Long-Lived Levels in Multiply and Highly Charged Ions
Abstract
:1. Introduction
2. What Is “Metastability”?
3. Atomic Structure Concepts and Geometry
Line Ratio
4. H-, He-, and Li-like Atomic Systems
4.1. H-like Atoms and Ions
4.2. He-like Atoms and Ions
4.3. Ions with Hyperfine Interaction
4.4. Li-like Atoms and Ions
5. Examples of Many-Electron Ions
5.1. Intercombination Transitions
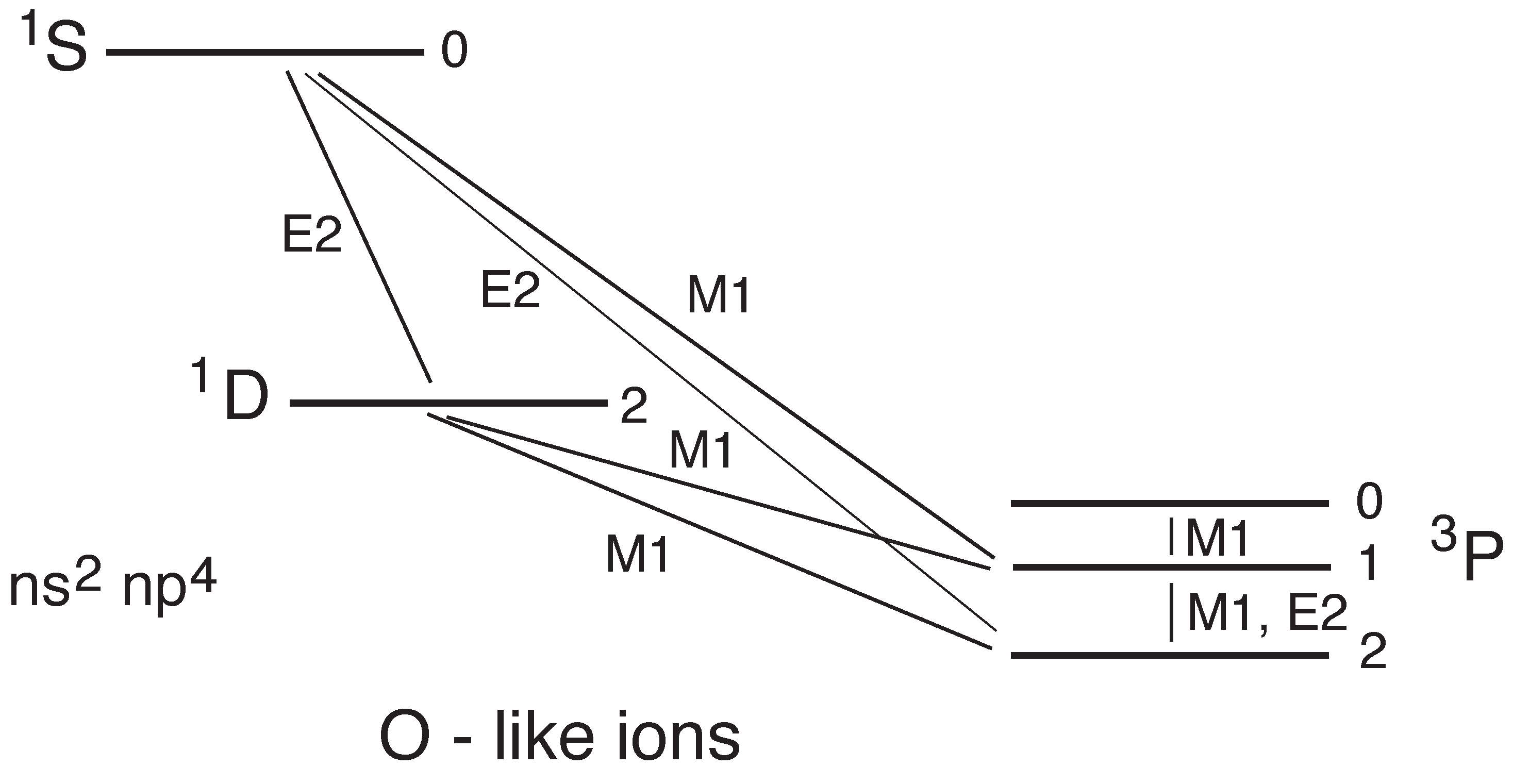
5.2. -Forbidden Transitions in the Ground Complex
5.3. Ne-like Ions
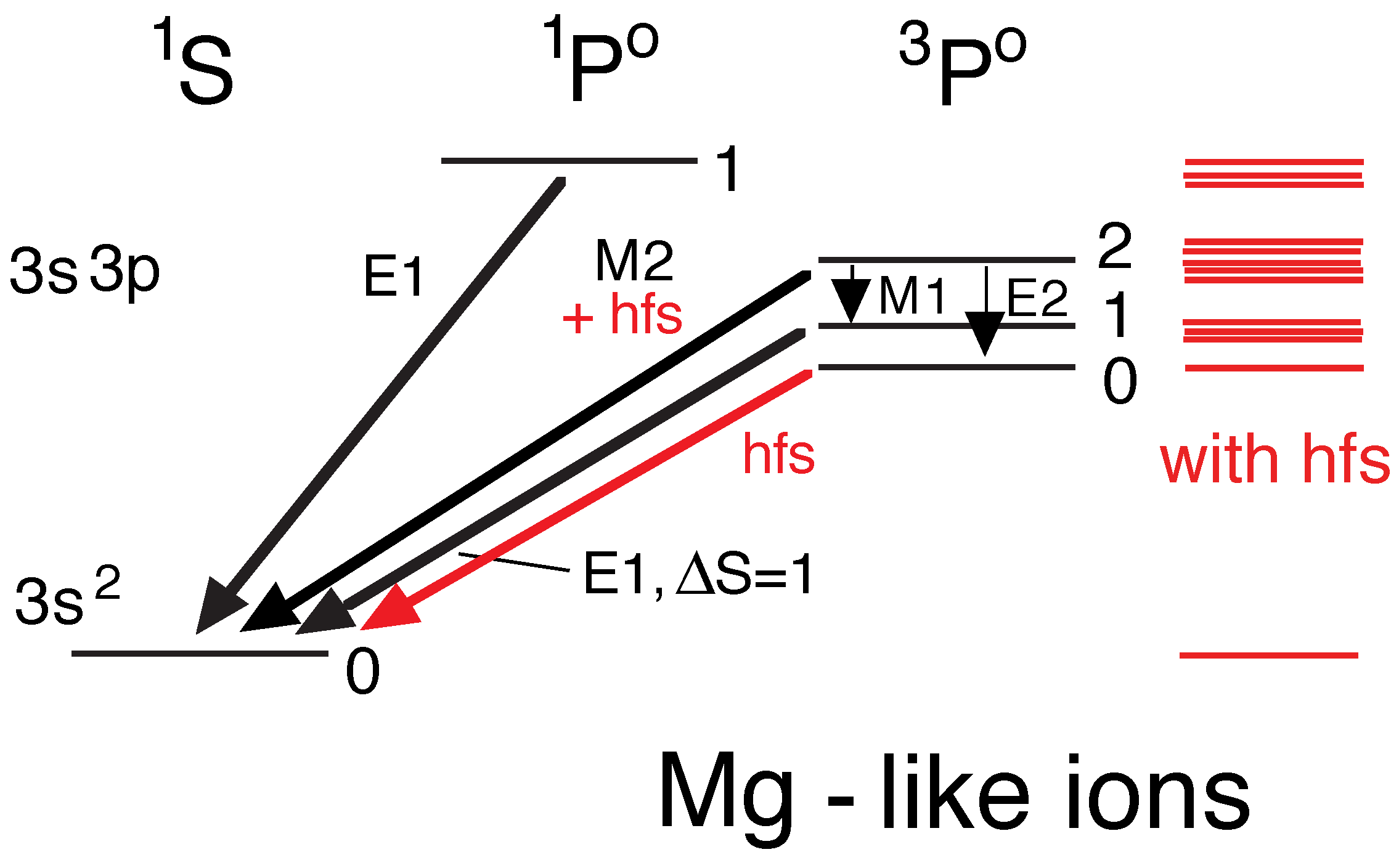
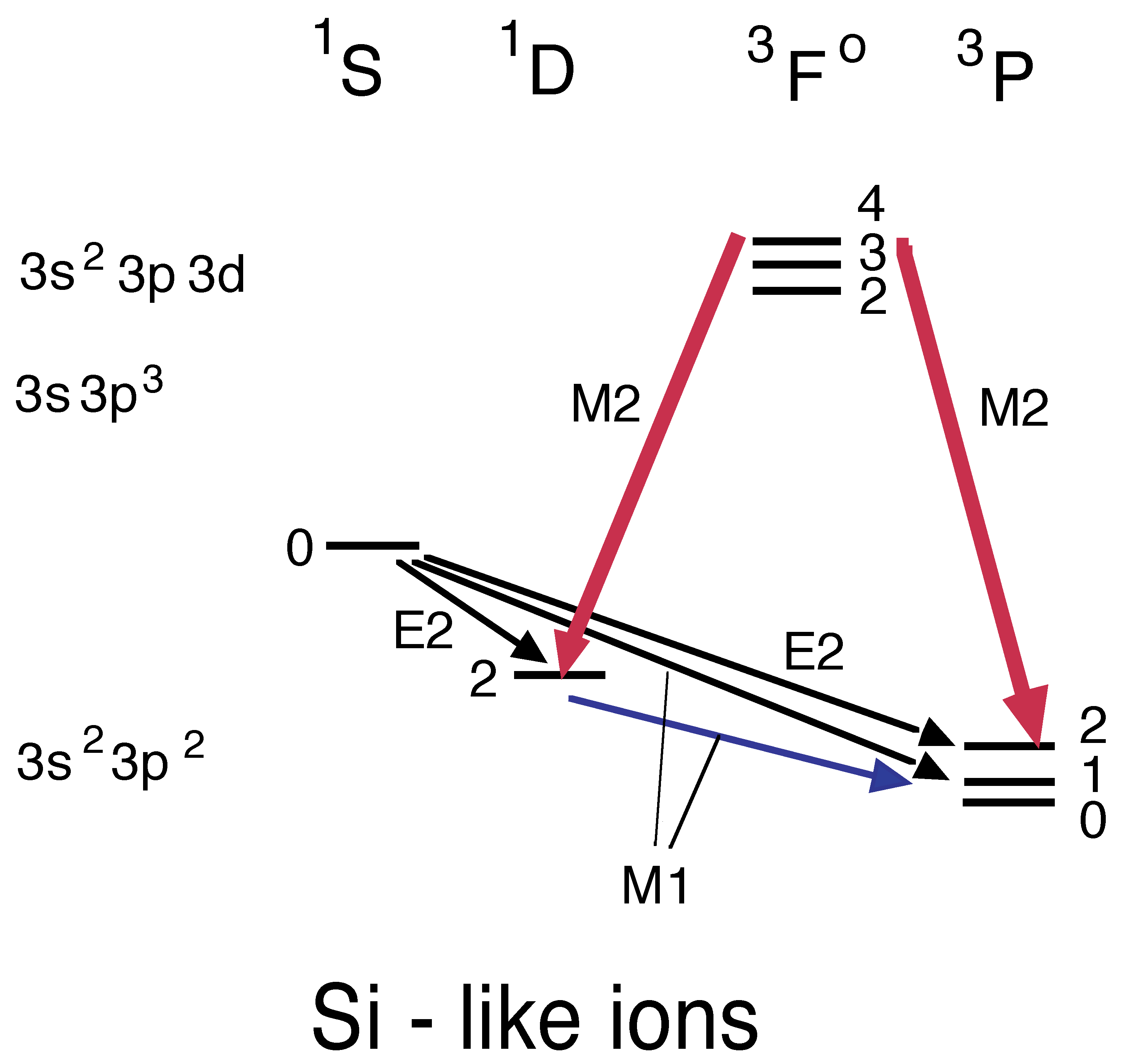
5.4. Mg- through Cl-Like Ions
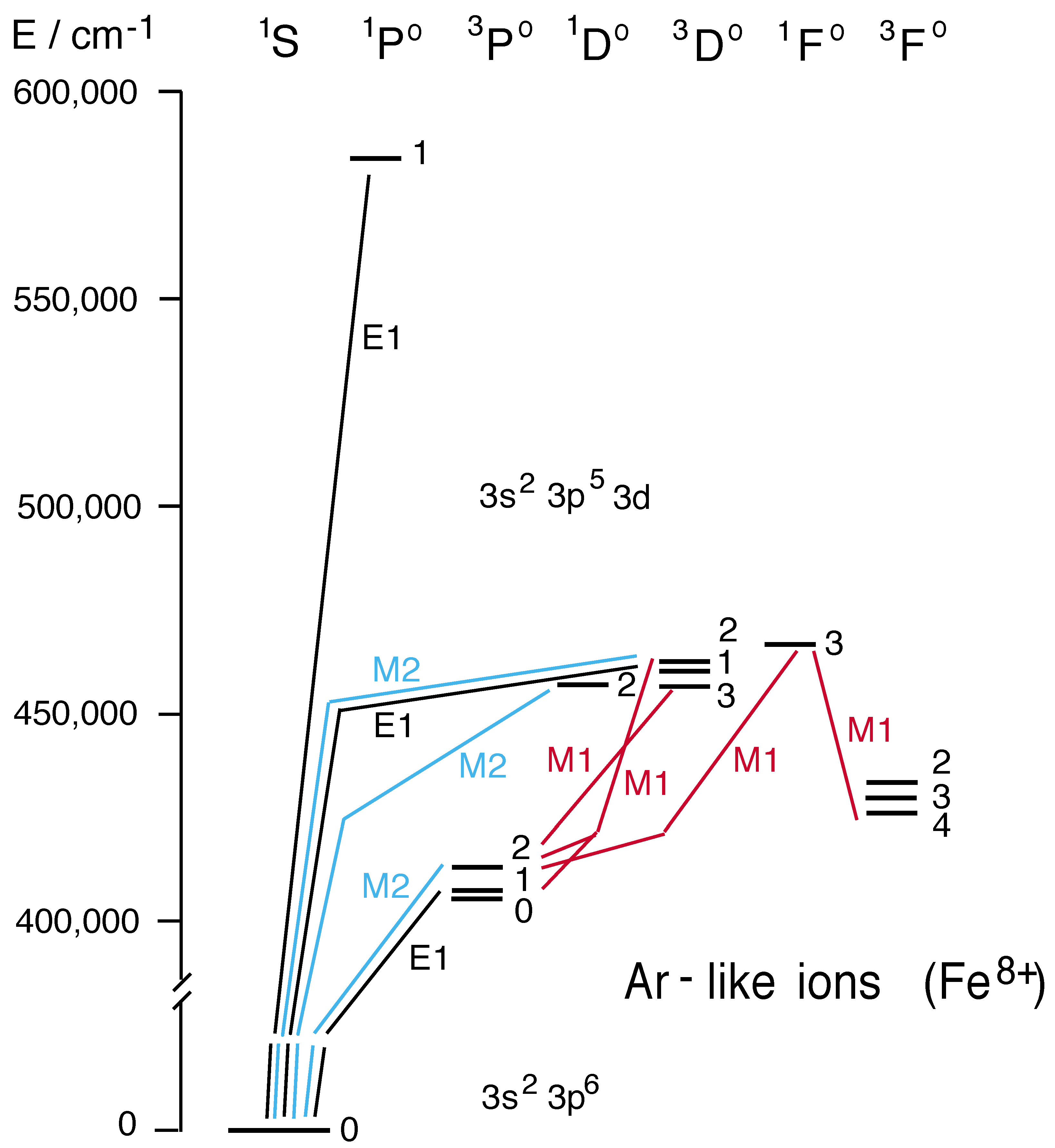
5.5. Ar-like Ions
5.6. K-like Ions
5.7. Ni-like Ions
5.8. Hyperfine Structure Again
5.9. Assorted Samples
6. Computations
7. Discussion
Funding
Data Availability Statement
Conflicts of Interest
References
- Träbert, E. On atomic lifetimes and environmental density. Atoms 2022, 10, 114. [Google Scholar] [CrossRef]
- Lange, R.; Peshkov, A.A.; Huntemann, N.; Tamm, C.; Surzhykov, A.; Peik, E. Lifetime of the 2F7/2 level in Yb+ for spontaneous emission of electric octupole radiation. Phys. Rev. Lett. 2021, 127, 213001. [Google Scholar] [CrossRef]
- Kramida, A.; Ralchenko, Y.; Reader, J.; NIST ASD Team. NIST Atomic Spectra Database; Version 5.7.1; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2019. Available online: https://physics.nist.gov/asd (accessed on 1 June 2021).
- Bertschinger, G.; Schmid, H.; von Buttlar, H. Herkunft des Nachleuchtens in einer gepulsten Argonentladung (Origin of the afterglow in a pulsed argon discharge). Z. Phys. A 1974, 268, 129–132. (In German) [Google Scholar] [CrossRef]
- Träbert, E.; Schmid, H.; von Buttlar, H. VUV-Nachleuchten einer gepulsten Koronaentladung in reinem Argon (VUV afterglow of a pulsed corona discharge in pure argon). Z. Phys. A 1975, 274, 27–32. (In German) [Google Scholar] [CrossRef]
- Laporte, O. Die Struktur des Eisenspektrums. Z. Phys. 1924, 23, 135–175. [Google Scholar] [CrossRef]
- Martin, W.C.; Wiese, W.L. Atomic Spectroscopy. Available online: http://sed.nist.gov/Pubs/AtSpec/total.html (accessed on 1 December 2023).
- Martin, W.C.; Wiese, W.L.; Kramida, A. Atomic Spectroscopy. In Springer Handbook of Atomic, Molecular, and Optical Physics, 2nd ed.; Drake, G.W.F., Ed.; Chapter 11; Springer Nature: Cham, Switzerland, 2023; pp. 177–197. [Google Scholar]
- Drake, G.W.F. (Ed.) Springer Handbook of Atomic, Molecular, and Optical Physics, 2nd ed.; Springer Nature: Cham, Switzerland, 2023. [Google Scholar]
- Träbert, E.; Saathoff, G.; Wolf, A. N II electric quadrupole transition rate measured at a heavy-ion storage ring. Phys. Scr. 2005, 72, 35–37. [Google Scholar] [CrossRef]
- Hylleraas, E.A. Über den Grundterm der Zweielektronenprobleme von H−, He, Li+, Be++ usw. Z. Phys. 1930, 65, 209–225. (In German) [Google Scholar] [CrossRef]
- White, H.E.; Eliason, A.Y. Relative intensity tables for spectrum lines. Phys. Rev. 1933, 44, 753–756. [Google Scholar] [CrossRef]
- Chen, H.; Beiersdorfer, P.; Gu, M.-F.; Heeter, L.A.; Lepson, J.K.; Liedahl, D.A.; Naranjo-Rivera, K.L.; Träbert, E. Experimental and theoretical evaluation of density-sensitive N VI, Ar XIV, and Fe XXII line ratios. Astrophys. J. 2004, 611, 598–604. [Google Scholar] [CrossRef]
- Jitrik, O.; Bunge, C.F. Transition probabilities for hydrogen-like atoms. J. Phys. Chem. Ref. Data 2004, 33, 1059–1070. [Google Scholar] [CrossRef]
- Lamb, W.E.; Retherford, R.C. Fine Structure of the Hydrogen Atom by a Microwave Method. Phys. Rev. 1947, 72, 241–243. [Google Scholar] [CrossRef]
- Pritchard, J.R.; Loeb, A. 21 cm cosmology in the 21st century. Rep. Prog. Phys. 2012, 75, 086901. [Google Scholar] [CrossRef]
- Hannen, V.; Vollbrecht, J.; Andelkovic, Z.; Brandau, C.; Dax, A.; Geithner, W.; Geppert, C.; Gorges, C.; Hammen, M.; Kaufmann, S.; et al. Lifetimes and g-factors of the HFS states in H-like and Li-like bismuth. J. Phys. B At. Mol. Opt. Phys. 2019, 52, 085003. [Google Scholar] [CrossRef]
- Drake, G.W.F. Spontaneous two-photon decay rates in hydrogenlike and heliumlike ions. Phys. Rev. A 1986, 34, 2871–2880. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W.R.; Plante, D.R.; Sapirstein, J. Relativistic calculations of transition amplitudes in the helium isoelectronic sequence. Adv. At. Mol. Opt. Phys. 1995, 35, 255–329. [Google Scholar]
- Träbert, E.; Pinnington, E.H. Spectroscopy of ions using fast beams and ion traps. In Springer Handbook of Atomic, Molecular, and Optical Physics, 2nd ed.; Drake, G.W.F., Ed.; Chapter 19; Springer Nature: Cham, Switzerland, 2023; pp. 269–281. [Google Scholar]
- Drake, G.W.F. Theoretical energies for the n = 1 and 2 states of the helium isooelectronic sequence up to Z = 100. Can. J. Phys. 1988, 66, 586–611. [Google Scholar] [CrossRef]
- Marrs, R.E.; Beiersdorfer, P.; Schneider, D.H. The electron beam ion trap. Phys. Today 1994, 47, 27–34. [Google Scholar] [CrossRef]
- Beiersdorfer, P. A “brief" history of spectroscopy on EBIT. Can. J. Phys. 2008, 86, 1–10. [Google Scholar] [CrossRef]
- Birkett, B.B.; Briand, J.-P.; Charles, P.; Dietrich, D.D.; Finlayson, K.; Indelicato, P.; Liesen, D.; Simionovici, A. Hyperfine quenching and measurement of the 2 3P0—2 3P1 fine-structure splitting in heliumlike silver (Ag45+). Phys. Rev. A 1993, 47, R2454–R2457. [Google Scholar] [CrossRef]
- Marrus, R.W.; Schmieder, R.W. Observation of the magnetic-quadrupole decay (2 3P2 → 1 1S0) of heliumlike Ar XVII and lifetime of the 2 3P2 state. Phys. Rev. Lett. 1979, 25, 1689–1691. [Google Scholar] [CrossRef]
- Indelicato, P.; Parente, F.; Marrus, R. Effect of hyperfine structure on the 2 3P1 and the 2 3P0 lifetime in heliumlike ions. Phys. Rev. A 1989, 40, 3505–3514. [Google Scholar] [CrossRef] [PubMed]
- Indelicato, P.; Birkett, B.B.; Briand, J.-P.; Charles, P.; Dietrich, D.D.; Marrus, R.; Simionovici, A. Hyperfine quenching and precision measurement of the 2 3P0 – 2 3P1 fine-structure splitting in heliumlike gadolinium (Gd62+). Phys. Rev. Lett. 1992, 68, 1307–1310. [Google Scholar] [CrossRef] [PubMed]
- Munger, C.T.; Gould, H. Lamb shift in heliumlike uranium (U90+). Phys. Rev. Lett. 1986, 57, 2927–2930. [Google Scholar] [CrossRef]
- Träbert, E. The allure of high total angular momentum levels in multiply-excited ions. Atoms 2019, 7, 103. [Google Scholar] [CrossRef]
- Edlén, B.; Tyrén, F. Atomic energy states of an unusual type. Nature 1939, 3631, 940–941. [Google Scholar] [CrossRef]
- Gabriel, A.H.; Jordan, C. Long wavelength satellites to the He-like ion resonance lines in the laboratory and the sun. Nature 1969, 221, 947–949. [Google Scholar] [CrossRef]
- Gabriel, A.H. Dielectronic satellite spectra for highly-charged helium-like ion lines. Mon. Not. R. Astr. Soc. 1972, 160, 99–119. [Google Scholar] [CrossRef]
- Pietenpol, J.L. Auto-ionization of the (1s2s2p) 4P5/2 level in Li and He−. Phys. Rev. Lett. 1961, 7, 64. [Google Scholar] [CrossRef]
- Beiersdorfer, P.; Bitter, M.; Hey, D.; Reed, K.J. Identification of the 1s2s2p 4P5/2 → 1s22s 2S1/2 magnetic quadrupole inner-shell satellite line in the Ar16+ K-shell X-ray spectrum. Phys. Rev. A 2002, 66, 032504. [Google Scholar] [CrossRef]
- Kaufman, V.; Sugar, J. Forbidden lines in ns2 npk ground configurations and nsnp excited configurations of beryllium through molybdenum atoms and ions. J. Phys. Chem. Ref. Data 1986, 15, 321–426. [Google Scholar] [CrossRef]
- Träbert, E. E1-forbidden transition rates in ions of astrophysical interest. Phys. Scr. T 2014, 89, 114003. [Google Scholar] [CrossRef]
- Church, D.A. Collision measurements and excited-level lifetime measurements on ions stored in Paul, Penning and Kingdon ion traps. Phys. Rep. 1993, 228, 253–358. [Google Scholar] [CrossRef]
- Träbert, E. Atomic lifetime measurements with ion traps of many sizes. Phys. Scr. 2000, 61, 257–286. [Google Scholar] [CrossRef]
- Träbert, E. Precise measurements of long atomic lifetimes using stored ion beams and ion traps. Can. J. Phys. 2002, 80, 1481–1503. [Google Scholar] [CrossRef]
- Mannervik, S. Experimental lifetime studies of metastable levels. Phys. Scr. T 2003, 105, 67–75. [Google Scholar] [CrossRef]
- Träbert, E. Atomic lifetime measurements using electron beam ion traps. Can. J. Phys. 2008, 86, 73–97. [Google Scholar] [CrossRef]
- Träbert, E. Problems with accurate atomic lifetime measurements of multiply charged ions. Phys. Scr. 2009, 79, 068101. [Google Scholar] [CrossRef]
- Träbert, E. In pursuit of high precision atomic lifetime measurements of multiply charged ions. J. Phys. B At. Mol. Opt. Phys. 2010, 43, 074034. [Google Scholar] [CrossRef]
- Träbert, E. Atomic lifetimes of astrophysical interest in ions of Fe. Atoms 2023, 11, 85. [Google Scholar] [CrossRef]
- Träbert, E.; Wolf, A.; Linkemann, J.; Tordoir, X. Measurement of the B+ and Al+ intercombination and Sc12+ forbidden transition rates at a heavy-ion storage ring. J. Phys. B At. Mol. Opt. Phys. 1999, 32, 537–552. [Google Scholar] [CrossRef]
- Doerfert, J.S.; Träbert, E.; Wolf, A.; Schwalm, D.; Uwira, O. Precision measurement of the electric dipole intercombination rate in C2+. Phys. Rev. Lett. 1997, 78, 4355–4358. [Google Scholar] [CrossRef]
- Träbert, E.; Knystautas, E.J.; Saathoff, G.; Wolf, A. EUV intercombination transition rates in Be-like nitrogen and oxygen ions measured at a heavy-ion storage ring. J. Phys. B At. Mol. Opt. Phys. 2005, 38, 2395–2405. [Google Scholar] [CrossRef]
- Träbert, E. Radiative-lifetime measurements on highly-charged ions. In Accelerator-Based Atomic Physics Techniques and Applications; Shafroth, S.M., Austin, J.C., Eds.; American Institute of Physics: Washington, DC, USA, 1997; pp. 567–607. [Google Scholar]
- Träbert, E.; Curtis, L.J. Isoelectronic trends of line strength data in the Li and Be isoelectronic sequences. Phys. Scr. 2006, 74, C46. [Google Scholar] [CrossRef]
- Jönsson, P.; Froese Fischer, C.; Träbert, E. On the status and perspectives of MCDF computations and measurements of transition data in the Be isoelectronic sequence. J. Phys. B At. Mol. Opt. Phys. 1998, 31, 3497–3511. [Google Scholar] [CrossRef]
- Chen, M.H.; Cheng, K.T.; Johnson, W.R. Large-scale relativistic configuration-interaction computation of the 2s2 1S0 – 2s2p 3P1 intercombination transition in C III. Phys. Rev. A 2001, 64, 042507. [Google Scholar] [CrossRef]
- Träbert, E.; Heckmann, P.H.; von Buttlar, H. Beam-foil lifetimes of highly ionized silicon. Z. Phys. A 1977, 281, 333–339. [Google Scholar] [CrossRef]
- Cheng, K.T.; Kim, Y.-K.; Desclaux, J.P. Electric dipole, quadrupole, and magnetic dipole transition probabilities of ions isoelectronic to the first-row atoms, Li through F. At. Data Nucl. Data Tab. 1979, 24, 111–189. [Google Scholar] [CrossRef]
- Träbert, E.; Gwinner, G.; Knystautas, E.J.; Tordoir, X.; Wolf, A. Precise intercombination transition rates in C+ and N2+ ions measured at a heavy-ion storage ring. J. Phys. B At. Mol. Opt. Phys. 1999, 32, L491–L499. [Google Scholar] [CrossRef]
- Curtis, L.J. Lifetime measurements in highly ionised atoms. Phys. Scr. 1984, 8, 77–83. [Google Scholar] [CrossRef]
- Moehs, D.P.; Church, D.A. Magnetic dipole transition rates from measured lifetimes of levels of Be-like and B-like argon ions. Phys. Rev. A 1998, 58, 1111–1114. [Google Scholar] [CrossRef]
- Träbert, E.; Beiersdorfer, P.; Utter, S.B.; Brown, G.V.; Chen, H.; Harris, C.L.; Neill, P.A.; Savin, D.W.; Smith, A.J. Experimental M1 transition rates of coronal lines from Ar X, Ar XIV, and Ar XV. Astrophys. J. 2000, 541, 506–511. [Google Scholar] [CrossRef]
- Lapierre, A.; Crespo López-Urrutia, J.R.; Braun, J.; Brenner, G.; Bruhns, H.; Fischer, D.; González-Martínez, A.J.; Mironov, V.; Osborne, C.J.; Sikler, G.; et al. Lifetime measurement of the Ar XIV 1s22s22p 2P metastable level at the Heidelberg electron-beam ion trap. Phys. Rev. A 2006, 73, 052507. [Google Scholar] [CrossRef]
- Pasternack, S. Transition probabilities of forbidden lines. Astrophys. J. 1940, 92, 129–155. [Google Scholar] [CrossRef]
- Nemouchi, M.; Godefroid, M.R. Irreducible tensor form of the relativistic corrections to the M1 transition operator. J. Phys. B At. Mol. Opt. Phys. 2009, 42, 175002. [Google Scholar] [CrossRef]
- Leopold, T.; King, S.A.; Micke, P.; Bautista-Salvador, A.; Heip, J.C.; Ospelkaus, C.; Crespo López-Urrutia, J.R.; Schmidt, P.O. A cryogenic radio-frequency ion trap for quantum logic spectroscopy of highly charged ions. Rev. Sci. Instrum. 2019, 90, 073201. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, M.G.; Safronova, M.S.; Crespo López-Urrutia, J.R.; Schmidt, P.O. Highly charged ions: Optical clocks and applications in fundamental physics. Rev. Mod. Phys. 2018, 90, 045005. [Google Scholar] [CrossRef]
- Bowen, I.S. The origin of the chief nebular lines. Proc. Astron. Soc. Pac. 1927, 39, 295B. [Google Scholar] [CrossRef]
- Stevenson, A.F. The intensities of certain nebular lines and the mean lives of atoms emitting them. Proc. R. Soc. A 1932, 137, 298–325. [Google Scholar]
- Boyce, J.C.; Menzel, D.H.; Payne, C.H. Forbidden lines in astrophysical sources. Proc. Nat. Acad. Sci. USA 1933, 19, 581–591. [Google Scholar] [CrossRef]
- Bowen, I.S. Forbidden lines. Rev. Mod. Phys. 1936, 8, 55–81. [Google Scholar] [CrossRef]
- Czyzak, S.J.; Krueger, T.K. Forbidden transition probabilities for some P, S, Cl, and A ions. Mon. Not. R. Astron. Soc. 1963, 126, 177–194. [Google Scholar] [CrossRef]
- Czyzak, S.J.; Keyes, C.D.; Aller, L.H. Atomic structure calculations and nebular diagnostics. Astroph. J. Suppl. 1986, 61, 159–175. [Google Scholar] [CrossRef]
- Träbert, E.; Calamai, A.G.; Gillaspy, J.D.; Gwinner, G.; Tordoir, X.; Wolf, A. Intercombination and forbidden transition rates in C- and N-like ions O2+, F3+, and S9+ measured at a heavy-ion storage ring. Phys. Rev. A 2000, 62, 022507. [Google Scholar] [CrossRef]
- Träbert, E.; Wolf, A.; Pinnington, E.H.; Linkemann, J.; Knystautas, E.J.; Curtis, A.; Bhattacharya, N.; Berry, H.G. Heavy-ion storage ring measurement of forbidden transition rates between ground-configuration levels in Si6+ and Si8+ ions. Can. J. Phys. 1998, 76, 899–906. [Google Scholar] [CrossRef]
- Träbert, E.; Grieser, M.; Hoffmann, J.; Krantz, C.; Repnow, R.; Wolf, A. Heavy-ion storage-ring-lifetime measurement of metastable levels in the C-, N-,and O-like ions of Si, P, and S. Phys. Rev. A 2012, 85, 042508. [Google Scholar] [CrossRef]
- Storey, P.J.; Zeippen, C.J. Theoretical values for the [O III] 5007/4959 line-intensity ratio and homologous cases. Mon. Not. R. Astron. Soc. 2000, 312, 813–816. [Google Scholar] [CrossRef]
- Crespo López-Urrutia, J.R.; Beiersdorfer, P. Measurement of the radiative decay rate of the metastable (2s22p3s1/2)(J=2) level in Fe XVII. Astrophys. J. 2010, 721, 576–581. [Google Scholar] [CrossRef]
- Beiersdorfer, P.; Crespo López-Urrutia, J.R.; Träbert, E. Measurement of the radiative decay rate and energy of the metastable (2s22p3s1/2)(J=0) level in Fe XVII. Astrophys. J. 2016, 817, 67. [Google Scholar] [CrossRef]
- Xu, G.; Yan, C.; Lu, Q.; Yang, Y.; Li, W.; Ma, S.; Zhao, Z.; Huang, S.; Song, L.; Si, R.; et al. First laboratory measurement of magnetic-field-induced transition effect in Fe X at different magnetic fields. Astrophys. J. 2022, 937, 48. [Google Scholar] [CrossRef]
- Li, W.; Li, M.; Wang, K.; Brage, T.; Hutton, R.; Landi, E. A theoretical investigation of the magnetic-field-induced transition in Fe X, of importance for measuring magnetic field strengths in the solar corona. Astrophys. J. 2021, 913, 135. [Google Scholar] [CrossRef]
- Träbert, E. Intercombination transition probabilities in two-electron spectra. Phys. Scr. 1990, 41, 675–680. [Google Scholar] [CrossRef]
- Träbert, E.; Hoffmann, J.; Krantz, C.; Wolf, A.; Ishikawa, Y.; Santana, J.A. Atomic lifetime measurements on forbidden transitions of Al-, Si-, P- and S-like ions at a heavy-ion storage ring. J. Phys. B At. Mol. Opt. Phys. 2009, 42, 025002. [Google Scholar] [CrossRef]
- Träbert, E.; Grieser, M.; Krantz, C.; Repnow, R.; Wolf, A.; Diaz, F.J.; Ishikawa, Y.; Santana, J.A. Isoelectronic trends of the E1-forbidden decay rates of Al-, Si-, P-, and S-like ions of Cl, Ti, Mn, Cu, and Ge. J. Phys. B At. Mol. Opt. Phys. 2012, 45, 215003. [Google Scholar] [CrossRef]
- Träbert, E.; Ishikawa, Y.; Santana, J.A.; Del Zanna, G. The 3s23p3d 3Fo term in the Si-like spectrum of Fe (Fe XIII). Can. J. Phys. 2011, 89, 403–412. [Google Scholar] [CrossRef]
- Beiersdorfer, P.; Träbert, E.; Pinnington, E.H. Experimental transition rate of the green coronal line of Fe XIV. Astrophys. J. 2003, 587, 836–840. [Google Scholar] [CrossRef]
- Träbert, E. On the transition rates of the Fe X and Fe XIV coronal lines. Astron. Astrophys. 2004, 415, L39–L42. [Google Scholar] [CrossRef]
- Brenner, G.; Crespo López-Urrutia, J.R.; Harman, Z.; Mokler, P.H.; Ullrich, J. Lifetime determination of the Fe XIV 3s23p 2Po metastable level. Phys. Rev. A 2007, 75, 032504. [Google Scholar] [CrossRef]
- Träbert, E. Differential observations in spectroscopic measurements using electron beam ion traps. J. Phys. B At. Mol. Opt. Phys. 2009, 42, 154019. [Google Scholar] [CrossRef]
- Wang, K.; Song, C.X.; Jönsson, P.; Del Zanna, G.; Schiffmann, S.; Godefroid, M.; Gaigalas, G.; Zhao, X.H.; Si, R.; Chen, C.Y.; et al. Benchmarking atomic data from large-scale multiconfiguration Dirac-Hartree-Fock calculations for astrophysics: S-like ions from Cr IX to Cu XIV. Astrophys. J. Suppl. Ser. 2018, 239, 30. [Google Scholar] [CrossRef]
- Träbert, E. EUV beam-foil spectra of germanium and a blind-spot problem in spectroscopy. Atoms 2023, 11, 45. [Google Scholar] [CrossRef]
- Träbert, E. A laboratory astrophysics problem: The lifetime of very long-lived levels in low-charge ions. Atoms 2020, 8, 21. [Google Scholar] [CrossRef]
- Träbert, E. Experimental checks on calculations for Cl-, S- and P-like ions of the iron group elements. J. Phys. B At. Mol. Opt. Phys. 1996, 29, L217–L224. [Google Scholar] [CrossRef]
- Shirai, T.; Funatake, Y.; Mori, K.; Sugar, J.; Wiese, W.L.; Nakai, Y. Spectral data and Grotrian diagrams for highly ionized iron, Fe VIII-XXVI. J. Phys. Chem. Ref. Data 1990, 19, 127–275. [Google Scholar] [CrossRef]
- Del Zanna, G.; Storey, P.J.; Badnell, N.R.; Mason, H.E. Atomic data for astrophysics: Fe IX. Astron. Astrophys. 2014, 565, A77. [Google Scholar] [CrossRef]
- Träbert, E.; Beiersdorfer, P.; Brown, G.V.; Hell, N.; Lepson, J.K.; Fairchild, A.J.; Hahn, M.; Savin, D.W. Laboratory search for Fe IX solar diagnostic lines using an electron beam ion trap. Atoms 2022, 10, 115. [Google Scholar] [CrossRef]
- Storey, P.J.; Zeippen, C.J. Coronal Fe IX line intensities and electron density diagnostics. Mon. Not. R. Astron. Soc. 2001, 324, L7–L10. [Google Scholar] [CrossRef]
- Biémont, E.; Hansen, J.E. Energy levels and transition probabilities in 3d and 3d9 configurations. Phys. Scr. 1989, 39, 308–313. [Google Scholar] [CrossRef]
- Czyzak, S.J.; Krueger, T.K. On the excited levels of Fe VIII. Astrophys. J. 1966, 144, 381–407. [Google Scholar] [CrossRef]
- Beiersdorfer, P.; Osterheld, A.L.; Scofield, J.; Wargelin, B.; Marrs, R.E. Observation of magnetic octupole decay in atomic spectra. Phys. Rev. Lett. 1991, 67, 2272–2275. [Google Scholar] [CrossRef]
- Biémont, E. Multipole transitions in nickel-like and palladium-like spectra. J. Phys. B At. Mol. Opt. Phys. 1997, 30, 4207–4222. [Google Scholar] [CrossRef]
- Träbert, E.; Beiersdorfer, P.; Brown, G.V.; Terracol, S.; Safronova, U.I. On the metastable level in Ni-like ions. Nucl. Instrum. Meth. Phys. Res. B 2005, 235, 23–27. [Google Scholar] [CrossRef]
- Träbert, E.; Beiersdorfer, P.; Brown, G.V.; Boyce, K.; Kelley, R.L.; Kilbourne, C.A.; Porter, F.S.; Szymkowiak, A. Time-resolved soft-x-ray spectroscopy of a magnetic octupole transition in nickel-like xenon, cesium, and barium ions. Phys. Rev. A 2006, 73, 022508. [Google Scholar] [CrossRef]
- Träbert, E.; Beiersdorfer, P.; Brown, G.V. Observation of hyperfine mixing in measurements of a magnetic octupole decay in isotopically pure nickel-like 129Xe and 132Xe ions. Phys. Rev. Lett. 2007, 98, 263001. [Google Scholar] [CrossRef]
- Yao, K.; Andersson, M.; Brage, T.; Hutton, R.; Jönsson, P.; Zou, Y. MF-dependent lifetimes due to hyperfine induced interference effects. Phys. Rev. Lett. 2006, 97, 183001, Erratum Phys. Rev. Lett. 2007, 98, 269903; Reply Phys. Rev. Lett. 2007, 98, 269304. [Google Scholar] [CrossRef]
- Andersson, M.; Yao, K.; Hutton, R.; Zou, Y.; Chen, C.Y.; Brage, T. Hyperfine-state-dependent lifetimes along the Ni-like isoelectronic sequence. Phys. Rev. A 2008, 77, 042509. [Google Scholar] [CrossRef]
- Du, W.; Andersson, M.; Yao, K.; Brage, T.; Hutton, R.; Zou, Y. Lifetimes of the hyperfine levels of 3d94s 3D3 in high-Z Ni-like ions. J. Phys. B At. Mol. Opt. Phys. 2013, 46, 145001. [Google Scholar] [CrossRef]
- Träbert, E.; Beiersdorfer, P.; Gu, M.F. Spectra of Ni- and Co-like ions of Xe in an electron-beam ion trap. Can. J. Phys. 2008, 86, 467–475. [Google Scholar] [CrossRef]
- Sakoda, J.; Komatsu, A.; Kikuchi, H.; Nakamura, N. Visible spectroscopy of Rh-like ions. Phys. Scr. 2011, 144, 014011. [Google Scholar] [CrossRef]
- Kilbane, D.; Gillaspy, J.D.; Ralchenko, Y.; Reader, J.; O’Sullivan, G. Extreme ultraviolet spectra from N-shell ions of Gd, Dy and W. Phys. Scr. 2013, 156, 014012. [Google Scholar]
- Qiu, M.L.; Zhao, R.F.; Guo, X.L.; Zhao, Z.Z.; Li, W.X.; Du, S.Y.; Xiao, J.; Yao, K.; Chen, C.Y.; Hutton, R.; et al. Investigation of transitions between metastable levels of the first excited configuration of palladium-like tungsten. J. Phys. B At. Mol. Opt. Phys. 2014, 47, 175002. [Google Scholar] [CrossRef]
- Marques, J.P.; Parente, F.; Indelicato, P. Hyperfine quenching of the 1s22s2p 3P0 level in berylliumlike ions. Phys. Rev. A 1993, 47, 929–935. [Google Scholar] [CrossRef]
- Cheng, K.T.; Chen, M.H.; Johnson, W.R. Hyperfine quenching of the 2s2p 3P0 state of berylliumlike ions. Phys. Rev. A 2008, 77, 052504. [Google Scholar] [CrossRef]
- Andersson, M.; Zou, Y.; Hutton, R. Hyperfine-dependent lifetimes in Be-like ions. Phys. Rev. A 2009, 79, 032501. [Google Scholar] [CrossRef]
- Andersson, M.; Hutton, R.; Zou, Y. Hyperfine dependent lifetimes in Neon like ions. J. Phys. Conf. Ser. 2009, 163, 012013. [Google Scholar] [CrossRef]
- Marques, J.P.; Parente, F.; Indelicato, P. Hyperfine quenching of the 1s2 2s2 2p6 3s3p 3P0 level in magnesiumlike ions. At.Data Nucl. Data Tab. 1993, 55, 157–170. [Google Scholar] [CrossRef]
- Kang, H.; Li, J.G.; Dong, C.Z.; Jönsson, P.; Gaigalas, G. Hyperfine quenching of the 3s3p 3P0 level in Mg-like ions. J. Phys. B At. Mol. Opt. Phys. 2009, 42, 195002. [Google Scholar] [CrossRef]
- Kang, H.; Li, J.G.; Dong, C.Z.; Jönsson, P.; Gaigalas, G. THe effect of hyperfine interaction on the lifetime of the 3s3p 3P2 level of Mg-like ions. J. Phys. B At. Mol. Opt. Phys. 2010, 43, 095003. [Google Scholar] [CrossRef]
- Andersson, M.; Zou, Y.; Hutton, R.; Brage, T. Hyperfine dependent lifetimes in Mg-like ions. J. Phys. B At. Mol. Opt. Phys. 2010, 43, 095001. [Google Scholar] [CrossRef]
- Marques, J.P.; Parente, F.; Indelicato, P. Hyperfine quenching of the 4s4p 3P0 level in Zn-like ions. Eur. Phys. J. D 2007, 41, 457–465. [Google Scholar] [CrossRef]
- Andersson, M.; Liu, Z.; Chen, C.Y.; Hutton, R.; Zou, Y. Hyperfine-interaction-dependent 4s4p 3P2 lifetimes in Zn-like ions. Phys. Rev. A 2008, 78, 062505. [Google Scholar] [CrossRef]
- Brage, T.; Judge, P.G.; Proffitt, C.R. Determination of hyperfine-induced transition rates from observations of a planetary nebula. Phys. Rev. Lett. 2002, 89, 281101. [Google Scholar] [CrossRef]
- Schippers, S.; Bernhardt, D.; Müller, A.; Lestinsky, M.; Hahn, M.; Novotný, O.; Savin, D.W.; Grieser, M.; Krantz, C.; Repnow, R.; et al. Storage-ring measurement of the hyperfine-induced 2s2p 3P0 → 2s2 1S0 transition rate in berylliumlike sulfur. Phys. Rev. A 2012, 85, 012513. [Google Scholar] [CrossRef]
- Schippers, S.; Schmidt, E.W.; Bernhardt, D.; Yu, D.; Müller, A.; Lestinsky, M.; Orlov, D.A.; Grieser, M.; Repnow, R.; Wolf, A. Storage-ring measurement of the hyperfine induced 47Ti18+ (2s2p 3P0 → 2s2 1S0) transition rate. Phys. Rev. Lett. 2007, 98, 033001. [Google Scholar] [CrossRef]
- Träbert, E.; Grieser, M.; Hoffmann, J.; Krantz, C.; Reinhardt, S.; Repnow, R.; Wolf, A.; Indelicato, P. M1, M2 and hyperfine-induced decay rates in Mg-like ions of Co, Ni and Cu measured at a heavy-ion storage ring. New J. Phys. 2011, 13, 023017. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Kato, D.; Sakaue, H.A.; Murakami, I.; Nakamura, N. Spectroscopic study of promethiumlike bismuth with an electron-beam ion trap: Search for alkali-metal-like resonance lines. Phys. Rev. A 2014, 89, 010501(R). [Google Scholar] [CrossRef]
- Kobayashi, Y.; Kubota, K.; Omote, K.; Komatsu, A.; Sakoda, J.; Minoshima, M.; Kato, D.; Li, J.; Sakaue, H.A.; Murakami, I.; et al. Extreme ultraviolet and visible spectroscopy of promethiumlike heavy ions. Phys. Rev. A 2015, 92, 022510. [Google Scholar] [CrossRef]
- Kimura, N.; Priti; Kuma, S.; Azuma, T.; Nakamura, N. Electric-quadrupole transition-rate measurement of a highly charged ion in an electron-beam ion trap using pulsed laser excitation from a metastable state. Phys. Rev. A 2023, 107, 022805. [Google Scholar] [CrossRef]
- Feldman, U.; Indelicato, P.; Sugar, J. Magnetic dipole line from U LXXI ground-term levels predicted at 3200 Å. J. Opt. Soc. Am. B 1991, 8, 3–5. [Google Scholar] [CrossRef]
- Biémont, E.; Träbert, E.; Zeippen, C.J. Calculated transition probabilities in highly charged Ti-like ions. J. Phys. B At. Mol. Opt. Phys. 2001, 34, 1941–1951. [Google Scholar] [CrossRef]
- Tu, B.; Yao, K.; Shen, Y.; Yang, Y.; Li, M.C.; Xu, T.H.; Lu, Q.F.; Lu, D.; Wang, X.; Chen, C.Y.; et al. Observation of an extremely-long-lived metastable level in a Ti-like system via an L-shell dielectronic recombination measurement in highly charged 3dn ions of tungsten. Phys. Rev. A 2017, 96, 032705. [Google Scholar] [CrossRef]
- Adler, A.; Meyer, E.S.; Serpa, F.G.; Takacs, E.; Gillaspy, J.D.; Brown, C.M.; Feldman, U. Fabry-Perot spectroscopy of a visible magnetic dipole transition in Ba34+. Nucl. Instrum. Meth. Phys. Res. B 1995, 98, 581–584. [Google Scholar] [CrossRef]
- Schüssler, R.X.; Bekker, H.; Braß, M.; Cakir, H.; Crespo López-Urrutia, J.R.; Door, M.; Filianin, P.; Harman, Z.; Haverkort, M.W.; Huang, W.J.; et al. Detection of metastable electronic states by Penning-trap mass spectrometry. Nature 2020, 581, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Kromer, K.; Lyu, C.; Door, M.; Filianin, P.; Harman, Z.; Herkenhoff, J.; Indelicato, P.; Keitel, C.H.; Lange, D.; Novikov, Y.N.; et al. Observation of a low-lying metastable electronic state in highly charged lead by Penning-trap mass spectrometry. Phys. Rev. Lett. 2023, 131, 223002. [Google Scholar] [CrossRef] [PubMed]
- Tu, B.; Si, R.; Shen, Y.; Wang, J.; Wei, B.; Chen, C.-Y.; Yao, K.; Zou, Y.-M. Experimental access to observing decay from extremely long-lived metastable electronic states via Penning trap spectrometry. Phys. Rev. Res. 2023, 5, 043014. [Google Scholar] [CrossRef]
- Yudin, V.I.; Taichenachev, A.V.; Derevianko, A. Magnetic-dipole transitions in highly charged ions as a basis of ultraprecise optical clocks. Phys. Rev. Lett. 2014, 113, 233003. [Google Scholar] [CrossRef]
- Bohman, M.A.; Porsev, S.G.; Hume, D.B.; Leibrandt, D.R.; Safronova, M.S. Enhancing divalent optical atomic clocks with the 1S0 ↔3P2 transition. Phys. Rev. A 2023, 108, 053120. [Google Scholar] [CrossRef]
- Rehbehn, N.-H.; Rosner, M.K.; Berengut, J.C.; Schmidt, P.O.; Pfeifer, T.; Gu, M.F.; Crespo López-Urrutia, J.R. Narrow and ultra-narrow transitions in highly charged Xe ions as probes of fifth forces. arXiv 2023, arXiv:2309.17141v1. [Google Scholar]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Träbert, E. Long-Lived Levels in Multiply and Highly Charged Ions. Atoms 2024, 12, 12. https://doi.org/10.3390/atoms12030012
Träbert E. Long-Lived Levels in Multiply and Highly Charged Ions. Atoms. 2024; 12(3):12. https://doi.org/10.3390/atoms12030012
Chicago/Turabian StyleTräbert, Elmar. 2024. "Long-Lived Levels in Multiply and Highly Charged Ions" Atoms 12, no. 3: 12. https://doi.org/10.3390/atoms12030012




