.H.; Kennard, O.; Watson, D.G.; Taylor, R. Typical Interatomic Distances in Organic Compounds and Organometallic Compounds and Coordination Complexes of the d- and f-Block Metals. In Structure Correlation; Burgi, H.-B., Dunitz, J.D., Eds.; Wiley-VCH: Weinheim, Germany, 1994; Volume 2, pp. 741–926. [Google Scholar]
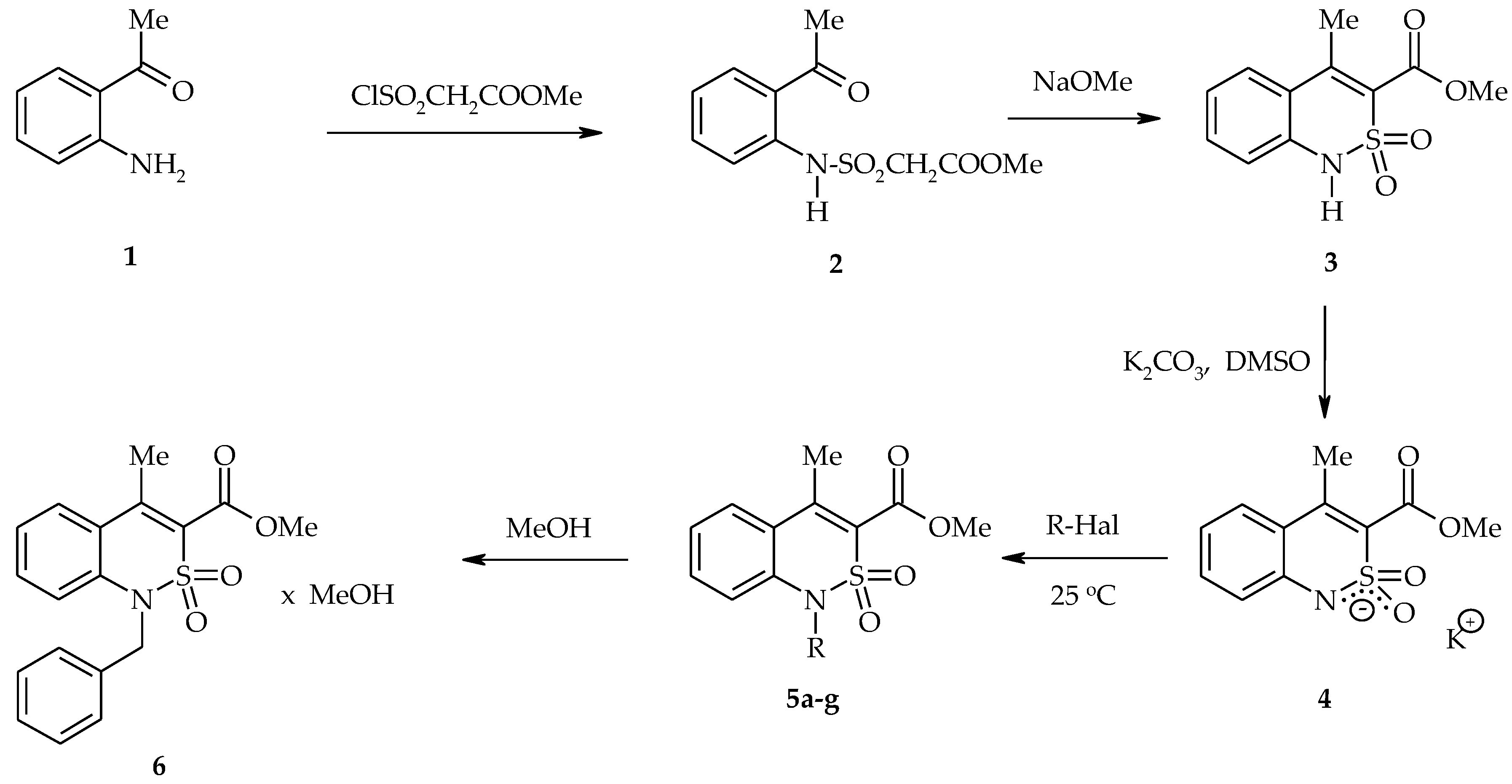
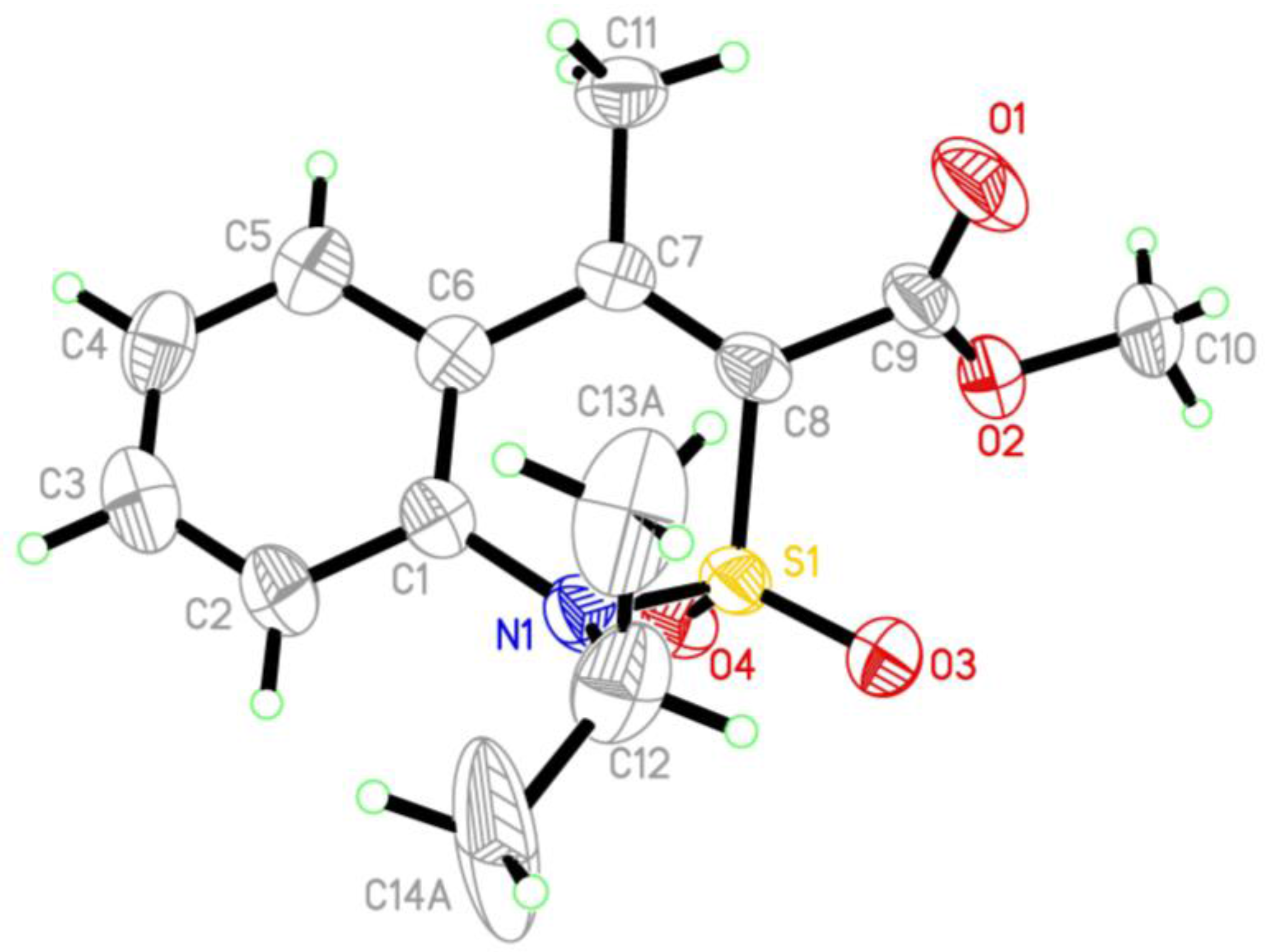
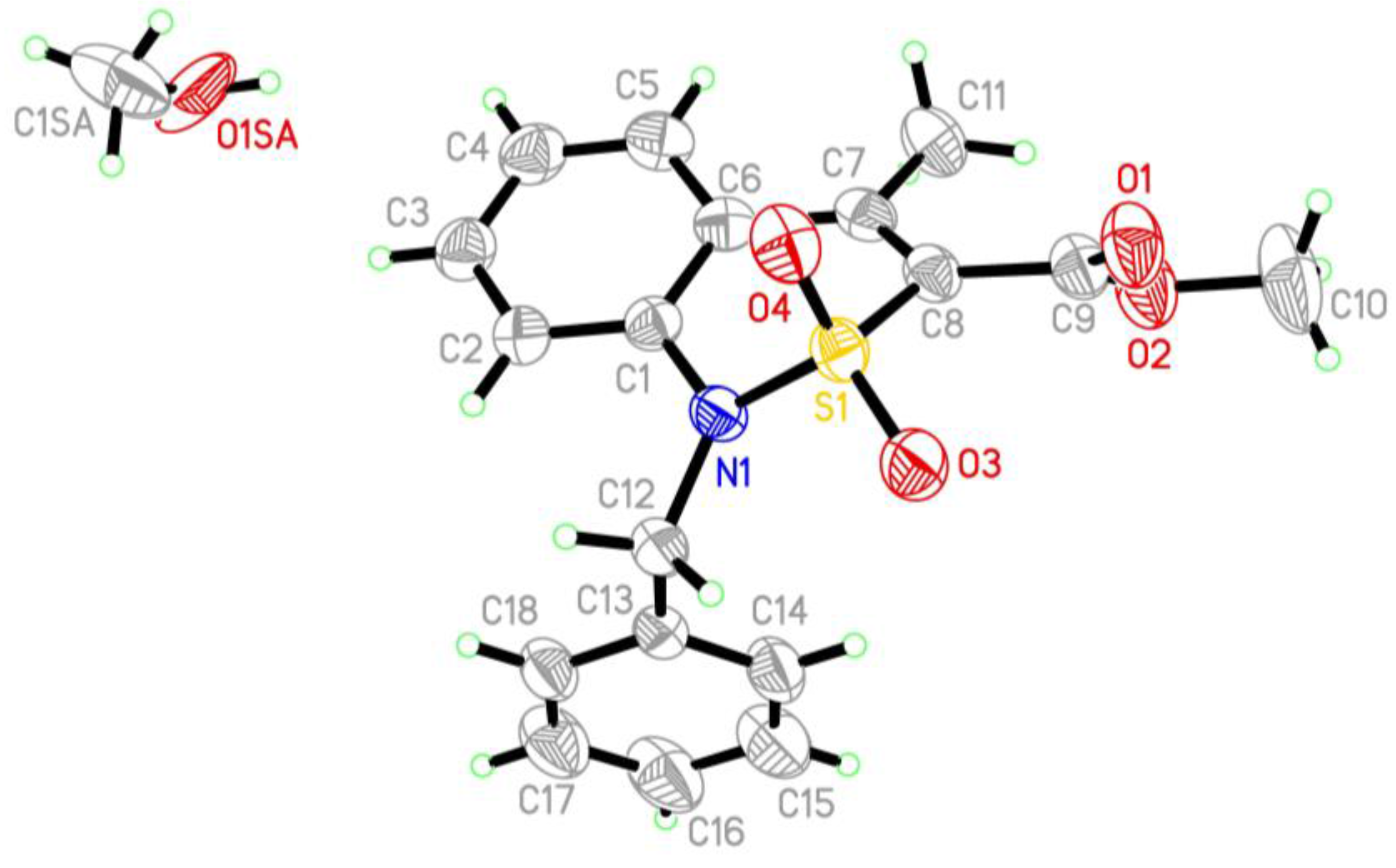
| Entry | Product | Alkyl Halide | Duration of the Reaction (h) |
|---|---|---|---|
| 1 | 5a | CH3–I | 1.3 |
| 2 | 5b | C2H5–I | 2.0 |
| 3 | 5c | H2C=CH–CH2–Br | 0.5 |
| 4 | 5d | C3H7–I | 2.4 |
| 5 | 5e | (CH3)2CH–I | 5.0 |
| 6 | 5f | C4H9–I | 2.5 |
| 7 | 5g | C6H5–CH2–Cl 1 | 1.5 |
| Entry | Product | R | Latent Period in 2 h after Introduction of the Compounds (s) 1 | Change of the Latent Period, Compared to Control (%) 2 |
|---|---|---|---|---|
| 1 | 3 | H | 8.47 ± 0.38 | +17.6 |
| 2 | 5a | –CH3 | 12.51 ± 1.04 | +73.7 (+10.9) |
| 3 | 5b | –C2H5 | 10.29 ± 0.61 | +42.9 (+23.6) |
| 4 | 5c | –CH2–CH=CH2 | 9.56 ± 0.44 | +32.8 (+71.1) |
| 5 | 5d | –C3H7 | 8.22 ± 0.35 | +14.2 |
| 6 | 5e | –CH(CH3)2 | 9.21 ± 0.43 | +27.9 |
| 7 | 5f | –C4H9 | 8.60 ± 0.39 | +19.5 |
| 8 | 5g | –CH2–C6H5 | 9.85 ± 0.46 | +36.8 |
| 9 | Meloxicam | – | 11.59 ± 1.02 | +61.1 |
| 10 | Piroxicam | – | 9.24 ± 0.45 | +28.4 |
| 11 | Control | – | 7.20 ± 0.31 | – |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azotla-Cruz, L.; Lijanova, I.V.; Ukrainets, I.V.; Likhanova, N.V.; Olivares-Xometl, O.; Bereznyakova, N.L. New Synthesis, Structure and Analgesic Properties of Methyl 1-R-4-Methyl-2,2-Dioxo-1H-2λ6,1-Benzothiazine-3-Carboxylates. Sci. Pharm. 2017, 85, 2. https://doi.org/10.3390/scipharm85010002
Azotla-Cruz L, Lijanova IV, Ukrainets IV, Likhanova NV, Olivares-Xometl O, Bereznyakova NL. New Synthesis, Structure and Analgesic Properties of Methyl 1-R-4-Methyl-2,2-Dioxo-1H-2λ6,1-Benzothiazine-3-Carboxylates. Scientia Pharmaceutica. 2017; 85(1):2. https://doi.org/10.3390/scipharm85010002
Chicago/Turabian StyleAzotla-Cruz, Liliana, Irina V. Lijanova, Igor V. Ukrainets, Natalya V. Likhanova, Octavio Olivares-Xometl, and Natalya L. Bereznyakova. 2017. "New Synthesis, Structure and Analgesic Properties of Methyl 1-R-4-Methyl-2,2-Dioxo-1H-2λ6,1-Benzothiazine-3-Carboxylates" Scientia Pharmaceutica 85, no. 1: 2. https://doi.org/10.3390/scipharm85010002







