Hydrogen Sulfide Releasing 2-Mercaptoacrylic Acid-Based Derivative Possesses Cytoprotective Activity in a Small Intestine of Rats with Medication-Induced Enteropathy
Abstract
:1. Introduction
2. Methods
2.1. Animals
2.2. Models of Medication-Induced Enteropathies
2.3. Test Drugs
2.4. Study Protocol
2.5. Biochemical Assessment
2.6. Statistics
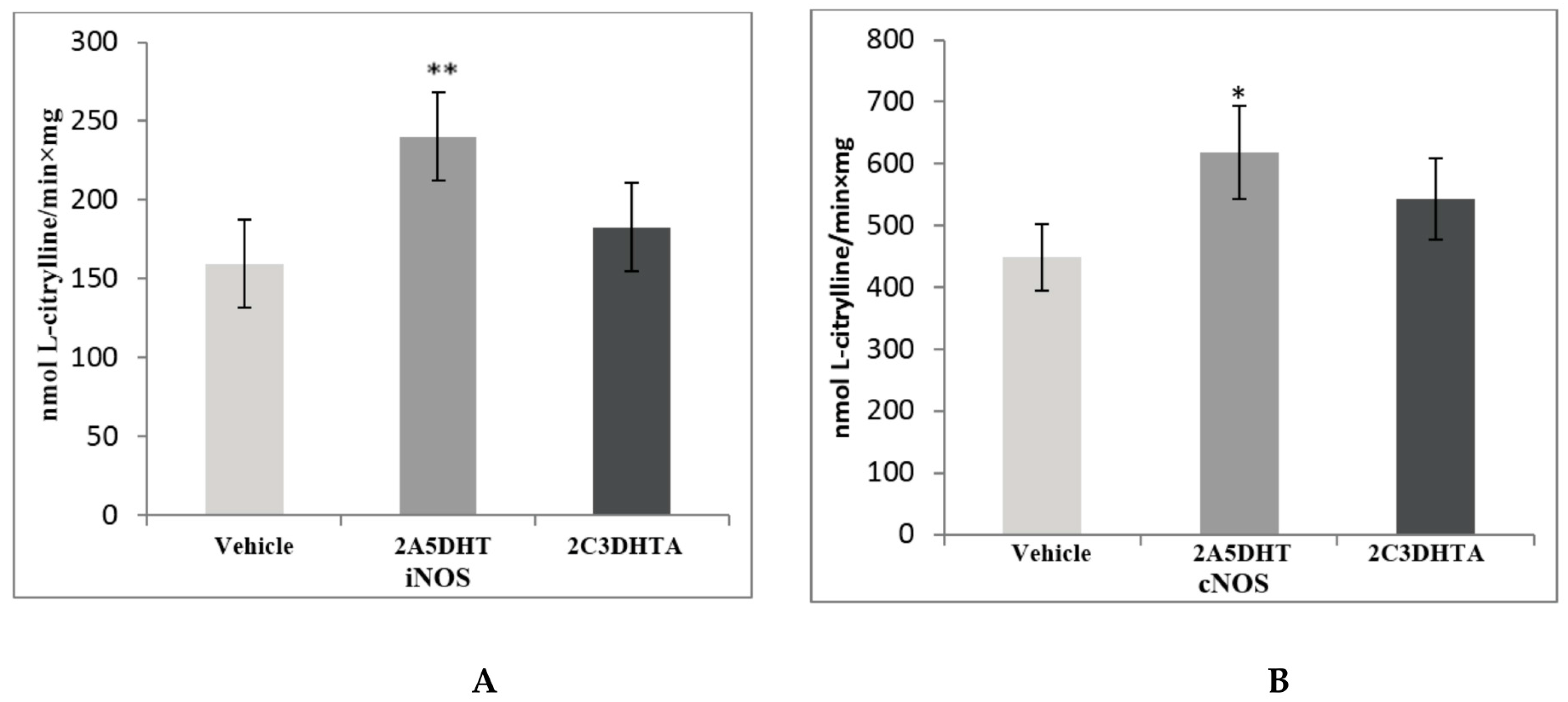
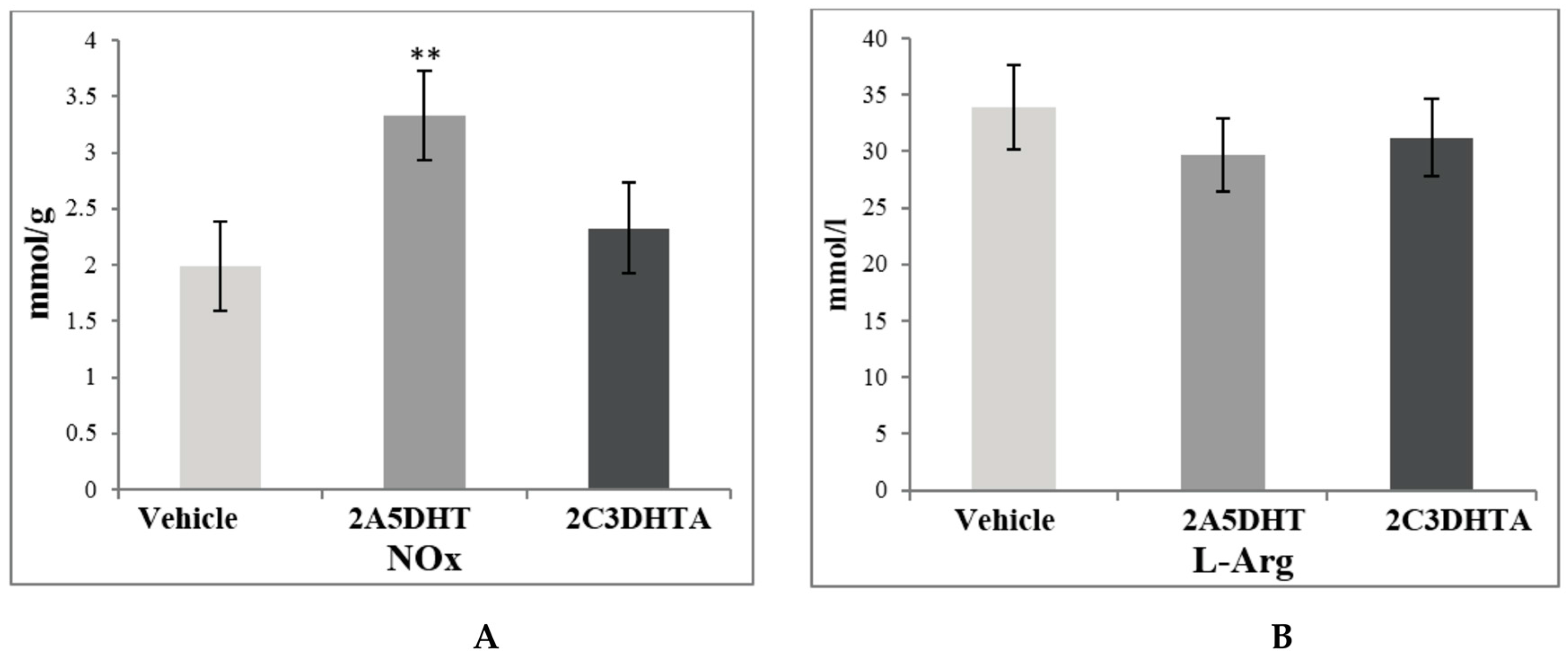
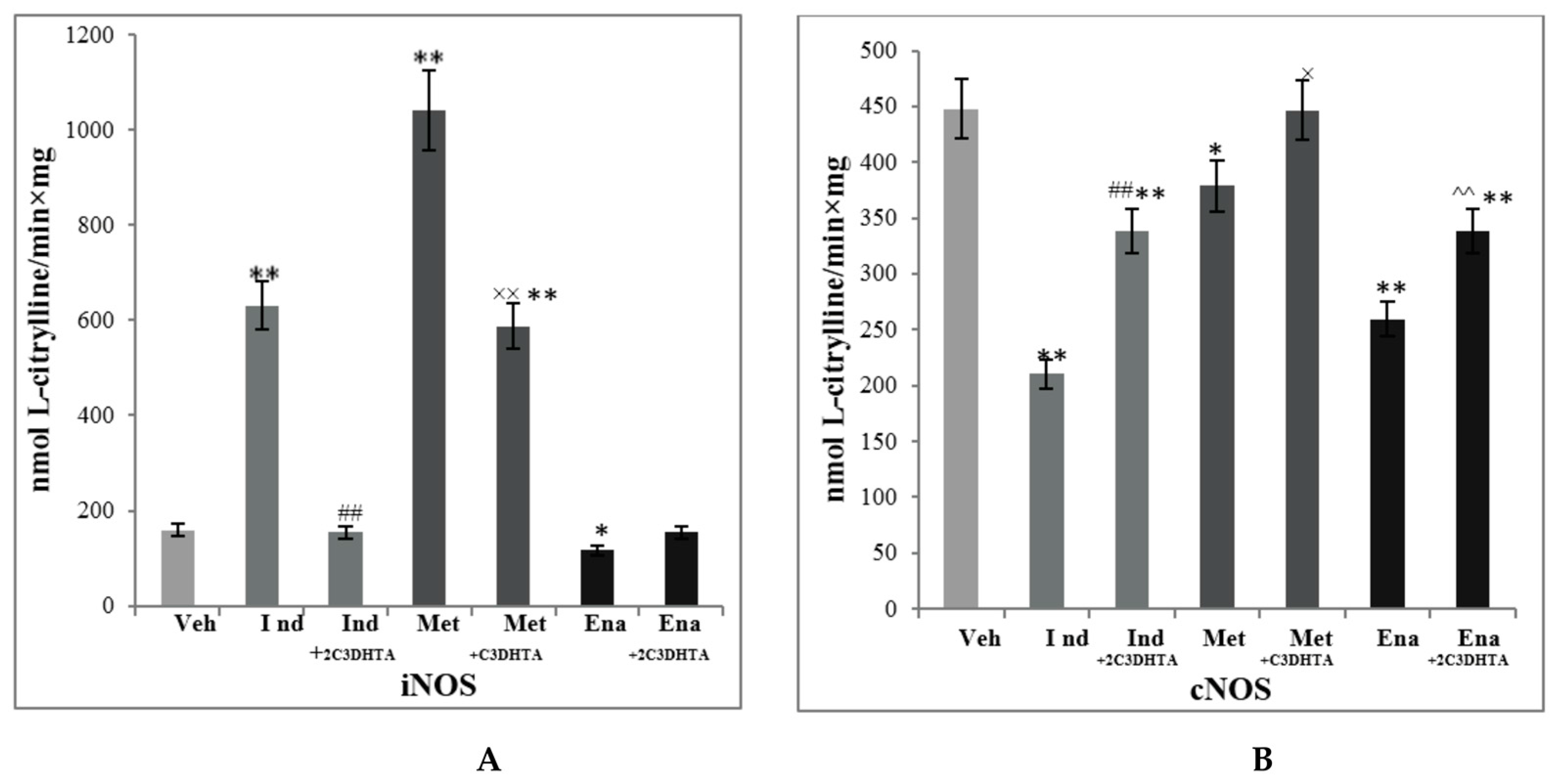
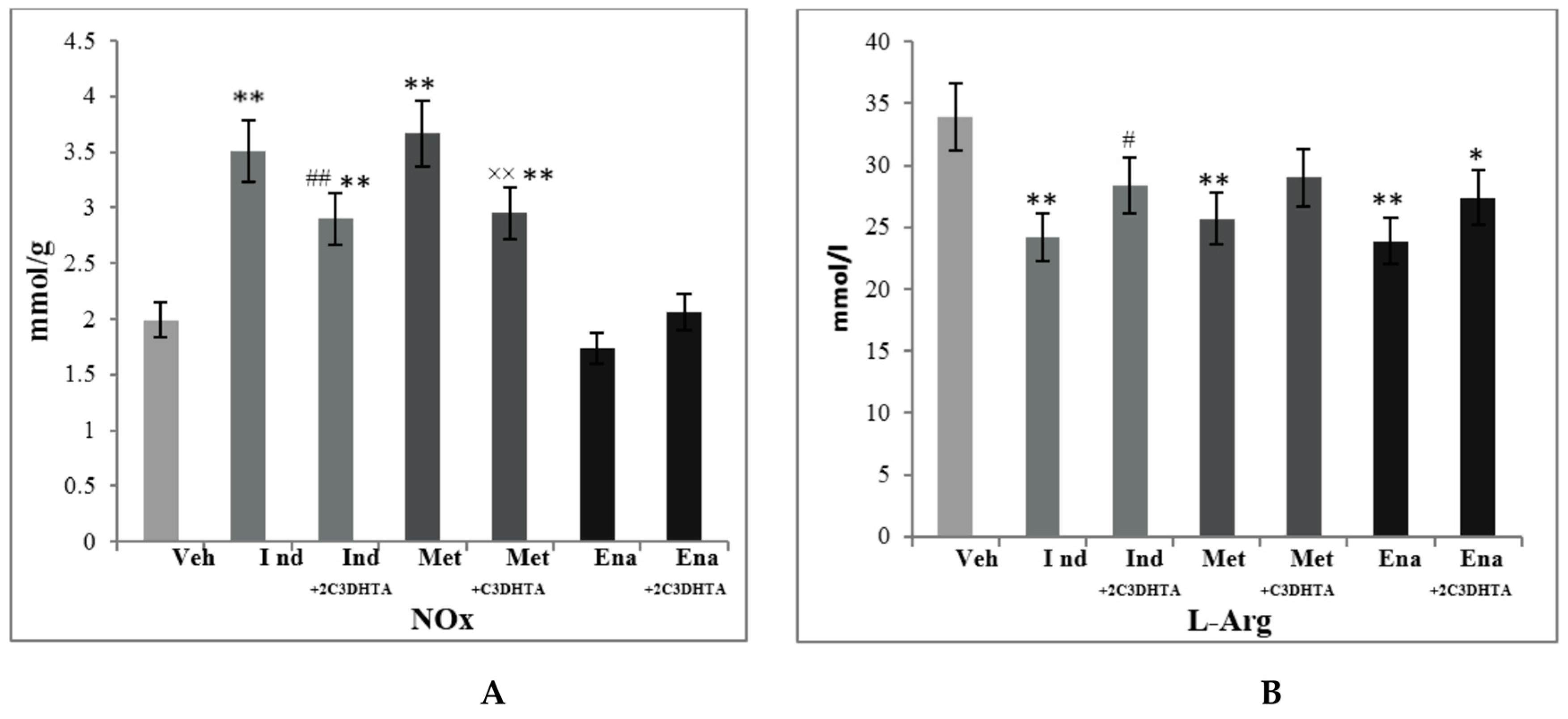
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- McGettigan, M.J.; Menias, C.O.; Gao, Z.J.; Mellnick, V.M.; Hara, A.K. Imaging of drug-induced complications in the gastrointestinal system. Radiographics 2016, 36, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Pusztaszeri, M.P.; Genta, R.M.; Cryer, B.L. Drug-induced injury in the gastrointestinal tract: Clinical and pathologic considerations. Nat. Clin. Pract. Gastroenterol. Hepatol. 2007, 4, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Blackler, R.W.; Gemici, B.; Manko, A.; Wallace, J.L. NSAID-gastroenteropathy: New aspects of pathogenesis and prevention. Curr. Opin. Pharmacol. 2014, 19, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Utzeri, E.; Usai, P. Role of non-steroidal anti-inflammatory drugs on intestinal permeability and nonalcoholic fatty liver disease. World J. Gastroenterol. 2017, 23, 3954–3963. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.L. Mechanisms, prevention and clinical implications of nonsteroidal anti-inflammatory drug-enteropathy. World J. Gastroenterol. 2013, 19, 1861–1876. [Google Scholar] [CrossRef] [PubMed]
- Freeman, H.J. Drug-induced Sprue-like Intestinal Disease. Int. J. Celiac Dis. 2014, 2, 49–53. [Google Scholar] [CrossRef]
- Jahovic, N.; Sener, G.; Cevik, H.; Ersoy, Y.; Arbak, S.; Yeğen, B.C. Amelioration of methotrexate-induced enteritis by melatonin in rats. Cell Biochem. Funct. 2004, 22, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Farkas, H. Current pharmacotherapy of bradykinin-mediated angioedema. Exp. Opin. Pharmacother. 2013, 14, 571–586. [Google Scholar] [CrossRef] [PubMed]
- Havrylyuk, D.; Zimenkovsky, B.; Vasylenko, O.; Zaprutko, L.; Gzella, A.; Lesyk, R. Synthesis of novel thiazolone-based compounds containing pyrazoline moiety and evaluation of their anticancer activity. Eur. J. Med. Chem. 2009, 44, 1396–1404. [Google Scholar] [CrossRef] [PubMed]
- Lozynskyi, A.V.; Kaminskyy, D.V.; Romanchyshyn, K.B.; Semenciv, N.G.; Ogurtsov, V.V.; Nektegayev, I.O.; Lesyk, R.B. Screening of antioxidant and anti-inflammatory activities among thiopyrano[2,3-d]thiazoles. Biopolym. Cell 2015, 31, 131–137. [Google Scholar] [CrossRef]
- Vigorita, M.G.; Ottana, R.; Monforte, F.; Maccari, R.; Trovato, A.; Monforte, M.T.; Taviano, M.F. Synthesis and antiinflammatory, analgesic activity of 3,3′-(1,2-Ethanediyl)-bis[2-aryl-4-thiazolidinone] chiral compounds. Part 10. Bioorg. Med. Chem. Lett. 2001, 11, 2791–2794. [Google Scholar] [CrossRef]
- Charlier, C.; Michaux, C. Dual inhibition of cyclooxygenase-2 (COX-2) and 5-lipoxygenase (5-LOX) as a new strategy to provide safer non-steroidal anti-inflammatory drugs. Eur. J. Med. Chem. 2003, 38, 645–659. [Google Scholar] [CrossRef]
- Bonde, C.G.; Gaikwad, N.J. Synthesis and preliminary evaluation of some pyrazine containing thiazolines and thiazolidinones as antimicrobial agents. Bioorg. Med. Chem. 2004, 12, 2151–2161. [Google Scholar] [CrossRef] [PubMed]
- Leone, S.; Ottani, A.; Bertolini, A. Dual acting anti-inflammatory drugs. Curr. Top. Med. Chem. 2007, 7, 65–75. [Google Scholar] [CrossRef]
- Sklyarov, A.Y.; Lesyk, R.B.; Panasyuk, N.B.; Fomenko, I.S.; Havryluk, D.Y. Comparison of dual acting drugs and conventional NSAIDs towards parameters of NO-synthase system and oxidative stress in mucosal membrane of large intestine of rats with experimental ulcerative colitis. Biopolym. Cell 2011, 27, 147–153. [Google Scholar] [CrossRef]
- Ilkiv, I.I.; Lesyk, R.B.; Sklyarov, O.Y. The influence of novel 4-thiazolidinone derivaties in cytoprotective mechanisms of small intestine under NSAID-induced damage. Ukr. Biochem. J. 2016, 88, 99–104. [Google Scholar] [CrossRef]
- Ilkiv, I.; Lesyk, R.; Sklyarov, O. Evaluation of novel 4-thiazolidinone-based derivatives as possible cytoprotective agents against stress model in rats. J. Appl. Pharm. Sci. 2017, 7, 199–203. [Google Scholar] [CrossRef]
- Singh, S.B.; Lin, H.C. Hydrogen Sulfide in Physiology and Diseases of the Digestive Tract. Microorganisms 2015, 3, 866–889. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.L.; Ianaro, A.; de Nucci, G. Gaseous mediators in gastrointestinal mucosal defense and injury. Dig. Dis. Sci. 2017, 62, 2223–2230. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef]
- Cho, H.; Yun, C.W.; Park, W.K.; Kong, J.Y.; Kim, K.S.; Park, Y.; Lee, S.; Kim, B.K. Modulation of the activity of pro-inflammatory enzymes, COX-2 and iNOS, by chrysin derivatives. Pharmacol. Res. 2004, 49, 37–43. [Google Scholar] [CrossRef]
- Marks, S.L.; Cook, A.K.; Griffey, S.; Kass, P.H.; Rogers, Q.R. Dietary modulation of methotrexate-induced enteritis in cats. Am. J. Vet. Res. 1997, 58, 989–996. [Google Scholar] [PubMed]
- Wang, W.; Xiao, W.; Sun, L.; Zhang, C.; Chen, G.; Yang, H. Inhibition of ACE activity contributes to the intestinal structural compensation in a massive intestinal resection rat model. Pediatr. Surg. Int. 2012, 28, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Ravaeva, M.Y.; Chuyan, E.N. Changes in the synthesis of nitric oxide system activity under the influence of low-intensity millimeter radiation. Uchenye Zapiski Tavricheskogo Natsional’nogo Universiteta im VI Vernadskogo Seriya Biologiya Himiya 2011, 24, 201–210. (In Ukrainian) [Google Scholar]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Alejnikova, T.L.; Rubtsova, G.V.; Pavlova, N.A. Manuals for Practical Lessons in Biochemistry; Medicine: Moscow, Russia, 2000; 128p. (In Russian) [Google Scholar]
- Olkhovskiy, O.S.; Zaichko, N. Influence propargyl glycine and sodium content of hydrogen and H2S-indices of antioxidant system in the myocardium of rats of different ages. Med. Chem. 2013, 15, 10–15. (In Ukrainian) [Google Scholar]
- Bradley, P.P.; Christensen, R.D.; Rothstein, G. Cellular and extracellular myeloperoxidase in pyrogenic inflammation. Blood 1982, 60, 618–622. [Google Scholar] [PubMed]
- Timirbulatov, M.A.; Seleznev, E.I. Method for increasing the intensity of free radical oxidation of lipid-containing components of the blood and its diagnostic significance. Lab. Delo 1981, 4, 209–211. (In Russian) [Google Scholar]
- Chevari, S.; Andial, T.; Shtrenger, I. Determination of antioxidant parameters of blood and their diagnostical significance. Lab. Delo 1991, 10, 9–13. (In Russian) [Google Scholar]
- Koroliuk, M.A.; Ivanova, L.I.; Maĭorova, I.G.; Tokarev, V.E. A method of determining catalase activity. Lab. Delo 1988, 1, 16–19. (In Russian) [Google Scholar]
- Sparatore, A.; Santus, G.; Giustarini, D.; Rossi, R.; Del Soldato, P. Therapeutic potential of new hydrogen sulfide-releasing hybrids. Exp. Rev. Clin. Pharmacol. 2011, 4, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Gemici, B.; Elsheikh, W.; Feitosa, K.B.; Costa, S.K.; Muscara, M.N.; Wallace, J.L. H2S-releasing drugs: Anti-inflammatory, cytoprotective and chemopreventative potential. Nitric Oxide 2015, 46, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Fomenko, I.; Bondarchuk, T.; Emelyanenko, V.; Denysenko, N.; Sklyarov, P.; Ilkiv, I.; Lesyk, R.; Sklyarov, A. Changes of nitric oxide system and lipid peroxidation parameters in the digestive system of rats under conditions of acute stress, and use of nonsteroidal anti-inflammatory drugs. Curr. Issues Pharm. Med. Sci. 2015, 28, 37–41. [Google Scholar] [CrossRef]
- Fomenko, I.; Sklyarov, A.; Bondarchuk, T.; Biletska, L.; Panasyuk, N.; Wallace, J.L. Effects of conventional and hydrogen sulfide-releasing non-steroidal anti-inflammatory drugs in rats with stress-induced and epinephrine-induced gastric damage. Stress 2014, 17, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Fomenko, I.; Sklyarov, A.; Denysenko, N.; Hrycevych, N.; Dranitsyna, A.; Wallace, J. Interactions between nitric oxide and hydrogen sulfide generating systems in gastric mucosa under condition of the combined action of stress and NDAIDs. J. Appl. Pharm. Sci. 2017, 7, 13–19. [Google Scholar] [CrossRef]
- Paul-Clark, M.; Elsheikh, W.; Kirkby, N.; Chan, M.; Devchand, P.; Agbor, T.A.; Flannigan, K.L.; Cheadle, C.; Freydin, M.; Ianaro, A.; et al. Profound chemopreventative effects of a hydrogen sulfide-releasing NSAID in the APCMin/+ mouse model of intestinal tumorigenesis. PLoS ONE 2016, 11, e0147289. [Google Scholar] [CrossRef] [PubMed]
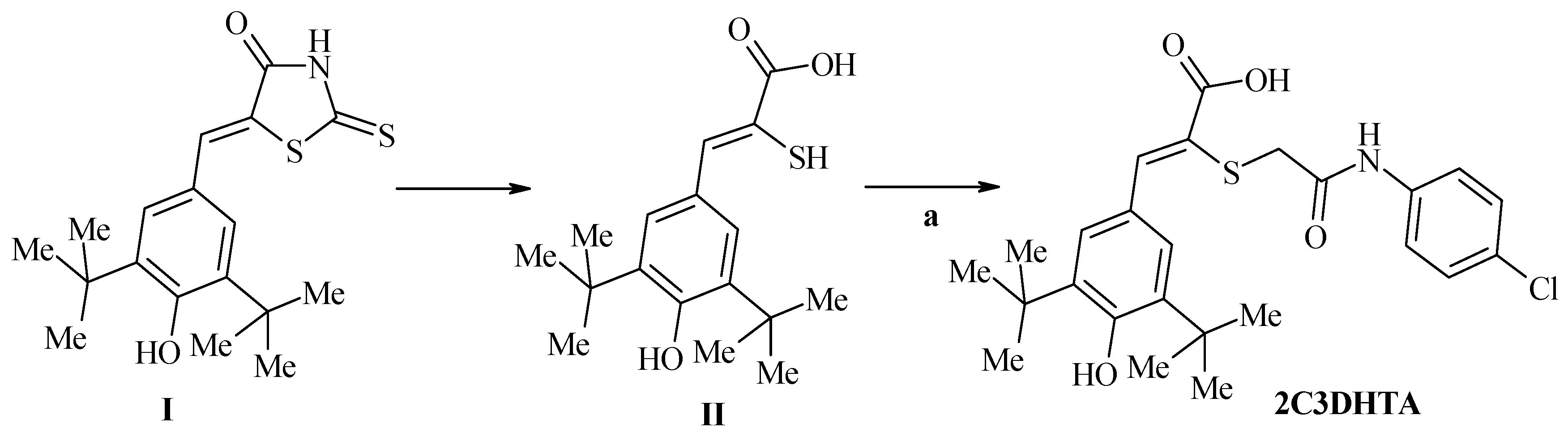
| Stage 1. Evaluation of effects of dual COX/LOX inhibitors in the small intestine of rats under condition of physiological norm (in noninflamed mucosa) | |||||
| Control group | Action of dual COX/LOX inhibitor | Action of H2S releasing COX/LOX inhibitor | |||
| Vehicle | 10 mg/kg/day 2A5DHT intraperitoneally | 10 mg/kg/day 2C3DHTA intraperitoneally | |||
| (n = 8) | (n = 6) | (n = 8) | |||
| Group 1 | Group 2 | Group 3 | |||
| Stage 2. Determination of 2C3DHTA action in the small intestine based on the background of drug-induced enteropathy | |||||
| Indomethacin 35 mg/kg subcutaneously | Indomethacin 35 mg/kg subcutaneously | Metothrexate 10 mg/kg intraperitoneally | Metothrexate 10 mg/kg Intraperitoneally | Enalapril 2 mg/kg/day intraperitoneally | Enalapril 2 mg/kg/day intraperitoneally |
| Vehicle | 10 mg/kg/day 2C3DHTA intraperitoneally | Vehicle | 10 mg/kg/day 2C3DHTA intraperitoneally | Vehicle | 10 mg/kg/day 2C3DHTA intraperitoneally |
| (n = 8) | (n = 8) | (n = 8) | (n = 8) | (n = 8) | (n = 8) |
| Group 4 | Group 5 | Group 6 | Group 7 | Group 8 | Group 9 |
| Experimental Groups | Н2S (µmol/L) | MPO (U/mg) | MDA (µmol/g) | SOD (mmol/min × mg) | CAT (mmol H2O2/min × mg) |
|---|---|---|---|---|---|
| Control group | 72.37 ± 2.48 | 1.49 ± 0.43 | 191.2 ± 32.7 | 25.63 ± 1.71 | 35.97 ± 2.48 |
| 2A5DHT | 67.21 ± 2.53 | 2.24 ± 0.41 ** | 220.7 ± 18.9 ** | 23.35 ± 3.45 | 32.81 ± 3.41 |
| 2C3DHTA | 75.36 ± 5.27 | 1.55 ± 0.35 | 236.2 ± 28.4 ** | 25.9 ± 2.39 | 30.29 ± 3.87 |
| Experimental Groups | Н2S (µmol/L) | MPO (U/mg) | MDA (µmol/g) | SOD (mmol/min × mg) | CAT(mmol H2O2/min × mg) |
|---|---|---|---|---|---|
| Control group | 72.37 ± 2.48 | 1.49 ± 0.43 | 191.2 ± 32.7 | 25.63 ± 1.71 | 35.97 ± 2.48 |
| Indomethacin | 60.22 ± 6.55 ** | 5.16 ± 0.98 ** | 268.2 ± 34.5 ** | 16.33 ± 2.45 | 17.78 ± 2.33 ** |
| 2C3DHTA + indomethacin | 79.23 ± 8.58 ## | 1.45 ± 0.75 ## | 254.7 ± 29.65 ** | 19.4 ± 1.28 #* | 17.21 ± 1.99 ** |
| Metothrexate | 58.20 ± 5.41 ** | 2.18 ± 0.19 ** | 307.7 ± 52.1 ** | 14.18 ± 1.92 ** | 29.71 ± 1.11 ** |
| 2C3DHTA + metothrexate | 69.34 ± 13.9 | 1.64 ± 0.84 × | 192.2 ± 40.43 ×× | 24.78 ± 4.1 ×× | 26.50 ± 4.35 ** |
| Enalapril | 69.61 ± 6.1 | 4.92 ± 1.73 ** | 250.4 ± 29.6 ** | 26.53 ± 2.41 | 26.08 ± 3.23 ** |
| 2C3DHTA + enalapril | 73.8 ± 8,9 | 3.86 ± 0.89 ^** | 218.0 ± 19.1 ^** | 20.97 ± 3.02 ^** | 24.99 ± 2.98 ** |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sklyarova, Y.; Fomenko, I.; Lozynska, I.; Lozynskyi, A.; Lesyk, R.; Sklyarov, A. Hydrogen Sulfide Releasing 2-Mercaptoacrylic Acid-Based Derivative Possesses Cytoprotective Activity in a Small Intestine of Rats with Medication-Induced Enteropathy. Sci. Pharm. 2017, 85, 35. https://doi.org/10.3390/scipharm85040035
Sklyarova Y, Fomenko I, Lozynska I, Lozynskyi A, Lesyk R, Sklyarov A. Hydrogen Sulfide Releasing 2-Mercaptoacrylic Acid-Based Derivative Possesses Cytoprotective Activity in a Small Intestine of Rats with Medication-Induced Enteropathy. Scientia Pharmaceutica. 2017; 85(4):35. https://doi.org/10.3390/scipharm85040035
Chicago/Turabian StyleSklyarova, Yulia, Iryna Fomenko, Iryna Lozynska, Andrii Lozynskyi, Roman Lesyk, and Alexandr Sklyarov. 2017. "Hydrogen Sulfide Releasing 2-Mercaptoacrylic Acid-Based Derivative Possesses Cytoprotective Activity in a Small Intestine of Rats with Medication-Induced Enteropathy" Scientia Pharmaceutica 85, no. 4: 35. https://doi.org/10.3390/scipharm85040035







