Theoretical and Experimental Studies on Inclusion Complexes of Pinostrobin and β-Cyclodextrins
Abstract
:1. Introduction
2. Materials and Methods
2.1. Phase Solubility Study
2.2. Preparation of Solid Inclusion Complexes
2.3. Characterization of Inclusion Complexes
2.4. Determination of Biological Activity
2.5. Computational Studies
Steered Molecular Dynamics Simulation
3. Results and Discussion
3.1. Phase Solubility Study
3.2. Characterization of Inclusion Complexes
3.2.1. Differential Scanning Calorimetriy
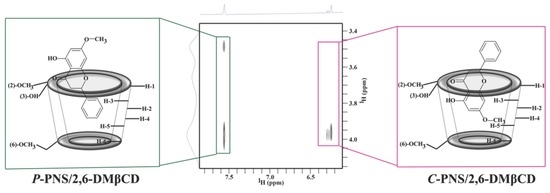
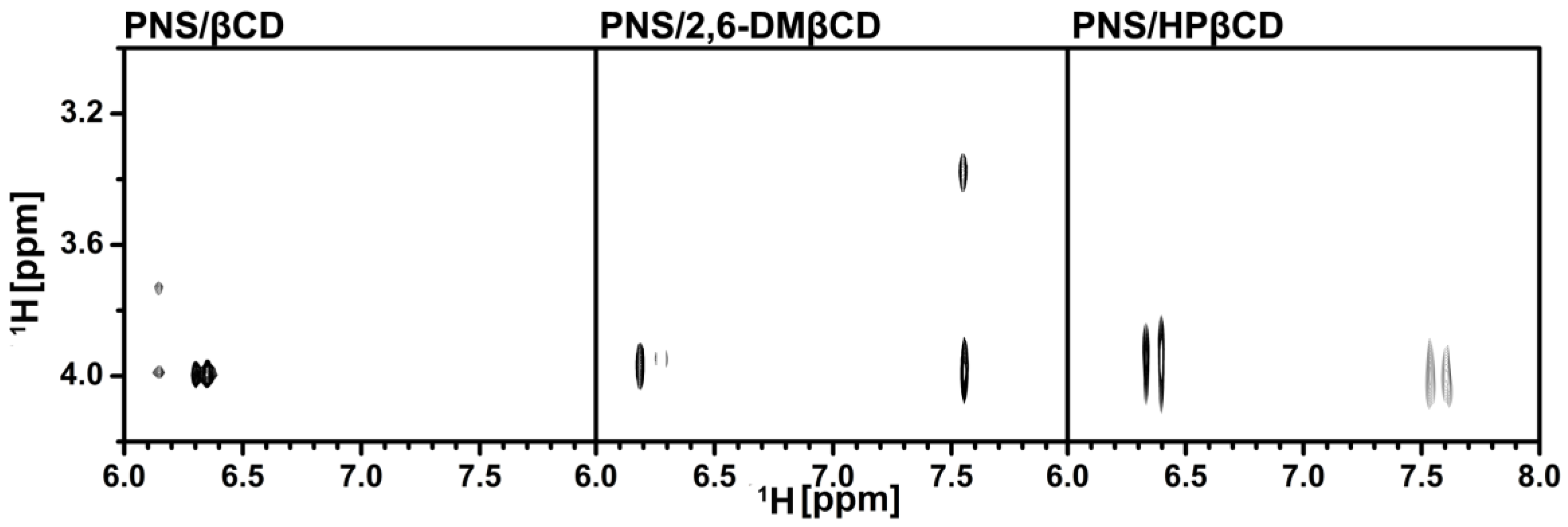
3.2.2. Two-Dimensional Nuclear Magnetic Resonance
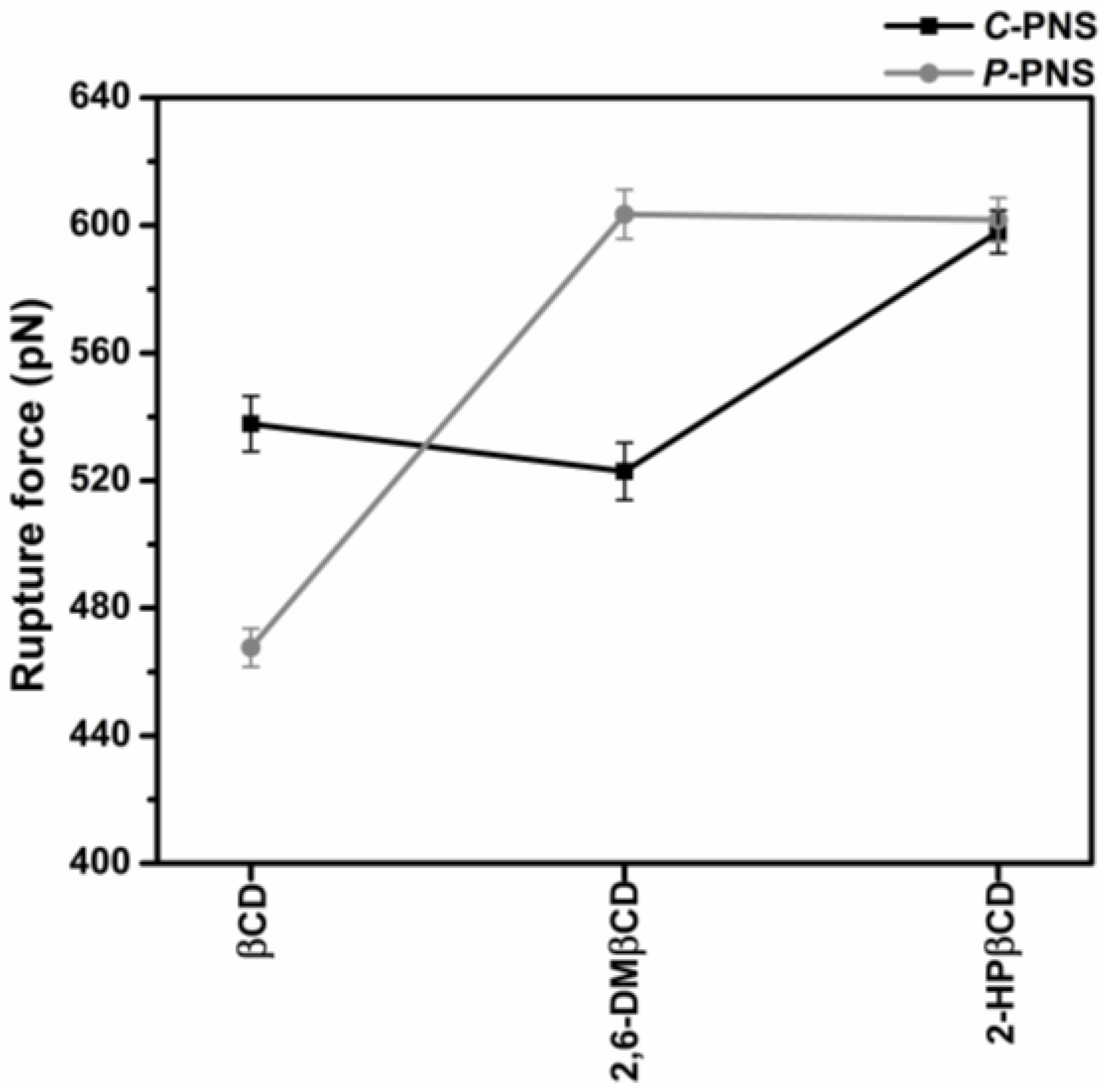
3.2.3. Steered Molecular Dynamics
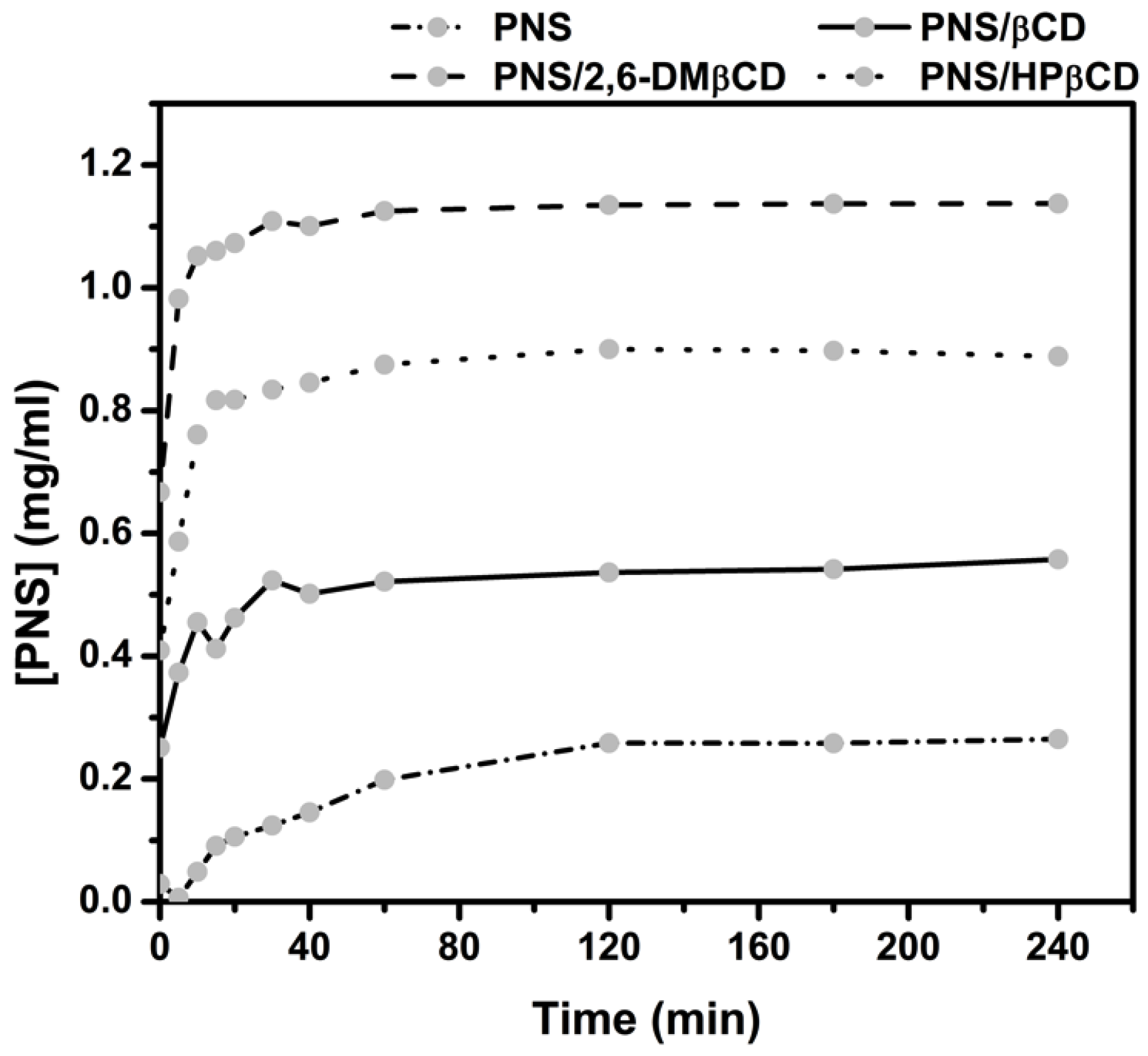
3.2.4. Dissolution Diagram
3.2.5. Determination of Inclusion Complexes Biological Activity
Anti-Inflammatory Activity
Cytotoxicity
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sher, A. Antimicrobial activity of natural products from medical plants. Gomal J. Med. Sci. 2009, 7, 72–78. [Google Scholar]
- Hollman, P.C.H.; Katan, M.B. Absorption, metabolism, and health effects of dietary flavonoids in man. Biomed. Pharmacother. 1997, 51, 305–310. [Google Scholar] [CrossRef]
- Smolinski, A.T.; Pestka, J.J. Modulation of lipopolysaccharide induce inflammatory cytokine production in vitro and in vivo by herbal constituents apigenin (chamomile), ginsenoside Rb-1 (ginseng) and parthenolide (feverfew). Food Chem. Toxicol. 2003, 41, 1381–1390. [Google Scholar] [CrossRef]
- Wu, N.; Kong, Y.; Zu, Y.; Fu, Y.; Liu, Z.; Meng, Z.R.; Liu, X.; Efferth, T. Activity investigation of pinostrobin towards herpes simplex virus-1 as determined by atomic force microscopy. Phytomedicine 2011, 13, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Atun, S.; Arianingrum, R.; Sulistyowati, E.; Aznam, N. Isolation and antimutagenic activity of some flavanone compounds from Kaempferia rotunda. Int. J. Chem. Anal. Sci. 2003, 4, 3–8. [Google Scholar] [CrossRef]
- Geibel, M. Sensitivity of the fungus Cytospora persoonii to the flavonoids of Prunus cerasus. Phytochemistry 1995, 38, 599–601. [Google Scholar] [CrossRef]
- Kaur, K.; Jain, M.; Kaur, T.; Jain, R. Antimalarials from nature. Bioorg. Med. Chem. 2009, 17, 3229–3256. [Google Scholar] [CrossRef] [PubMed]
- Abdelwahab, S.I.; Mohan, S.; Abdulla, M.A.; Sukari, M.A.; Abdul, A.B.; Taha, M.M.E.; Syam, S.; Ahmad, S.; Lee, K.H. The methanolic extract of Boesenbergia rotunda (L.) Mansf. and its majorcompound pinostrobin induces anti-ulcerogenic property in vivo: Possible involvement of indirect antioxidant action. J. Ethnopharmacol. 2011, 137, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Fu, K.; Fu, Y.-J.; Zu, Y.-G.; Chang, R.-R.; Chen, Y.-H.; Liu, X.-L.; Kong, Y.; Liu, W.; Gu, C.-B. Antioxidant activities of extracts and main components of pigeonpea [Cajanus cajan (L.) Millsp.] leaves. Molecules 2009, 14, 1032–1043. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.K.; Bhutani, K.K. Pinostrobin and Cajanus lactone isolated from Cajanus cajan (L.) leaves inhibits TNF-α and IL-1β production: In vitro and in vivo experimentation. Phytomedicine 2014, 21, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Bail, J.C.L.; Aubourg, L.; Habrioux, G. Effects of pinostrobin on estrogen metabolism and estrogen receptor transactivation. Cancer Lett. 2000, 156, 37–44. [Google Scholar] [CrossRef]
- Cao, X.D.; Ding, Z.S.; Jiang, F.S.; Ding, X.H.; Chen, J.Z.; Chen, S.H.; Lv, G.Y. Antitumor constituents from the leaves of Carya cathayensis. Nat. Prod. Res. 2012, 26, 2089–2094. [Google Scholar] [CrossRef] [PubMed]
- Charoensin, S.; Punvittayagul, C.; Pompimon, W.; Mevatee, U.; Wongpoomchai, R. Toxicological and clastogenic evaluation of pinocembrin and pinostrobin isolated from Boesenbergia pandurata in Wistar rats. Thai J. Toxicol. 2010, 25, 29–40. [Google Scholar]
- Pápaya, Z.E.; Sebestyéna, Z.K.; Ludányia, K.N.; Kállaia, N.E.; Balogha, E.A.; Kósab, A.S.; Somavarapuc, S.B.; Böddib, B.I.; Antala, I. Comparative evaluation of the effect of cyclodextrins and pH on aqueous solubility of apigenin. J. Pharm. Biomed. Anal. 2016, 117, 210–216. [Google Scholar] [CrossRef] [PubMed]
- García, A.; Leonardi, D.; Salazar, M.O.; Lamas, M.C. Modified β-Cyclodextrin Inclusion Complex to Improve the Physicochemical Properties of Albendazole. Complete In Vitro Evaluation and Characterization. PLoS ONE 2014, 9, e88234. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tan, T.; Heteényi, C.; Spoel, D. Quantification of Solvent Contribution to the Stability of Noncovalent Complexes. J. Chem. Theory Comput. 2013, 9, 4542–4551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kicuntod, J.; Khuntawee, W.; Wolschann, P.; Pongsawasdi, P.; Chavasiri, W.; Kungwang, N.; Rungrotmongkol, T. Inclusion complexation of pinostrobin with various cyclodextrin derivatives. J. Mol. Graph. Model. 2016, 63, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Semalty, A.; Tanwar, Y.S.; Semalty, M. Preparation and characterization of cyclodextrin inclusion complex of naringenin and critical comparison with phospholipid complexation for improving solubility and dissolution. J. Therm. Anal. Calorim. 2014, 115, 2471–2478. [Google Scholar] [CrossRef]
- Upadhyay, S.K.; Ali, S.M. Solution structure of loperamide and β-cyclodextrin inclusion complexes using NMR spectroscopy. J. Chem. Sci. 2009, 121, 521–527. [Google Scholar] [CrossRef]
- Hsu, C.M.; Yu, S.C.; Tsai, F.J.; Tsai, Y. Enhancement of rhubarb extract solubility and bioactivity by 2-hydroxypropyl-β-cyclodextrin. Carbohydr. Polym. 2013, 98, 1422–1429. [Google Scholar] [CrossRef] [PubMed]
- Caira, M.R.; Bourne, S.A.; Samsodien, H.; Smith, V.J. Inclusion complexes of 2-methoxyestradiol with dimethylated and permethylated β-cyclodextrins: Models for cyclodextrin-steroid interaction. Beilstein J. Org. Chem. 2015, 11, 2616–2630. [Google Scholar] [CrossRef] [PubMed]
- Hatziagapiou, K.; Bethanis, K.; Lambrou, G.I.; Yannakopoulou, K.; Karpusas, M.; Braoudaki, M.; Christoforides, E.; Tsorteki, F.; Milionis, V.; Kavantzas, N.; et al. Enhanced Gefitinib Cytotoxicity in the Presence of Cyclodextrins: In-Vitro and Biophysical Studies Towards Potential Therapeutic Interventions for Cancer. J. Biomed. Nanotechnol. 2017, 13, 522–533. [Google Scholar] [CrossRef]
- Sangpheak, W.; Khuntawee, W.; Wolschann, P.; Pongsawasdi, P.; Rungrotmongkol, T. Enhanced stability of a naringenin/2,6-dimethyl β-cyclodextrin inclusion complex: Molecular dynamics and free energy calculations based on MM- and QM-PBSA/GBSA. J. Mol. Graph. Model. 2014, 50, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Nutho, B.; Khuntawee, W.; Rungnim, C.; Pongsawasdi, P.; Wolschann, P.; Karpfen, A.; Kungwan, N.; Rungrotmongkol, T. Binding mode and free energy prediction of fisetin/β-cyclodextrin inclusion complexes. Beilstein J. Org. Chem. 2014, 10, 2789–2799. [Google Scholar] [CrossRef] [PubMed]
- Rungnim, C.; Phunpee, S.; Kunaseth, M.; Namuangruk, S.; Rungsardthong, K.; Rungrotmongkol, T.; Ruktanonchai, U. Co-solvation effect on the binding mode of the alpha-mangostin/β-cyclodextrin inclusion complex. Beilstein J. Org. Chem. 2015, 11, 2306–2317. [Google Scholar] [CrossRef] [PubMed]
- Wangkangwan, W.; Boonkerd, S.; Chavasiri, W.; Sukapirom, K.; Pattanapanyasat, K.; Kongkathip, N.; Miyakawa, T.; Yompakdee, C. Pinostrobin from Boesenbergia pandurata is an inhibitor of Ca2+-signal-mediated cell-cycle regulation in the yeast Saccharomyces cerevisiae. Biosci. Biotechnol. Biochem. 2009, 73, 1679–1682. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T.; Connors, K.A. Phase solubility techniques. Adv. Anal. Chem. Instrum. 1965, 4, 117–122. [Google Scholar]
- Bax, A.; Davis, D.G. Practical aspects of two-dimensional transverse NOE spectroscopy. J. Magn. Reson. 1985, 63, 207–213. [Google Scholar] [CrossRef]
- Hwang, T.L.; Shaka, A.J. Cross relaxation without TOCSY: Transverse rotating-frame Overhauser effect spectroscopy. J. Am. Chem. Soc. 1992, 114, 3157–3159. [Google Scholar] [CrossRef]
- Smolarz, H.D.; Mendyk, E.; Bogucka-Kocka, A.; Kocki, J. Pinostrobin—An anti-leukemic flavonoid from Polygonum lapathifolium L. ssp. nodosum (Pers.). Dans. Z. Naturforsch. C 2006, 61, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Belica, S.; Jeziorska, D.; Urbaniak, P.; Buko, V.U.; Zavodnik, I.B.; Pałecz, B. Calorimetric and spectroscopic characterization of complexes between β-cyclodextrin or heptakis (2,6-di-O-methyl)-β-cyclodextrin and sertraline hydrochloride in aqueous solution. J. Chem. Thermodyn. 2014, 70, 160–167. [Google Scholar] [CrossRef]
- Songngam, S.; Sukwattanasinit, M.; Siralertmukul, K.; Sawasdee, P. A 5,7-dimethoxyflavone/hydroxypropyl-β-cyclodextrin inclusion complex with anti-butyrylcholinesterase activity. AAPS PharmSciTech 2014, 15, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Mueller, M.; Hobiger, S.; Jungbauer, A. Anti-inflammatory activity of extracts from fruits, herbs and spices. Food Chem. 2010, 122, 987–996. [Google Scholar] [CrossRef]
- Sangpheak, W.; Kicuntod, J.; Schuster, R.; Rungrotmongkol, T.; Wolschann, P.; Kungwan, N.; Viernstein, H.; Mueller, M.; Pongsawasdi, P. Physical properties and biological activities of hesperetin and naringenin in complex with methylated β-cyclodextrin. Beilstein J. Org. Chem. 2015, 11, 2763–2773. [Google Scholar] [CrossRef] [PubMed]
- Cezard, C.; Trivelli, X.; Aubry, F.; Djedaïni-Pilard, F.; Dypradeau, F. Molecular dynamics studies of native and substituted cyclodextrins in different media: 1. Charge derivation and force field performances. Phys. Chem. Chem. Phys. 2011, 13, 15103–15121. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.; Vranken, W. ACPYPE—AnteChamber PYthon Parser interfacE. BioMed Cent. 2012, 5, 1–8. [Google Scholar]
- Szente, L.; Szejtli, J. Highly soluble cyclodextrin derivatives: Chemistry, properties, and trends in development. Adv. Drug Deliv. Rev. 1999, 36, 17–28. [Google Scholar] [CrossRef]
- Chadhaa, R.; Gupta, S.; Shukla, G.; Jain, D.V.S.; Pissurlenkar, R.R.S.; Coutinho, E.C. Interaction of artesunate with β-cyclodextrin : Characterization, thermodynamic parameters, molecular modeling, effect of PEG on complexation and antimalarial activity. Results Pharma Sci. 2011, 1, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Tommasini, S.; Raneri, D.; Ficarra, R.; Calabrò, M.L.; Stancanelli, R.; Ficarra, P. Improvement in solubility and dissolution rate of flavonoids by complexation with β-cyclodextrin. J. Pharm. Biomed. Anal. 2004, 35, 379–387. [Google Scholar] [CrossRef]
- Charumanee, S.; Titwan, A.; Sirithunyalug, J.; Weiss-Greiler, P.; Wolschann, P.; Viernstein, H.; Okonogi, S. Thermodynamics of the encapsulation by cyclodextrins. J. Chem. Technol. Biotechnol. 2006, 81, 523–529. [Google Scholar] [CrossRef]
- Viernstein, H.; Weiss-Greiler, P.; Wolschann, P. Solubility enhancement of low soluble biologically active compounds by β-cyclodextrin and dimethyl-β-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2002, 44, 235–239. [Google Scholar] [CrossRef]
- Liu, B.; Li, W.; Zhao, J.; Liu, Y.; Zhu, X.; Liang, G. Physicochemical characterisation of the supramolecular structure of luteolin/cyclodextrin inclusion complex. Food Chem. 2013, 141, 900–906. [Google Scholar] [CrossRef] [PubMed]
- Karathanos, V.T.; Mourtzinos, I.; Yannakopoulou, K.; Andrikopoulos, N.K. Study of the solubility, antioxidant activity and structure of inclusion complex of vanillin with β-cyclodextrin. Food Chem. 2007, 101, 652–658. [Google Scholar] [CrossRef]
- Ribeiro, L.; Loftsson, T.; Ferreira, D.; Veiga, F. Investigation and physicochemical characterization of vinpocetine-sulfobutyl ether β-cyclodextrin binary and ternary complexes. Chem. Pharm. Bull. 2003, 51, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Rajendiran, N.; Siva, S. Inclusion complex of sulfadimethoxine with cyclodextrins: Preparation and characterization. Carbohydr. Polym. 2014, 101, 828–836. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.W.; Ouyang, C.B.; Liu, Q.; Yuan, H.L.; Liu, X.H. Inclusion of quinestrol and 2,6-di-O-methyl-β-cyclodextrin: Preparation, characterization, and inclusion mode. Carbohydr. Polym. 2013, 93, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Pose-Vilarnovo, B.; Rodríguez-Tenreiro Sánchez, C.; Diéguez Moure, N.; Vila-Jato, J.L.; Torres-Labandeir, J.J. Effect of hydroxypropylmethyl cellulose on the complexation of diclofenac with cyclodextrins. J. Therm. Anal. Calorim. 2003, 73, 661–670. [Google Scholar] [CrossRef]
- Folch-Cano, C.; Guerrero, J.; Speisky, H.; Jullian, C.; Olea-Azar, C. NMR and molecular fluorescence spectroscopic study of the structure and thermodynamic parameters of EGCG/β-cyclodextrin inclusion complexes with potential antioxidant activity. J. Incl. Phenom. Macrocycl. Chem. 2014, 78, 287–298. [Google Scholar] [CrossRef]
- Ludemann, S.K.; Lounnas, V.; Wade, R.C. How do substrates enter and products exit the buried active site of cytochrome P450cam? J. Mol. Biol. 2000, 303, 813–830. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Gullingsrud, J.; McCammon, J.A. Potentials of mean force for acetylcholine unbinding from the alpha7 nicotinic acetylcholine receptor ligand-binding domain. J. Am. Chem. Soc. 2006, 128, 3019–3026. [Google Scholar] [CrossRef] [PubMed]
- Colizzi, F.; Perozzo, R.; Scapozza, L.; Recanatini, M.; Cavalli, A. Single-molecule pulling simulations can discern active from inactive enzyme inhibitors. J. Am. Chem. Soc. 2010, 132, 7361–7371. [Google Scholar] [CrossRef] [PubMed]
- Meeprasert, A.; Rungrotmongkol, T.; Li, M.S.; Hannongbua, S. In silico screening for potent inhibitors against the NS3/4A protease of hepatitis C virus. Curr. Pharm. Des. 2014, 20, 3465–3477. [Google Scholar] [CrossRef] [PubMed]
- Nutho, B.; Meeprasert, A.; Chulapa, M.; Kungwan, N.; Rungrotmongkol, T. Screening of Hepatitis C NS5B polymerase inhibitors containing benzothiadiazine core: A steered molecular dynamics study. J. Biomol. Struct. Dyn. 2016, 35, 1743–1757. [Google Scholar] [CrossRef] [PubMed]
- Schonbeck, C.; Westh, P.; Madsen, J.C.; Larsen, K.L.; Stade, L.W.; Holm, R. Hydroxypropyl-substituted β-cyclodextrins: Influence of degree of substitution on the thermodynamics of complexation with tauroconjugated and glycoconjugated bile salts. Langmuir 2010, 26, 17949–17957. [Google Scholar] [CrossRef] [PubMed]
- Caballero, J.; Zamora, C.; Aguayo, D.; Yanez, C.; González-Nilo, F.D. Study of the interaction between progesterone and β-cyclodextrin by electrochemical techniques and steered molecular dynamics. J. Phys. Chem. B 2008, 112, 10194–10201. [Google Scholar] [CrossRef] [PubMed]
- Bikádi, R.; Kurdi, S.; Balogh, J.S.; Zeman, E.H. Aggregation of cyclodextrins as an important factor to determine their complexation behavior. Chem. Biodivers. 2006, 3, 1266–1278. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Liu, W.; Wang, L.; Wen, Y. Characterization of inclusion complexation between fenoxaprop-p-ethyl and cyclodextrin. J. Agric. Food Chem. 2005, 53, 7193–7197. [Google Scholar] [CrossRef] [PubMed]
- Nacsa, Á.; Berkesi, O.; Szabó-Révész, P.; Aigner, Z. Achievement of pH-independence of poorly soluble: Ionizable loratadine by inclusion complex formation with dimethyl-β-cyclodextrin. J. Incl. Phenom. Macrocylc. Chem. 2009, 64, 249–254. [Google Scholar] [CrossRef]
- Ashidi, J.S.; Houghton, P.J.; Hylands, P.J.; Efferth, T. Ethnobotanical survey and cytotoxicity testing of plants of South-western Nigeria used to treat cancer, with isolation of cytotoxic constituents from Cajanus cajan Millsp. Leaves. J. Ethnopharmacol. 2010, 128, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Isa, N.M.; Abdelwahab, S.I.; Mohan, S.; Abdul, A.B.; Sukari, M.A.; Taha, M.M.E.; Syam, S.; Narrima, P.; Cheah, S.C.; Ahmad, S.; et al. In vitro anti-inflammatory, cytotoxic and antioxidant activities of boesenbergin A, a chalcone isolated from Boesenbergia rotunda (L.) (fingerroot). Braz. J. Med. Biol. Res. 2012, 45, 524–530. [Google Scholar] [CrossRef] [PubMed]
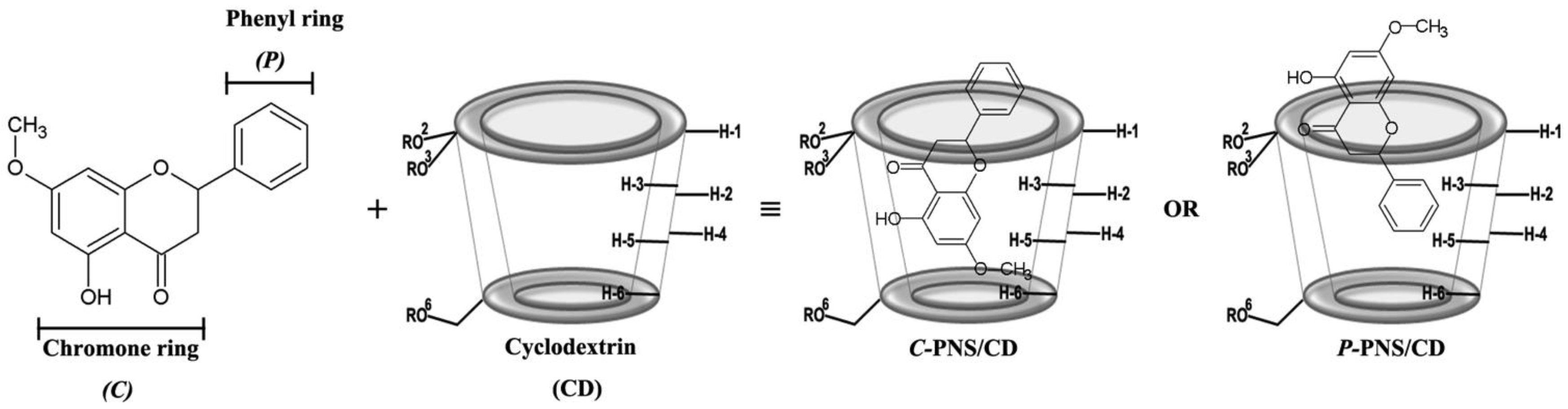
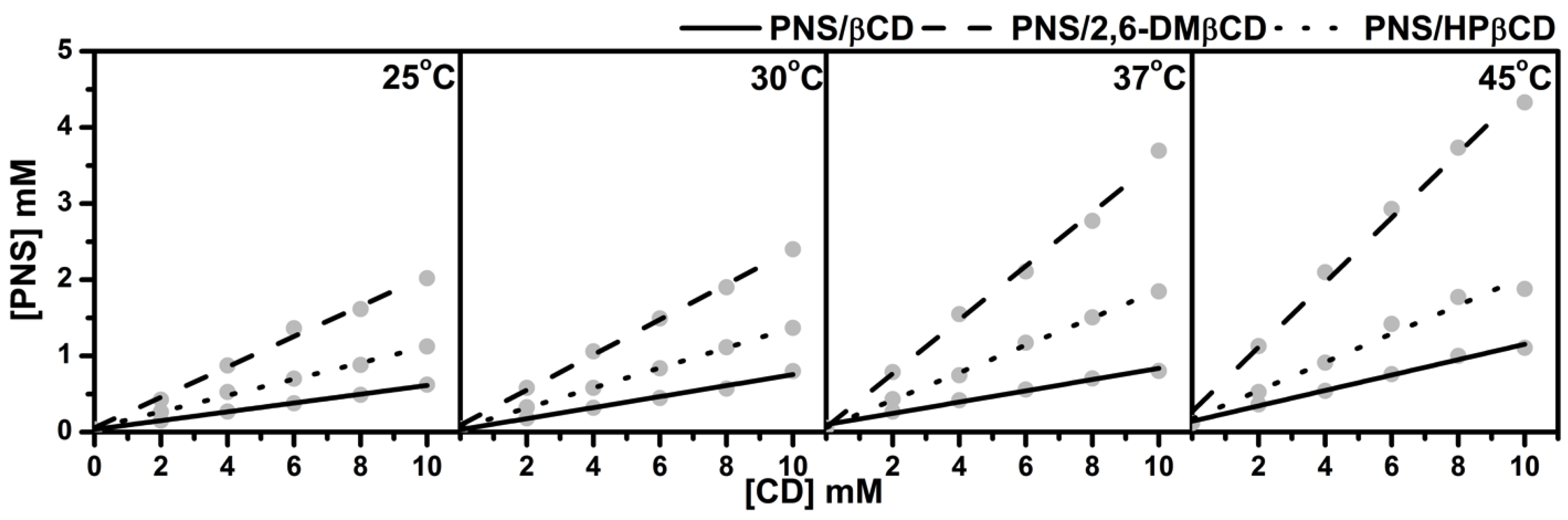
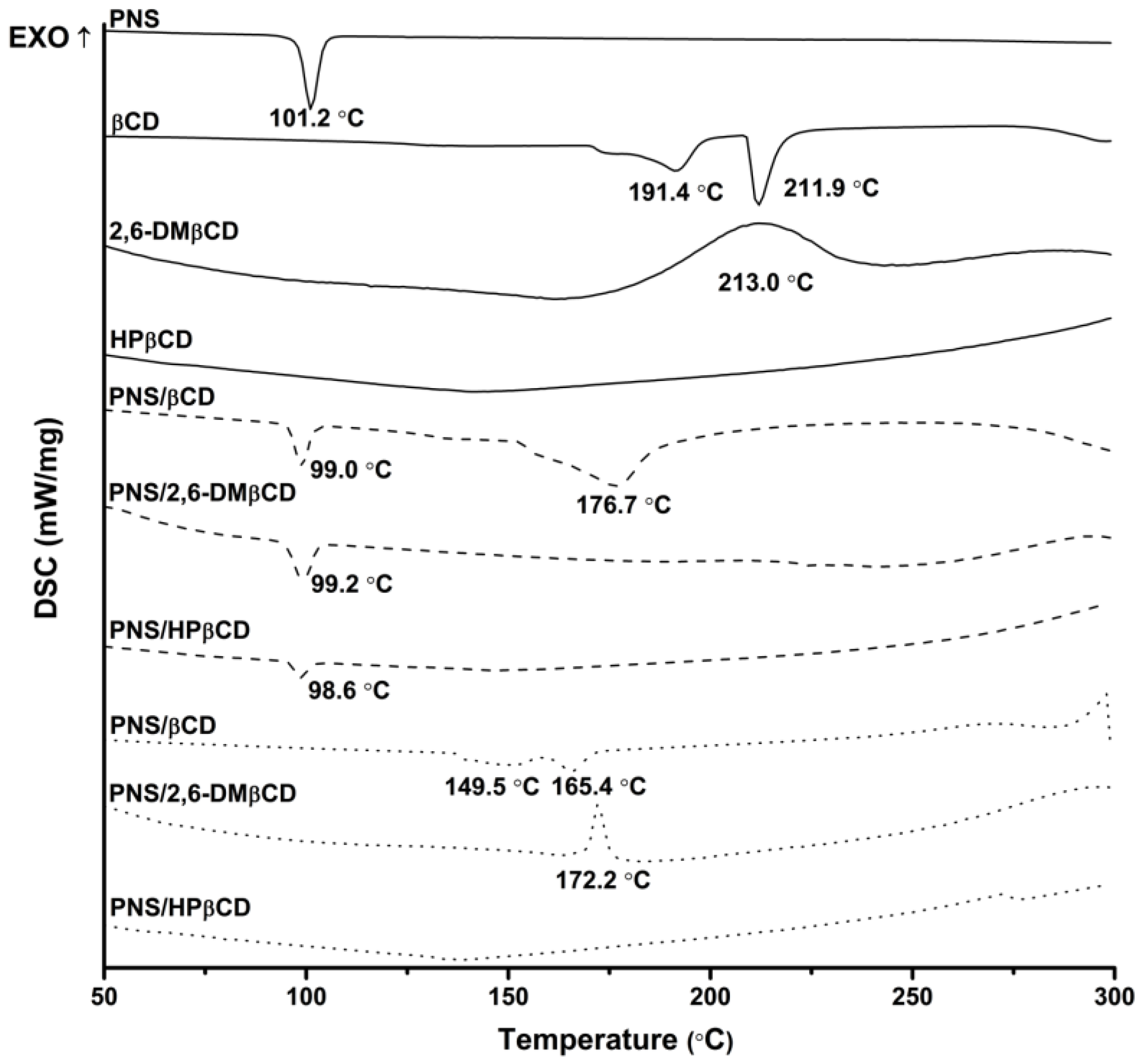
| Temperature (°C) | Stability Constant ( in M−1) | ||
|---|---|---|---|
| PNS/βCD | PNS/2,6-DMβCD | PNS/HPβCD | |
| 25 | 1800 | 7320 | 3500 |
| 30 | 1580 | 6070 | 3070 |
| 37 | 1300 | 7560 | 2840 |
| 45 | 1190 | 6930 | 2370 |
| Inclusion Complex | (kJ mol−1) | (kJ mol−1) | (kJ mol−1) |
|---|---|---|---|
| PNS/βCD | −17.2 | 1.7 | −18.8 |
| PNS/2,6-DMβCD | 1.0 | 22.9 | −21.9 |
| PNS/HPβCD | −15.1 | 5.4 | −20.5 |
| IC50 [μM] | |||
|---|---|---|---|
| IL-6 Secretion | Cytotoxicity Hela | Cytotoxicity MCF-7 | |
| PNS | 30 | 79 | 22 |
| PNS/βCD | 61 | 72 | 28 |
| PNS/2,6-DMβCD | 49 | 95 | 18 |
| PNS/HPβCD | 27 | 65 | 29 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kicuntod, J.; Sangpheak, K.; Mueller, M.; Wolschann, P.; Viernstein, H.; Yanaka, S.; Kato, K.; Chavasiri, W.; Pongsawasdi, P.; Kungwan, N.; et al. Theoretical and Experimental Studies on Inclusion Complexes of Pinostrobin and β-Cyclodextrins. Sci. Pharm. 2018, 86, 5. https://doi.org/10.3390/scipharm86010005
Kicuntod J, Sangpheak K, Mueller M, Wolschann P, Viernstein H, Yanaka S, Kato K, Chavasiri W, Pongsawasdi P, Kungwan N, et al. Theoretical and Experimental Studies on Inclusion Complexes of Pinostrobin and β-Cyclodextrins. Scientia Pharmaceutica. 2018; 86(1):5. https://doi.org/10.3390/scipharm86010005
Chicago/Turabian StyleKicuntod, Jintawee, Kanyani Sangpheak, Monika Mueller, Peter Wolschann, Helmut Viernstein, Saeko Yanaka, Koichi Kato, Warinthorn Chavasiri, Piamsook Pongsawasdi, Nawee Kungwan, and et al. 2018. "Theoretical and Experimental Studies on Inclusion Complexes of Pinostrobin and β-Cyclodextrins" Scientia Pharmaceutica 86, no. 1: 5. https://doi.org/10.3390/scipharm86010005
APA StyleKicuntod, J., Sangpheak, K., Mueller, M., Wolschann, P., Viernstein, H., Yanaka, S., Kato, K., Chavasiri, W., Pongsawasdi, P., Kungwan, N., & Rungrotmongkol, T. (2018). Theoretical and Experimental Studies on Inclusion Complexes of Pinostrobin and β-Cyclodextrins. Scientia Pharmaceutica, 86(1), 5. https://doi.org/10.3390/scipharm86010005