Methods to Evaluate Skin Penetration In Vitro
Abstract
:1. Introduction
1.1. Structure and Function of the Skin
1.2. Modifying Factors of Skin Penetration
1.3. General Guidelines of Skin Penetration Testing
2. Techniques for Modelling Penetration/Permeation through Human Skin
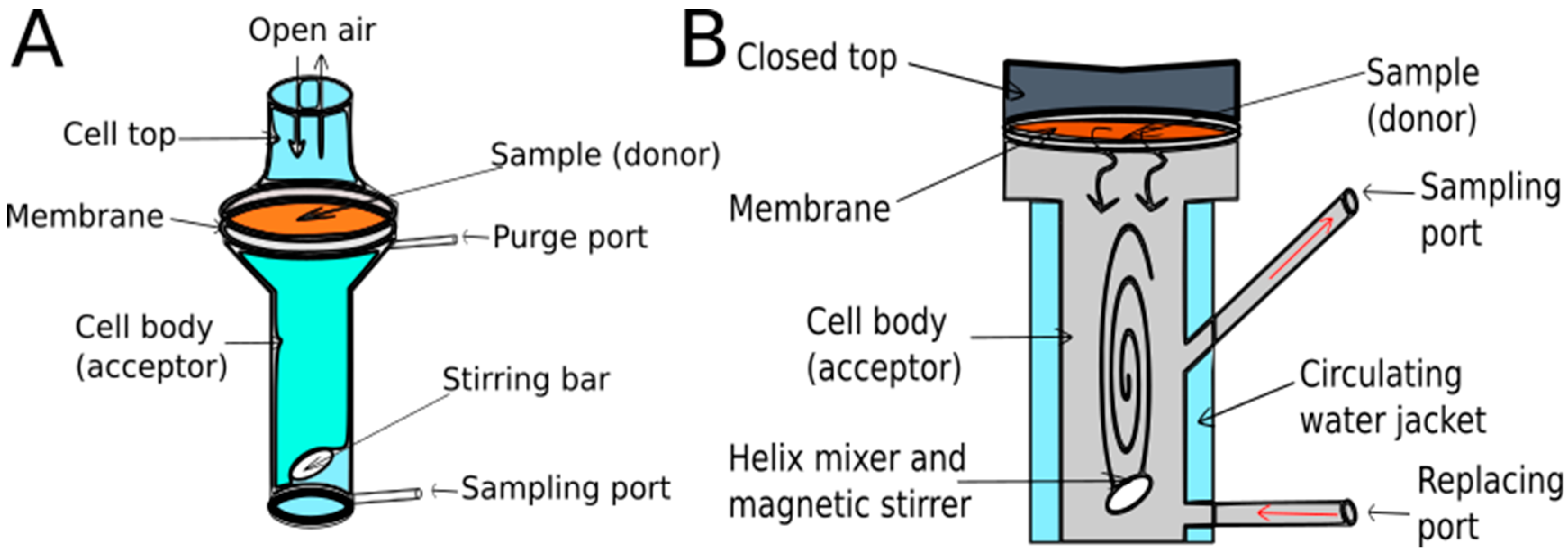
2.1. Diffusion Cells
2.1.1. Types and Properties of Diffusion Cells
2.1.2. Diffusion Test Types
2.2. Skin-PAMPA
2.3. The Most Important Experimental Considerations in the Case of Quantitative Methods
- the sink condition
- the incubation time
- the incubation temperature
- the mixing
- the hydration of the membrane
- the amount of dose [73].
2.4. Tape Stripping
2.4.1. Method Description
2.4.2. Possibility of Sample Analysis
2.4.3. Possibilities for Optimizing the Experimental Protocol
2.5. Microscopic and Spectroscopic Methods Utilised for the Percutaneous Penetration of APIs
2.5.1. Two-Photon Microscopy Method
2.5.2. Confocal Laser Scanning Microscopy Method
2.5.3. Confocal Raman Microscopic Method
3. Conclusions
Funding
Conflicts of Interest
References
- Transdermal Drug Delivery Systems Market-Industry Analysis, Market Size, Share, Trends, Application Analysis, Growth and Forecast 2019–2024. Available online: https://industryarc.com/Research/Transdermal-Drug-Delivery-Systems-Market-Research (accessed on 1 April 2019).
- Shah, V.P.; Yacobi, A.; Rădulescu, F.Ş.; Miron, D.S.; Lane, M.E. A science based approach to topical drug classification system (TCS). Int. J. Pharm. 2015, 491, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Wiedersberg, S.; Guy, R.H. Transdermal drug delivery: 30+ years of war and still fighting! J. Control. Release 2014, 190, 150–156. [Google Scholar] [CrossRef] [PubMed]
- So, J.; Ahn, J.; Lee, T.-H.; Park, K.-H.; Paik, M.-K.; Jeong, M.; Cho, M.-H.; Jeong, S.-H. Comparison of International Guidelines of Dermal Absorption Tests Used in Pesticides Exposure Assessment for Operators. Toxicol. Res. 2014, 30, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Groeber, F.; Holeiter, M.; Hampel, M.; Hinderer, S.; Schenke-Layland, K. Skin tissue engineering—in vivo and in vitro applications. Adv. Drug Deliv. Rev. 2011, 63, 352–366. [Google Scholar] [CrossRef] [PubMed]
- Feldmann, R.J.; Maibach, H.I. Absorption of Some Organic Compounds through the Skin in Man. J. Investig. Derm. 1970, 54, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Blank, I.H. Factors Which Influence the Water Content of the Stratum Corneum. J. Investig. Derm. 1952, 18, 433–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinson, L.J.; Singer, E.J.; Koehler, W.R.; Lehman, M.D.; Masurat, T. The nature of the epidermal barrier and some factors influencing skin permeability. Toxicol. Appl. Pharm. 1965, 7, 7–19. [Google Scholar] [CrossRef]
- Elias, P.M. The permeability barrier in mammalian epidermis. J. Cell Biol. 1975, 65, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, T.M.; Downing, D.T. The Role of Lipids in the Epidermal Barrier to Water Diffusion. J. Investig. Derm. 1970, 55, 135–140. [Google Scholar] [CrossRef]
- Anissimov, Y.G.; Jepps, O.G.; Dancik, Y.; Roberts, M.S. Mathematical and pharmacokinetic modelling of epidermal and dermal transport processes. Adv. Drug Deliv. Rev. 2013, 65, 169–190. [Google Scholar] [CrossRef]
- Bo Forslind. A domain mosaic model of the skin barrier. Acta Derm. Venereol. 1994, 74, 1–6. [Google Scholar]
- Godin, B.; Touitou, E. Transdermal skin delivery: Predictions for humans from in vivo, ex vivo and animal models. Adv. Drug Deliv. Rev. 2007, 59, 1152–1161. [Google Scholar] [CrossRef] [PubMed]
- Hadgraft, J. Skin deep. Eur. J. Pharm. Biopharm. 2004, 58, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Ruela, A.L.M.; Perissinato, A.G.; de Lino, M.E.S.; Mudrik, P.S.; Pereira, G.R. Evaluation of skin absorption of drugs from topical and transdermal formulations. Braz. J. Pharm. Sci. 2016, 52, 527–544. [Google Scholar] [CrossRef] [Green Version]
- Jepps, O.G.; Dancik, Y.; Anissimov, Y.G.; Roberts, M.S. Modeling the human skin barrier—Towards a better understanding of dermal absorption. Adv. Drug Deliv. Rev. 2013, 65, 152–168. [Google Scholar] [CrossRef] [PubMed]
- Barry, B.W. Novel mechanisms and devices to enable successful transdermal drug delivery. Eur. J. Pharm. Sci. 2001, 14, 101–114. [Google Scholar] [CrossRef]
- Blume-Peytavi, U.; Massoudy, L.; Patzelt, A.; Lademann, J.; Dietz, E.; Rasulev, U.; Garcia Bartels, N. Follicular and percutaneous penetration pathways of topically applied minoxidil foam. Eur. J. Pharm. Biopharm. 2010, 76, 450–453. [Google Scholar] [CrossRef]
- Patzelt, A.; Richter, H.; Knorr, F.; Schäfer, U.; Lehr, C.-M.; Dähne, L.; Sterry, W.; Lademann, J. Selective follicular targeting by modification of the particle sizes. J. Control. Release 2011, 150, 45–48. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA); Buist, H.; Craig, P.; Dewhurst, I.; Hougaard Bennekou, S.; Kneuer, C.; Machera, K.; Pieper, C.; Court Marques, D.; Guillot, G.; et al. Guidance on dermal absorption. EFSA J. 2017, 15, e04873. [Google Scholar] [Green Version]
- Kalia, Y.N.; Guy, R.H. Modeling transdermal drug release. Adv. Drug Deliv. Rev. 2001, 48, 159–172. [Google Scholar] [CrossRef]
- Machado, A.C.H.R.; Lopes, P.S.; Raffier, C.P.; Haridass, I.N.; Roberts, M.; Grice, J.; Leite-Silva, V.R. Skin Penetration. In Cosmetic Science and Technology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 741–755. ISBN 978-0-12-802005-0. [Google Scholar]
- Moser, K.; Kriwet, K.; Naik, A.; Kalia, Y.N.; Guy, R.H. Passive skin penetration enhancement and its quantification in vitro. Eur. J. Pharm. Biopharm. 2001, 10, 103–112. [Google Scholar] [CrossRef]
- Delgado-Charro, M.B.; Guy, R.H. Effective use of transdermal drug delivery in children. Adv. Drug Deliv. Rev. 2014, 73, 63–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dragicevic, N.; Maibach, H.I. Percutaneous Penetration Enhancers Drug Penetration Into/Through the Skin; Springer: Berlin/Heidelberg, Germany, 2017; ISBN 978-3-662-53268-3. [Google Scholar]
- Machado, M.; Hadgraft, J.; Lane, M.E. Assessment of the variation of skin barrier function with anatomic site, age, gender and ethnicity: Assessment of the variation of skin barrier function. Int. J. Cosmet. Sci. 2010, 32, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Waller, J.M.; Maibach, H.I. Age and skin structure and function, a quantitative approach (I): Blood flow, pH, thickness, and ultrasound echogenicity. Skin Res. Technol. 2005, 11, 221–235. [Google Scholar] [CrossRef] [PubMed]
- Behl, C.R.; Flynn, G.L.; Barrett, M.; Walters, K.A.; Linn, E.E.; Mohamed, Z.; Kurihara, T.; Ho, N.F.H.; Higuchi, W.I.; Pierson, C.L. Permeability of thermally damaged skin II: Immediate influences of branding at 60 °C on hairless mouse skin permeability. Burns 1981, 7, 389–399. [Google Scholar] [CrossRef]
- Behl, C.R.; Flynn, G.L.; Kurihara, T.; Smith, W.; Gatmaitan, O.; Higuchi, W.I.; Ho, N.F.H.; Pierson, C.L. Permeability of Thermally Damaged Skin: I. Immediate Influences of 60 °C Scalding on Hairless Mouse Skin. J. Investig. Derm. 1980, 75, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Flaten, G.E.; Palac, Z.; Engesland, A.; Filipović-Grčić, J.; Vanić, Ž.; Škalko-Basnet, N. In vitro skin models as a tool in optimization of drug formulation. Eur. J. Pharm. Sci. 2015, 75, 10–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- OECD. Test Guideline 427: Skin absorption: In Vivo Method; OECD: Paris, France, 2004. [Google Scholar]
- OECD. Test Guideline 428: Skin absorption: In Vitro Method; OECD: Paris, France, 2004. [Google Scholar]
- OECD. Guidance Document for the Conduct of Skin Absorption Studies; OECD Series on Testing and Assessment; OECD: Paris, France, 2004; ISBN 978-92-64-07879-6. [Google Scholar]
- Kielhorn, J. International Programme on Chemical Safety Dermal Absorption; Environmental Health Criteria; WHO: Geneva, Switzerland, 2006; ISBN 978-92-4-157235-4. [Google Scholar]
- European Centre for Ecotoxicology and Toxicology of Chemicals. Percutaneous absorption, Monograph; No. 20; European Centre for Ecotoxicology and Toxicology of Chemicals: Bruxelles, Belgium, 1993; ISSN 0773-6347-20. [Google Scholar]
- EPAU.S. Environmental Protection Agency (EPA). Dermal Exposure Assessment: A Summary of EPA Approaches. Available online: http://www.epa.gov/ncea (accessed on 1 April 2019).
- Dumont, C.; Prieto, P.; Asturiol, D.; Worth, A. Review of the Availability of In Vitro and In Silico Methods for Assessing Dermal Bioavailability. Appl. Vitro. Toxicol. 2015, 1, 147–164. [Google Scholar] [CrossRef]
- Anissimov, Y.G.; Roberts, M.S. Diffusion Modelling of Percutaneous Absorption Kinetics: 4. Effects of a Slow Equilibration Process Within Stratum Corneum on Absorption and Desorption Kinetics. J. Pharm. Sci. 2009, 98, 772–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anissimov, Y.G.; Roberts, M.S. Diffusion Modeling of Percutaneous Absorption Kinetics: 3. Variable Diffusion and Partition Coefficients, Consequences for Stratum Corneum Depth Profiles and Desorption Kinetics. J. Pharm. Sci. 2004, 93, 470–487. [Google Scholar] [CrossRef] [PubMed]
- Anissimov, Y.G.; Roberts, M.S. Diffusion modeling of percutaneous absorption kinetics: 2. Finite vehicle volume and solvent deposited solids. J. Pharm. Sci. 2001, 90, 504–520. [Google Scholar] [CrossRef]
- Anissimov, Y.G.; Roberts, M.S. Diffusion modeling of percutaneous absorption kinetics. 1. Effects of flow rate, receptor sampling rate, and viable epidermal resistance for a constant donor concentration. J. Pharm. Sci. 1999, 88, 1201–1209. [Google Scholar] [CrossRef] [PubMed]
- Todo, H.; Oshizaka, T.; Kadhum, W.; Sugibayashi, K. Mathematical Model to Predict Skin Concentration after Topical Application of Drugs. Pharmaceutics 2013, 5, 634–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crank, J. The Mathematics of Diffusion, 2nd ed.; Clarendon Press: Oxford, UK, 1975; ISBN 978-0-19-853344-3. [Google Scholar]
- Franz, T.J. Percutaneous Absorption. On the Relevance of in Vitro Data. J. Investig. Derm. 1975, 64, 190–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franz, T.J. The Finite Dose Technique as a Valid in vitro Model for the Study of Percutaneous Absorption in Man. In Current Problems in Dermatology; Simon, G.A., Paster, Z., Klingberg, M.A., Kaye, M., Eds.; S. Karger AG: Basel, Switzerland, 1979; Volume 7, pp. 58–68. ISBN 978-3-8055-2797-2. [Google Scholar]
- Sesto Cabral, M.E.; Ramos, A.N.; Cabrera, C.A.; Valdez, J.C.; González, S.N. Equipment and method for in vitro release measurements on topical dosage forms. Pharm. Dev. Technol. 2015, 20, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Diffusion Measurements. Available online: www.hansonresearch.com (accessed on 1 April 2019).
- Bronaugh, R.L.; Stewart, R.F. Methods for In Vitro Percutaneous Absorption Studies IV: The Flow-Through Diffusion Cell. J. Pharm. Sci. 1985, 74, 64–67. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. FDA Draft Guideline on Quality and Equivalence of Topical Products EMA/CHMP/QWP/708282/2018; European Medicines Agency: Amsterdam, The Netherlands, 2018.
- Lee, J.D.; Kim, J.Y.; Jang, H.J.; Lee, B.M.; Kim, K.B. Percutaneous permeability of 1-phenoxy-2-propanol, a preservative in cosmetics. Regul. Toxicol. Pharm. 2019, 103, 56–62. [Google Scholar] [CrossRef]
- Zhang, Y.; Lane, M.E.; Hadgraft, J.; Heinrich, M.; Chen, T.; Lian, G.; Sinko, B. A comparison of the in vitro permeation of niacinamide in mammalian skin and in the Parallel Artificial Membrane Permeation Assay (PAMPA) model. Int. J. Pharm. 2019, 556, 142–149. [Google Scholar] [CrossRef]
- Trombino, S.; Russo, R.; Mellace, S.; Varano, G.P.; Laganà, A.S.; Marcucci, F.; Cassano, R. Solid lipid nanoparticles made of trehalose monooleate for cyclosporin-A topic release. J. Drug Deliv. Sci. Technol. 2019, 49, 563–569. [Google Scholar] [CrossRef]
- Taofiq, O.; Rodrigues, F.; Barros, L.; Barreiro, M.F.; Ferreira, I.C.; Oliveira, M.B. Mushroom ethanolic extracts as cosmeceuticals ingredients: Safety and ex vivo skin permeation studies. Food Chem. Toxicol. 2019, 127, 228–236. [Google Scholar] [CrossRef] [Green Version]
- Ameen, D.; Michniak-Kohn, B. Development and in vitro evaluation of pressure sensitive adhesive patch for the transdermal delivery of galantamine: Effect of penetration enhancers and crystallization inhibition. Eur. J. Pharm. Biopharm. 2019, 139, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Silva-Abreu, M.; Gonzalez-Pizarro, R.; Espinoza, L.C.; Rodríguez-Lagunas, M.J.; Espina, M.; García, M.L.; Calpena, A.C. Thiazolidinedione as an alternative to facilitate oral administration in geriatric patients with Alzheimer’s disease. Eur. J. Pharm. Sci. 2019, 129, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Intarakumhaeng, R.; Alsheddi, L.; Wanasathop, A.; Shi, Z.; Li, S.K. Skin Permeation of Urea Under Finite Dose Condition. J. Pharm. Sci. 2019, 108, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Rajithaa, P.; Shammika, P.; Aiswarya, S.; Gopikrishnan, A.; Jayakumar, R.; Sabitha, M. Chaulmoogra oil based methotrexate loaded topical nanoemulsion for the treatment of psoriasis. J. Drug Deliv. Sci. Technol. 2019, 49, 463–476. [Google Scholar] [CrossRef]
- Salehi, S.; Boddohi, S. Design and optimization of kollicoat ® IR based mucoadhesive buccal film for co-delivery of rizatriptan benzoate and propranolol hydrochloride. Mater. Sci. Eng. C 2019, 97, 230–244. [Google Scholar] [CrossRef] [PubMed]
- Soriano-Ruiz, J.L.; Suñer-Carbó, J.; Calpena-Campmany, A.C.; Bozal-de Febrer, N.; Halbaut-Bellowa, L.; Boix-Montañés, A.; Souto, E.B.; Clares-Naveros, B. Clotrimazole multiple W/O/W emulsion as anticandidal agent: Characterization and evaluation on skin and mucosae. Coll. Surf. B Biointerfaces 2019, 175, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Serpe, L.; Muniz, B.V. Full-Thickness Intraoral Mucosa Barrier Models for In Vitro Drug- Permeation Studies Using Microneedles. J. Pharm. Sci. 2019, 108, 1756–1764. [Google Scholar] [CrossRef]
- Lee, W.-R.; Hsiao, C.-Y.; Huang, T.-H.; Wang, C.-L.; Alalaiwe, A.; Chen, E.-L.; Fang, J.-Y. Post-irradiation recovery time strongly influences fractional laser-facilitated skin absorption. Int. J. Pharm. 2019, 564, 48–58. [Google Scholar] [CrossRef]
- Kansy, M.; Senner, F.; Gubernator, K. Physicochemical High Throughput Screening: Parallel Artificial Membrane Permeation Assay in the Description of Passive Absorption Processes. J. Med. Chem. 1998, 41, 1007–1010. [Google Scholar] [CrossRef]
- Ottaviani, G.; Martel, S.; Carrupt, P.-A. Parallel Artificial Membrane Permeability Assay: A New Membrane for the Fast Prediction of Passive Human Skin Permeability. J. Med. Chem. 2006, 49, 3948–3954. [Google Scholar] [CrossRef]
- Sinkó, B.; Garrigues, T.M.; Balogh, G.T.; Nagy, Z.K.; Tsinman, O.; Avdeef, A.; Takács-Novák, K. Skin–PAMPA: A new method for fast prediction of skin penetration. Eur. J. Pharm. Sci. 2012, 45, 698–707. [Google Scholar] [CrossRef] [PubMed]
- Karadzovska, D.; Riviere, J.E. Assessing vehicle effects on skin absorption using artificial membrane assays. Eur. J. Pharm. Sci. 2013, 50, 569–576. [Google Scholar]
- Sinkó, B.; Pálfi, M.; Béni, S.; Kökösi, J.; Takács-Novák, K. Synthesis and Characterization of Long-Chain Tartaric Acid Diamides as Novel Ceramide-Like Compounds. Molecules 2010, 15, 824–833. [Google Scholar] [CrossRef]
- Sinkó, B.; Vizserálek, G.; Takács-Novák, K. Skin PAMPA: Application in practice. Admet Dmpk 2015, 2, 191–198. [Google Scholar] [CrossRef] [Green Version]
- Lee, P.H.; Conradi, R.; Shanmugasundaram, V. Development of an in silico model for human skin permeation based on a Franz cell skin permeability assay. Bioorg. Med. Chem. Lett. 2010, 20, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Tsinman, K.; Tsinman, O.; Schalau, G.; Aliyar, H.; Huber, R.; Loubert, G. Application of Skin PAMPA to Differentiate between Topical Pharmaceutical Formulations of Ibuprofen. (R6058) in AAPS Annual Meeting and Exposition, Chicago, IL, USA, 2012. [Google Scholar]
- Vizserálek, G.; Balogh, T.; Takács-Novák, K.; Sinkó, B. PAMPA study of the temperature effect on permeability. Eur. J. Pharm. 2014, 53, 45–49. [Google Scholar] [CrossRef]
- Clough, M.; Richardson, N.; Langley, N.; Tsinman, K.; Tsinman, O. Assessment of Transdermal Penetration Enhancement by Topical Pharmaceutical Excipients Using Skin PAMPA Method. (T2267) in AAPS Annual Meeting and Exposition, San Antonio, TX, USA, 2013. [Google Scholar]
- Luo, L.; Sinkó, B.; Tsinman, K.; Abdalghafor, H.; Hadgraft, J.; Lane, M. A Comparison of Drug Permeation in the Skin PAMPA Model and the Franz Cell Model. (W5104) in AAPS Annual Meeting and Exposition, San Diego, CA, USA, 2014. [Google Scholar]
- Vizserálek, G.; Vizserálek, G. Examination of Permeability of Drugs by PAMPA Method in Theoretical and Practical Aspects. Ph.D. Thesis, Semmelweis University, Budapest, Hungary, 13 December 2016. [Google Scholar]
- Ueda, C.T.; Shah, V.P.; Derdzinski, K.; Ewing, G.; Flynn, G.; Maibach, H.; Marques, M.; Rytting, H.; Shaw, S.; Thakker, K.; et al. Topical and Transdermal Drug Products. Dissolution Technol. 2010, 17, 12–25. [Google Scholar] [CrossRef]
- Selzer, D.; Abdel-Mottaleb, M.M.A.; Hahn, T.; Schaefer, U.F.; Neumann, D. Finite and infinite dosing: Difficulties in measurements, evaluations and predictions. Adv. Drug Deliv. Rev. 2013, 65, 278–294. [Google Scholar] [CrossRef] [PubMed]
- Zhai, H.; Maibach, H.I. Effects of Skin Occlusion on Percutaneous Absorption: An Overview. Skin Pharm. Physiol. 2001, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Treffel, P.; Muret, P.; Muret-D’Aniello, P.; Coumes-Marquet, S.; Agache, P. Effect of occlusion on in vitro percutaneus absorption of two compounds with different physicochemical properties. Skin Pharm. Physiol. 1992, 5, 108–113. [Google Scholar] [CrossRef]
- Zhai, H.; Maibach, H.I. Occlusion vs. skin barrier function: Occlusion versus skin barrier function. Skin Res. Technol. 2002, 8, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Chavez, J.J.; Merino-Sanjuán, V.; López-Cervantes, M.; Urban-Morlan, Z.; Piñón-Segundo, E.; Quintanar-Guerrero, D.; Ganem-Quintanar, A. The Tape-Stripping Technique as a Method for Drug Quantification in Skin. J. Pharm. Pharm. Sci. 2008, 11, 104. [Google Scholar] [CrossRef] [PubMed]
- Klang, V.; Schwarz, J.C.; Lenobel, B.; Nadj, M.; Auböck, J.; Wolzt, M.; Valenta, C. In vitro vs. in vivo tape stripping: Validation of the porcine ear model and penetration assessment of novel sucrose stearate emulsions. Eur. J. Pharm. Biopharm. 2012, 80, 604–614. [Google Scholar] [CrossRef] [PubMed]
- Pailler-Mattei, C.; Guerret-Piecourt, C.; Zahouani, H.; Nicoli, S. Interpretation of the human skin biotribological behaviour after tape stripping. J. R. Soc. Interface 2011, 8, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Lademann, J.; Jacobi, U.; Surber, C.; Weigmann, H.-J.; Fluhr, J.W. The tape stripping procedure–evaluation of some critical parameters. Eur. J. Pharm. Biopharm. 2009, 72, 317–323. [Google Scholar] [CrossRef]
- DB ALM-Tape Stripping. Available online: https://ecvam-dbalm.jrc.ec.europa.eu/ (accessed on 2 April 2019).
- Nagelreiter, C.; Mahrhauser, D.; Wiatschka, K.; Skipiol, S.; Valenta, C. Importance of a suitable working protocol for tape stripping experiments on porcine ear skin: Influence of lipophilic formulations and strip adhesion impairment. Int. J. Pharm. 2015, 491, 162–169. [Google Scholar] [CrossRef]
- Zhang, L.W.; Monteiro-Riviere, N.A. Use of confocal microscopy for nanoparticle drug delivery through skin. J. Biomed. Opt. 2012, 18, 061214. [Google Scholar] [CrossRef]
- Förster, M.; Bolzinger, M.-A.; Montagnac, G.; Briançon, S. Confocal Raman microspectroscopy of the skin. Eur. J. Dermatol. 2011, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, V.; Pfeiffer, S.; Lanzendörfer, G.; Wenck, H.; Diembeck, W.; Gooris, G.S.; Proksch, E.; Bouwstra, J. Barrier Characteristics of Different Human Skin Types Investigated with X-Ray Diffraction, Lipid Analysis, and Electron Microscopy Imaging. J. Investig. Derm. 2000, 114, 654–660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hofland, H.E.J.; Bouwstra, J.A.; Boddé, H.E.; Spies, F.; Junginger, H.E. Interactions between liposomes and human stratum corneum in vitro: Freeze fracture electron microscopical visualization and small angle X-ray scattering studies. Br. J. Derm. 2010, 132, 853–866. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; An, Y.; Liao, Y. A novel peptide-based fluorescent chemosensor for Cd(II) ions and its applications in bioimaging. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 216, 61–68. [Google Scholar] [CrossRef] [PubMed]
- König, K.; Ehlers, A.; Stracke, F.; Riemann, I. In vivo Drug Screening in Human Skin Using Femtosecond Laser Multiphoton Tomography. Skin Pharm. Physiol. 2006, 19, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Ashtikar, M.; Matthäus, C.; Schmitt, M.; Krafft, C.; Fahr, A.; Popp, J. Non-invasive depth profile imaging of the stratum corneum using confocal Raman microscopy: First insights into the method. Eur. J. Pharm. Sci. 2013, 50, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Caspers, P.J.; Lucassen, G.W.; Carter, E.A.; Bruining, H.A.; Puppels, G.J. In Vivo Confocal Raman Microspectroscopy of the Skin: Noninvasive Determination of Molecular Concentration Profiles. J. Investig. Derm. 2001, 116, 434–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunn, K.W.; Young, P.A. Principles of Multiphoton Microscopy. Nephron Exp. Nephrol. 2006, 103, e33–e40. [Google Scholar] [CrossRef] [PubMed]
- Imanishi, Y.; Lodowski, K.H.; Koutalos, Y. Two-Photon Microscopy: Shedding Light on the Chemistry of Vision. Biochemistry 2007, 46, 9674–9684. [Google Scholar] [CrossRef] [Green Version]
- Plasencia, I.; Norlén, L.; Bagatolli, L.A. Direct Visualization of Lipid Domains in Human Skin Stratum Corneum’s Lipid Membranes: Effect of pH and Temperature. Biophys. J. 2007, 93, 3142–3155. [Google Scholar] [CrossRef]
- Umino, Y.; Ipponjima, S.; Denda, M. Modulation of lipid fluidity likely contributes to the fructose/xylitol-induced acceleration of epidermal permeability barrier recovery. Arch. Derm. Res. 2019, 311, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Batista, A.; Breunig, H.G.; Uchugonova, A.; Morgado, A.M.; König, K. Two-photon spectral fluorescence lifetime and second-harmonic generation imaging of the porcine cornea with a 12-femtosecond laser microscope. J. Biomed. Opt. 2016, 21, 036002. [Google Scholar] [CrossRef] [PubMed]
- Carrer, D.C.; Vermehren, C.; Bagatolli, L.A. Pig skin structure and transdermal delivery of liposomes: A two photon microscopy study. J. Control. Release 2008, 132, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Hanson, K.M.; Behne, M.J.; Barry, N.P.; Mauro, T.M.; Gratton, E.; Clegg, R.M. Two-Photon Fluorescence Lifetime Imaging of the Skin Stratum Corneum pH Gradient. Biophys. J. 2002, 83, 1682–1690. [Google Scholar] [CrossRef] [Green Version]
- Verma, D.D.; Verma, S.; Blume, G.; Fahr, A. Liposomes increase skin penetration of entrapped and non-entrapped hydrophilic substances into human skin: A skin penetration and confocal laser scanning microscopy study. Eur. J. Pharm. Biopharm. 2003, 55, 271–277. [Google Scholar] [CrossRef]
- Alvarez-Román, R.; Naik, A.; Kalia, Y.N.; Fessi, H.; Guy, R.H. Visualization of skin penetration using confocal laser scanning microscopy. Eur. J. Pharm. Biopharm. 2004, 58, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Vardaxis, N.J.; Brans, T.A.; Boon, M.E.; Kreis, R.W.; Marres, L.M. Confocal laser scanning microscopy of porcine skin: Implications for human wound healing studies. J. Anat. 1997, 190, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Simonetti, O.; Kempenaar, J.A.; Ponec, M.; Hoogstraate, A.J.; Bialik, W.; Schrijvers, A.H.G.J.; Boddé, H.E. Visualization of diffusion pathways across the stratum corneum of native and in-vitro-reconstructed epidermis by confocal laser scanning microscopy. Arch. Derm. Res. 1995, 287, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Zellmer, S.; Reissig, D.; Lasch, J. Reconstructed human skin as model for liposome–skin interaction. J. Control. Release 1998, 55, 271–279. [Google Scholar] [CrossRef]
- van Kuijk-Meuwissen, M.E.M.J.; Mougin, L.; Junginger, H.E.; Bouwstra, J.A. Application of vesicles to rat skin in vivo: A confocal laser scanning microscopy study. J. Control. Release 1998, 56, 189–196. [Google Scholar] [CrossRef]
- van Kuijk-Meuwissen, M.E.M.J.; Junginger, H.E.; Bouwstra, J.A. Interactions between liposomes and human skin in vitro, a confocal laser scanning microscopy study. Biochim. Et Biophys. Acta (Bba)-Biomembr. 1998, 1371, 31–39. [Google Scholar] [CrossRef] [Green Version]
- Touitou, E.; Godin, B.; Dayan, N.; Weiss, C.; Piliponsky, A.; Levi-Schaffer, F. Intracellular delivery mediated by an ethosomal carrier. Biomaterials 2001, 22, 3053–3059. [Google Scholar] [CrossRef]
- Grams, Y.Y.; Alaruikka, S.; Lashley, L.; Caussin, J.; Whitehead, L.; Bouwstra, J.A. Permeant lipophilicity and vehicle composition influence accumulation of dyes in hair follicles of human skin. Eur. J. Pharm. Sci. 2003, 18, 329–336. [Google Scholar] [CrossRef]
- Grams, Y.Y.; Whitehead, L.; Cornwell, P.; Bouwstra, J.A. Time and depth resolved visualisation of the diffusion of a lipophilic dye into the hair follicle of fresh unfixed human scalp skin. J. Control. Release 2004, 98, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Ji, C.; Miao, M.; Yang, K.; Luo, Y.; Hoptroff, M.; Collins, L.Z.; Janssen, H.-G. Ex-vivo measurement of scalp follicular infundibulum delivery of zinc pyrithione and climbazole from an anti-dandruff shampoo. J. Pharm. Biomed. Anal. 2017, 143, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Binder, L.; SheikhRezaei, S.; Baierl, A.; Gruber, L.; Wolzt, M.; Valenta, C. Confocal Raman spectroscopy: In vivo measurement of physiological skin parameters–A pilot study. J. Derm. Sci. 2017, 88, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, N.; Matsumoto, M.; Sakai, S. In vivo measurement of the water content in the dermis by confocal Raman spectroscopy. Skin Res. Technol. 2010, 16, 137–141. [Google Scholar] [CrossRef]
- Sigurdsson, S.; Philipsen, P.A.; Hansen, L.K.; Larsen, J.; Gniadecka, M.; Wulf, H.C. Detection of Skin Cancer by Classification of Raman Spectra. IEEE Trans. Biomed. Eng. 2004, 51, 1784–1793. [Google Scholar] [CrossRef] [Green Version]
- Bakonyi, M.; Gácsi, A.; Kovács, A.; Szűcs, M.-B.; Berkó, S.; Csányi, E. Following-up skin penetration of lidocaine from different vehicles by Raman spectroscopic mapping. J. Pharm. Biomed. Anal. 2018, 154, 1–6. [Google Scholar] [CrossRef]
- Berkó, S.; Zsikó, S.; Deák, G.; Gácsi, A.; Kovács, A.; Budai-Szűcs, M.; Pajor, L.; Bajory, Z.; Csányi, E. Papaverine hydrochloride containing nanostructured lyotropic liquid crystal formulation as a potential drug delivery system for the treatment of erectile dysfunction. Drug Des. Dev. 2018, 12, 2923–2931. [Google Scholar] [CrossRef]
- Ilchenko, O.; Pilgun, Y.; Makhnii, T.; Slipets, R.; Reynt, A.; Kutsyk, A.; Slobodianiuk, D.; Koliada, A.; Krasnenkov, D.; Kukharskyy, V. High-speed line-focus Raman microscopy with spectral decomposition of mouse skin. Vib. Spectrosc. 2016, 83, 180–190. [Google Scholar] [CrossRef]
- Pyatski, Y.; Zhang, Q.; Mendelsohn, R.; Flach, C.R. Effects of permeation enhancers on flufenamic acid delivery in Ex vivo human skin by confocal Raman microscopy. Int. J. Pharm. 2016, 505, 319–328. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, L.; Téllez, S.C.A.; Sousa, M.P.J.; Azoia, N.G.; Cavaco-Paulo, A.M.; Martin, A.A.; Favero, P.P. In vivo confocal Raman spectroscopy and molecular dynamics analysis of penetration of retinyl acetate into stratum corneum. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 174, 279–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, G.P.S.; McGoverin, C.M.; Fraser, S.J.; Gordon, K.C. Raman imaging of drug delivery systems. Adv. Drug Deliv. Rev. 2015, 89, 21–41. [Google Scholar] [CrossRef] [PubMed]
- Franzen, L.; Windbergs, M. Applications of Raman spectroscopy in skin research—From skin physiology and diagnosis up to risk assessment and dermal drug delivery. Adv. Drug Deliv. Rev. 2015, 89, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Vajna, B. Multivariate Curve Resolution and Regression Methods in Raman Chemical Imaging. Ph.D. Thesis, Budapest University of Technology and Economics, Budapest, Hungary, 30 November 2012. [Google Scholar]
- Zhang, G.; Moore, D.J.; Sloan, K.B.; Flach, C.R.; Mendelsohn, R. Imaging the Prodrug-to-Drug Transformation of a 5-Fluorouracil Derivative in Skin by Confocal Raman Microscopy. J. Investig. Derm. 2007, 127, 1205–1209. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Flach, C.R.; Mendelsohn, R. Tracking the dephosphorylation of resveratrol triphosphate in skin by confocal Raman microscopy. J. Control. Release 2007, 123, 141–147. [Google Scholar] [CrossRef] [Green Version]
- Freudiger, C.W.; Min, W.; Saar, B.G.; Lu, S.; Holtom, G.R.; He, C.; Tsai, J.C.; Kang, J.X.; Xie, X.S. Label-Free Biomedical Imaging with High Sensitivity by Stimulated Raman Scattering Microscopy. Science 2008, 322, 1857–1861. [Google Scholar] [CrossRef] [Green Version]
- Mélot, M.; Pudney, P.D.A.; Williamson, A.-M.; Caspers, P.J.; Van Der Pol, A.; Puppels, G.J. Studying the effectiveness of penetration enhancers to deliver retinol through the stratum cornum by in vivo confocal Raman spectroscopy. J. Control. Release 2009, 138, 32–39. [Google Scholar] [CrossRef]
- Gotter, B.; Faubel, W.; Neubert, R.H.H. FTIR microscopy and confocal Raman microscopy for studying lateral drug diffusion from a semisolid formulation. Eur. J. Pharm. Biopharm. 2010, 74, 14–20. [Google Scholar] [CrossRef]
- Saar, B.G.; Contreras-Rojas, L.R.; Xie, X.S.; Guy, R.H. Imaging Drug Delivery to Skin with Stimulated Raman Scattering Microscopy. Mol. Pharm. 2011, 8, 969–975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franzen, L.; Selzer, D.; Fluhr, J.W.; Schaefer, U.F.; Windbergs, M. Towards drug quantification in human skin with confocal Raman microscopy. Eur. J. Pharm. Biopharm. 2013, 84, 437–444. [Google Scholar] [CrossRef] [PubMed]









| IVRT | IVPT |
|---|---|
| Synthetic membrane | Human skin |
| Occluded dose | Unoccluded dose |
| Infinite dose | Finite dose |
| Release rate | Flux profile |
| µg to mg range | pg to ng range |
| Relative consistency | Donor variability |
| Researcher | Membrane/Skin Model | Main Topic |
|---|---|---|
| Jung Dae Lee et al. [50] | full-thickness Sprague-Dawley rat skin | permeability of 1-phenoxy-2-propanol in a shampoo and a cream |
| Yanling Zhang et al. [51] | heat separated human epidermis and full-thickness porcine ear skin; skin-PAMPA membrane | comparison of the Franz cell methods with different membranes and different doses to the skin-PAMPA method |
| Sonia Trombino et al. [52] | dialysis membranes and rabbit ear skin | cyclosporin-A incorporated in SLN for the topical treatment of psoriasis |
| Oludemi Taofiq et al. [53] | pig ear skin | studied mushroom ethanolic extracts |
| Dina Ameen et al. [54] | dermatomed human cadaver skin | matrix–type patches for the transdermal delivery of galantamine for the treatment of Alzheimer’s disease |
| Marcelle Silva-Abreu et al. [55] | dialysis membrane and porcine mucosa (buccal, sublingual, nasal and intestinal) | oral solution of pioglitazone for the treatment of Alzheimer’s disease |
| Rattikorn Intarakumhaeng et al. [56] | heat-separated torso split-thickness cadaver skin | skin permeation of urea at finite dose and comparison to infinite dose condition |
| Panonnummal Rajitha et al. [57] | cellophane membrane, isolated pig ear skin and full-thickness pig ear skin | methotrexate loaded topical nanoemulsion for the treatment of psoriasis |
| Sahar Salehi et al. [58] | rat buccal mucosa | mucoadhesive buccal film containing rizatriptan benzoate and propranolol hydrochloride |
| José L. Soriano-Ruiz et al. [59] | nylon, cellulose and polysulfone membranes; porcine buccal mucosa, porcine sublingual, vaginal mucosa | multiple emulsion for the topical application of clotrimazole |
| Luciano Serpe et al. [60] | porcine palatal mucosa | permeation profiles of lidocaine and prilocaine across the palatal mucosa without bone |
| Woan-Ruoh Lee et al. [61] | nude mouse skin | fractional CO2 laser effect on drug penetration and absorption |
| Ahmed O.H. El-Nezhawy et al. | cellophane membrane | amidoalkylating agent and SLNs with antimicrobial activity |
| Researcher | Year | Main Topic |
|---|---|---|
| Lee et al. [68] | 2010 |
|
| Tsinman et al. [69] | 2012 |
|
| Karadzovska and Riviere [65] | 2013 |
|
| Vizserálek et al. [70] | 2013 |
|
| Clough et al. [71] | 2013 |
|
| Luo et al. [72] | 2014 |
|
| Researcher | Year | Main Topic |
|---|---|---|
| Hanson et al. [99] | 2002 |
|
| Plasencia et al. [95] | 2007 |
|
| Carrer et al. [98] | 2008 |
|
| Batista et al. [97] | 2016 |
|
| Umino et al. [96] | 2019 |
|
| Researcher | Year | Main Topic |
|---|---|---|
| Simonetti et al. [103] | 1995 |
|
| Zellmer et al. [104] | 1998 |
|
| Kuijk-Meuwissen et al. [105,106] | 1998 |
|
| Touitou et al. [107] | 2001 |
|
| Grams et al. [108,109] | 2003 2004 |
|
| Alvarez-Roman et al. [101] | 2004 |
|
| Patzelt et al. [19] | 2011 |
|
| Researcher | Year | Main Topic |
|---|---|---|
| Zhang et al. [122,123] | 2007 |
|
| Freudiger et al. [124] | 2008 |
|
| Melot et al. [125] | 2009 |
|
| Gotter et al. [126] | 2010 |
|
| Saar et al. [127] | 2011 |
|
| Franzen et al. [128] | 2013 |
|
| Smith et al. [119] | 2015 |
|
| Ilchenko et al. [116] | 2016 |
|
| Berkó et al. [115] | 2018 |
|
| Bakonyi et al. [114] | 2018 |
|
| Method | Advantages | Disadvantages |
|---|---|---|
| Franz Diffusion |
|
|
| Skin-PAMPA |
|
|
| Tape-Stripping |
|
|
| Microscopic and Spectroscopic Methods |
|
|
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zsikó, S.; Csányi, E.; Kovács, A.; Budai-Szűcs, M.; Gácsi, A.; Berkó, S. Methods to Evaluate Skin Penetration In Vitro. Sci. Pharm. 2019, 87, 19. https://doi.org/10.3390/scipharm87030019
Zsikó S, Csányi E, Kovács A, Budai-Szűcs M, Gácsi A, Berkó S. Methods to Evaluate Skin Penetration In Vitro. Scientia Pharmaceutica. 2019; 87(3):19. https://doi.org/10.3390/scipharm87030019
Chicago/Turabian StyleZsikó, Stella, Erzsébet Csányi, Anita Kovács, Mária Budai-Szűcs, Attila Gácsi, and Szilvia Berkó. 2019. "Methods to Evaluate Skin Penetration In Vitro" Scientia Pharmaceutica 87, no. 3: 19. https://doi.org/10.3390/scipharm87030019
APA StyleZsikó, S., Csányi, E., Kovács, A., Budai-Szűcs, M., Gácsi, A., & Berkó, S. (2019). Methods to Evaluate Skin Penetration In Vitro. Scientia Pharmaceutica, 87(3), 19. https://doi.org/10.3390/scipharm87030019





