Detailed Investigation of the Electric Discharge Plasma between Copper Electrodes Immersed into Water
Abstract
:1. Introduction
2. Experimental Method
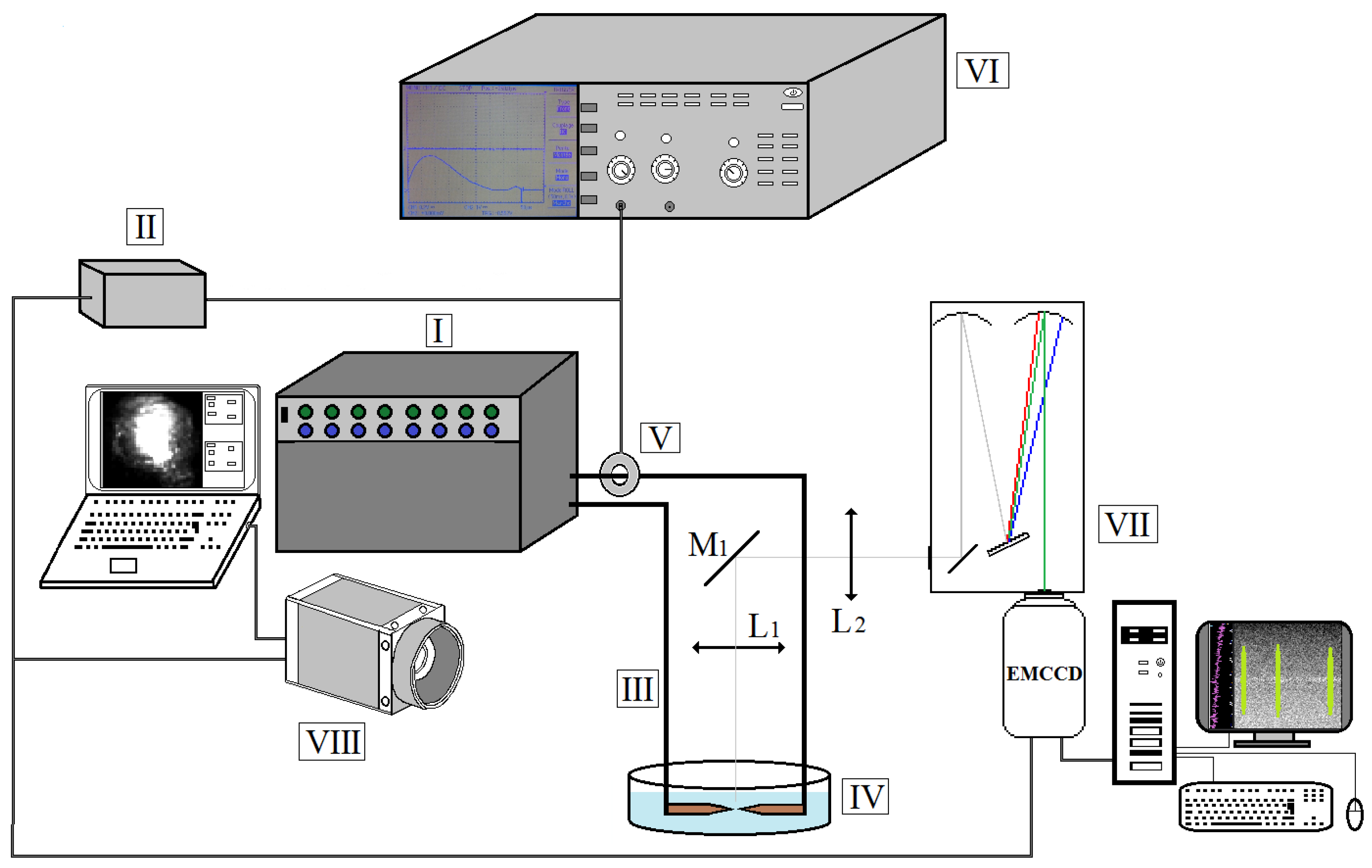
2.1. Experimental Setup
2.2. Optical Emission Spectroscopy (OES)
2.2.1. Registered Spectra
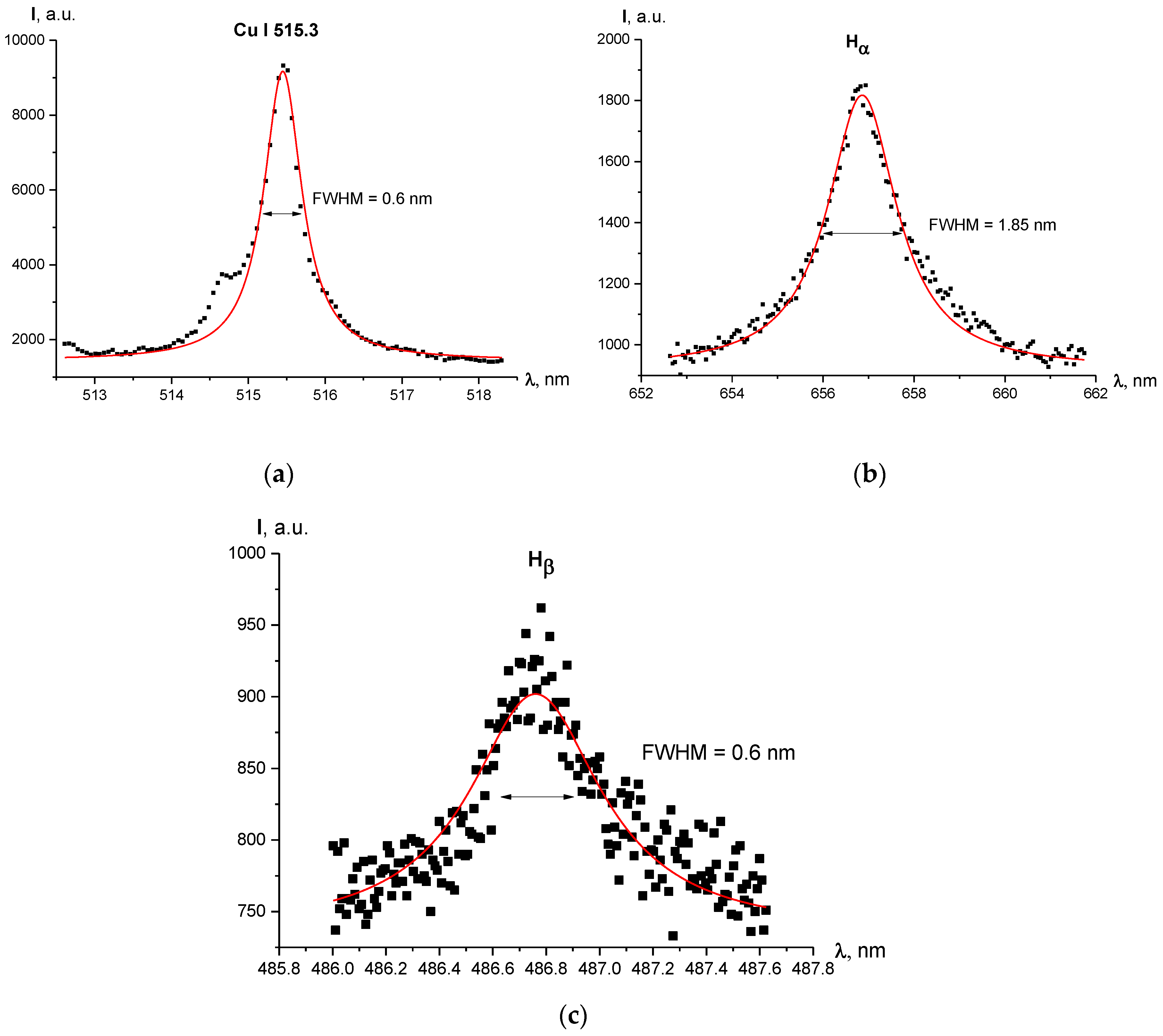
2.2.2. Broadening of Spectral Lines
2.2.3. Line Broadening Values in Our Configuration
3. Results
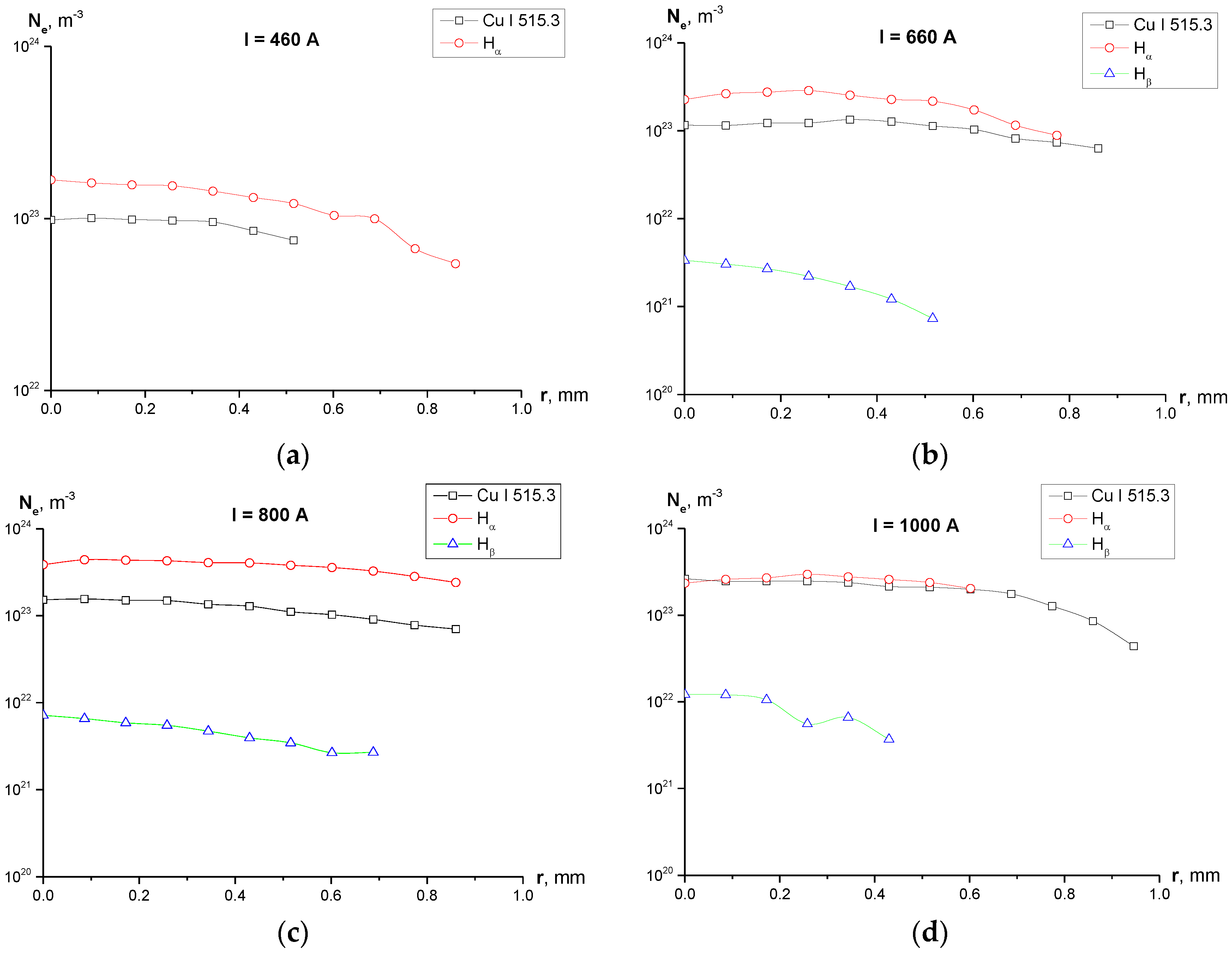
3.1. Electron Density
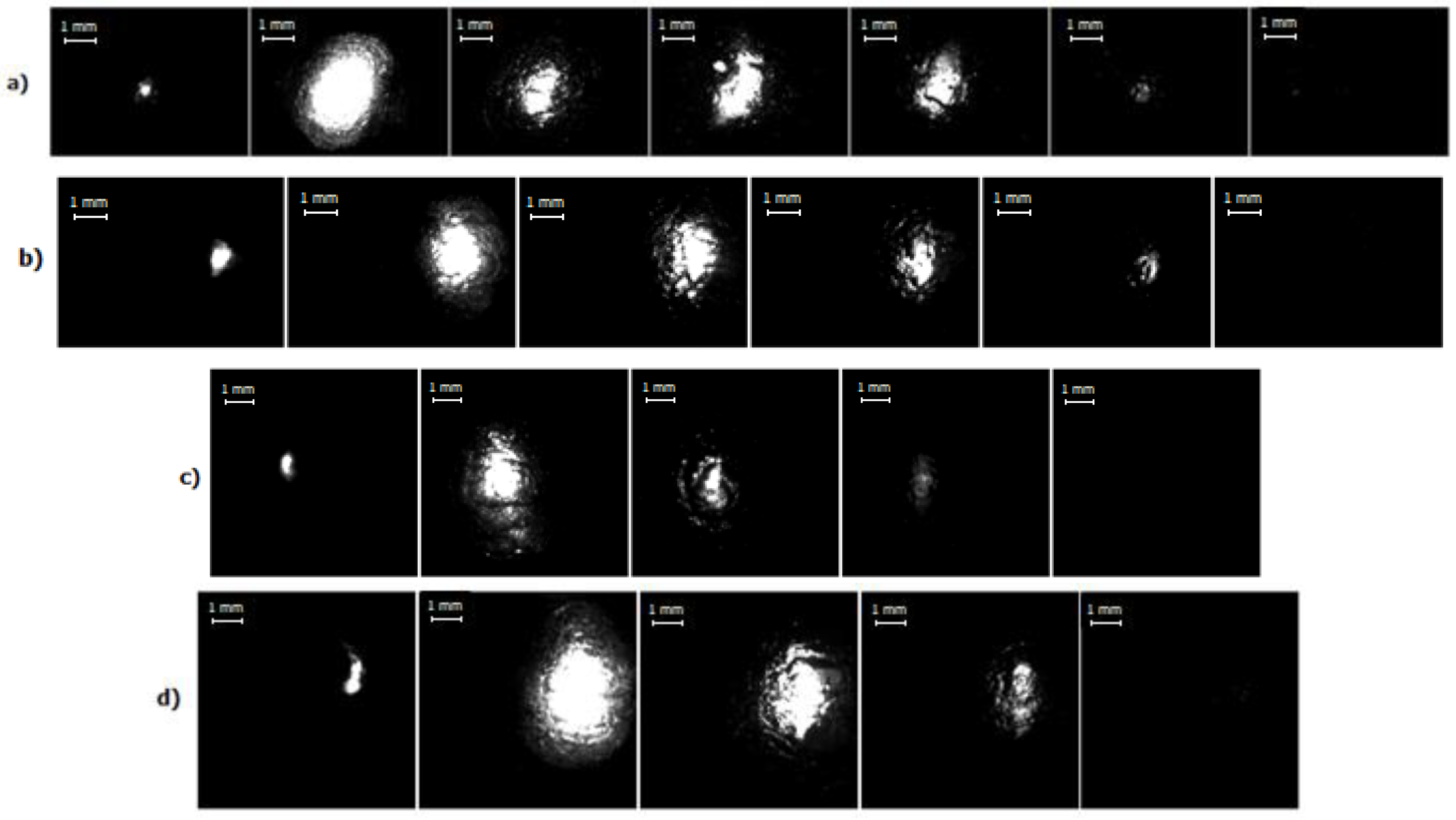
3.2. High-Speed Imaging
3.3. Erosion of Electrodes
4. Discussion and Conclusions
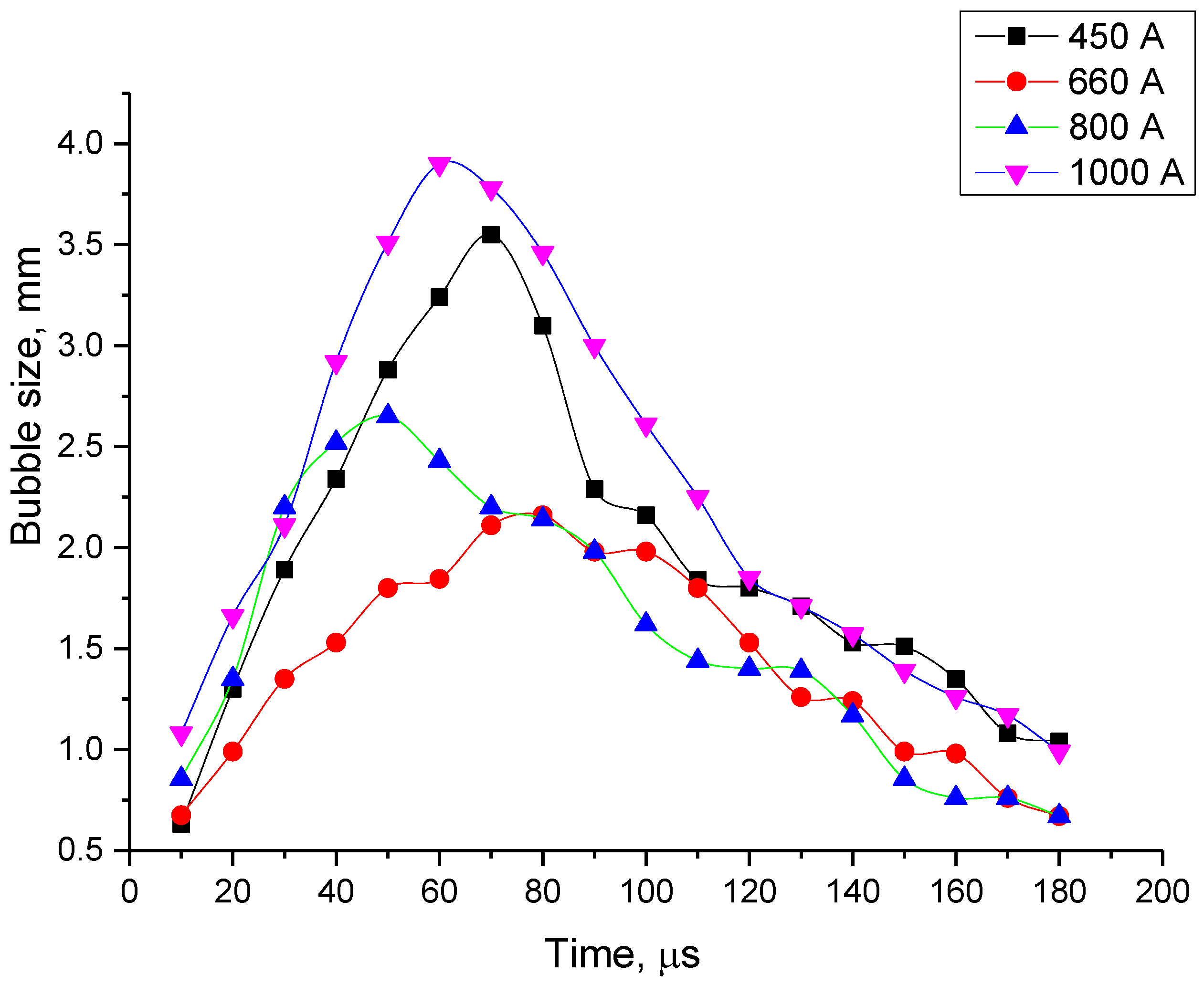
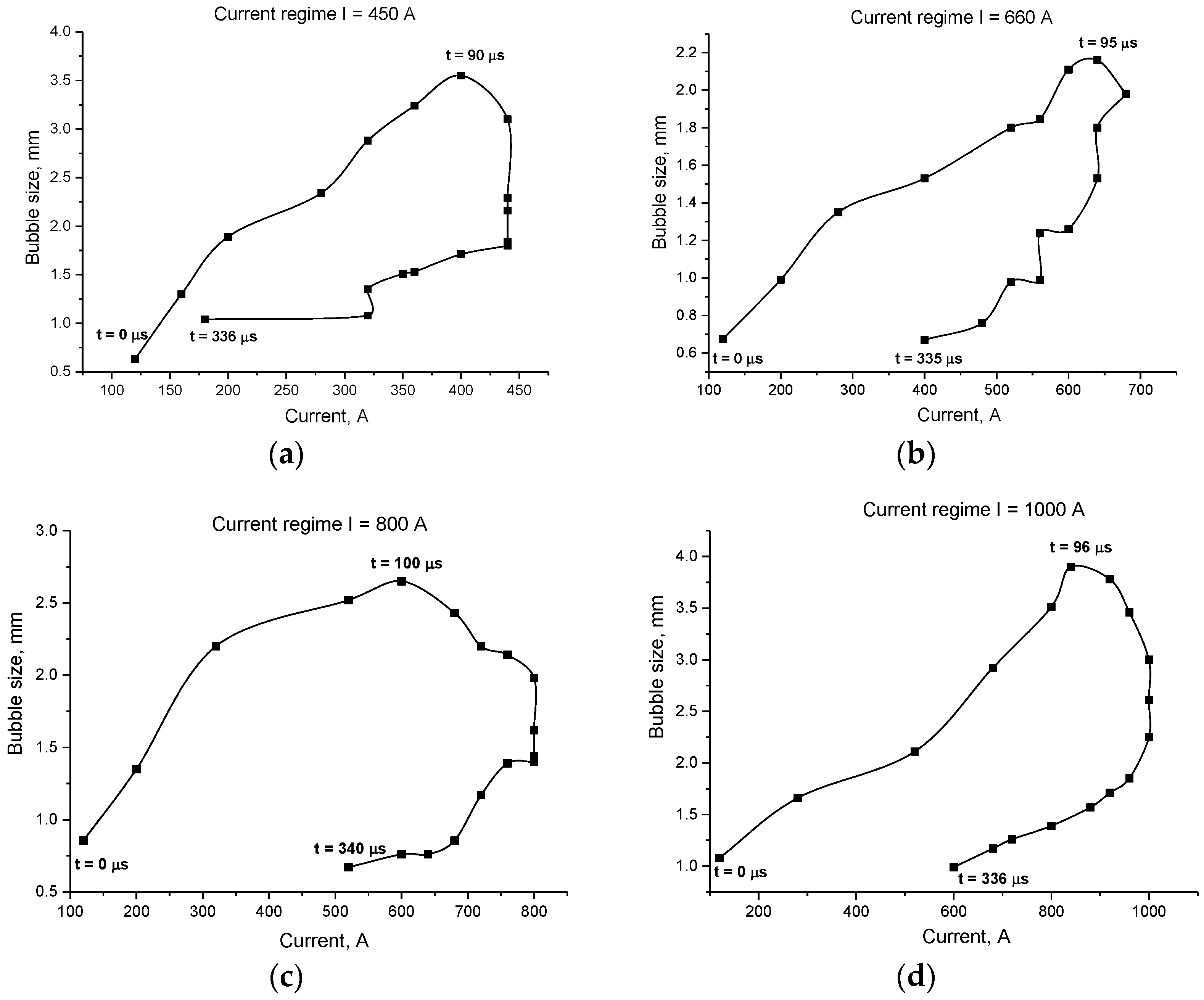
- For all the four current regimes studied in this work (I = 450 A, I = 660 A, I = 800 A and I = 1000 A), duration of the pulse in average shows to be 320–346 μs with the rise time of a current up to its maximum value of 90–100 μs (see Table 1). While raise time of a current up to its maximum value is the shortest for the current regime I = 450 A, the maximum bubble’s size is reached the fastest for the case of the current regime of I = 800 A, for which the current raise time is the longest. The only pattern that can be distinguished (see Figure 7 and Figure 9) is that the bubble reaches it maximum size not at the same time when the current value is on peak, but before that, maintaining its size more or less constant while current value equals to its maximum value for the given current regime. In order to be able to make any conclusions, more experiments must be performed, which will give the more reliable statistical data.
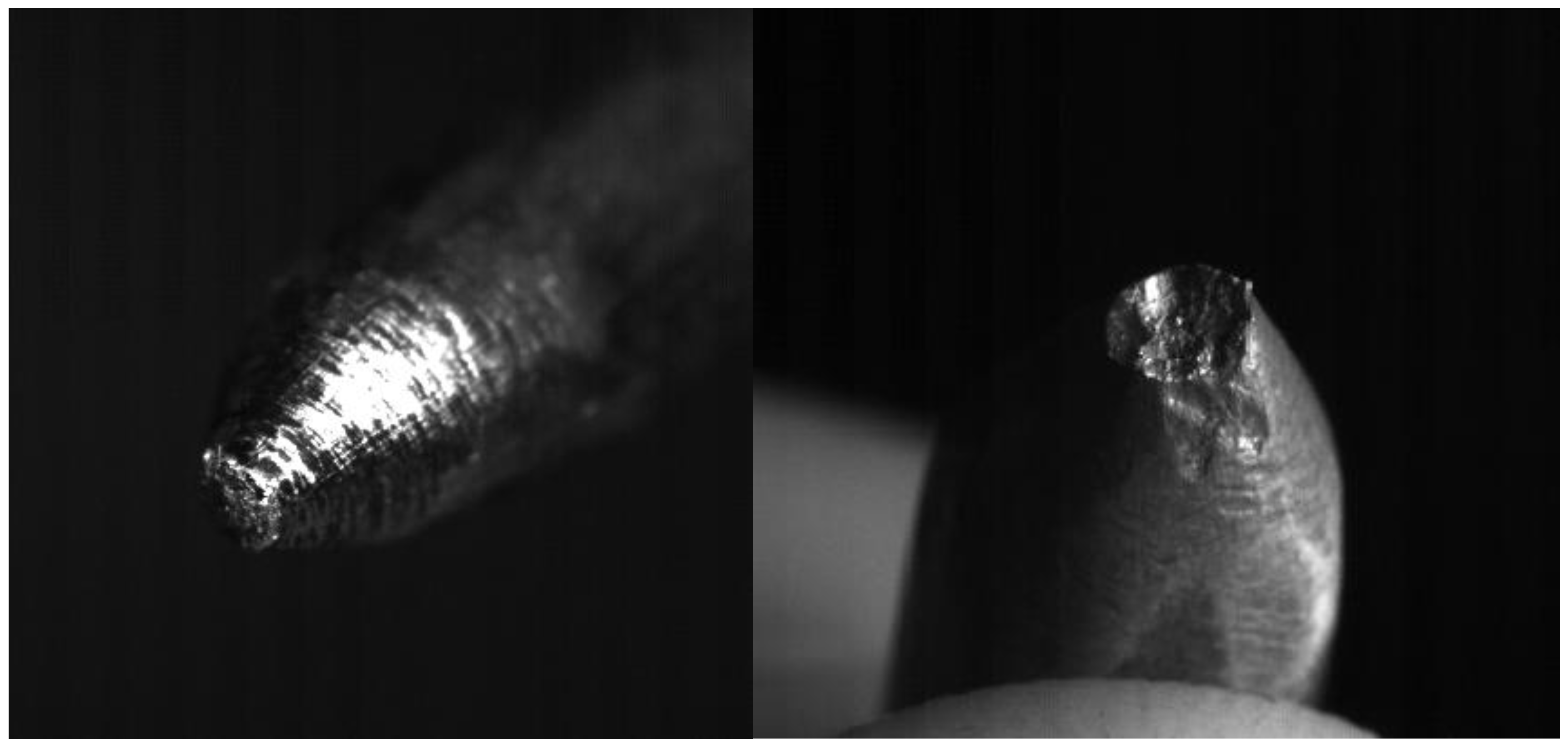
- The erosion does not change much for the different current regimes. Table 2 shows that for current of 800 A, which is approximately two times greater than the first current regime, increase of the eroded mass per pulse is ~20%. Erosion of anode shows to be greater than that of cathode (in our experiments anode is the moving electrode). This is consistent with the results obtained by other authors studying the submerged pulsed arc discharges [22], and may have one of the possible explanations that the energy dissipated in the anode is larger than in the cathode. As expected, the size of craters produced on the electrodes during arcing (Figure 10) increases with the measured erosion. The larger craters were formed on the anode where the erosion was larger.
- Calculation of the electron density shows (Figure 5) disagreement the values of Ne calculated using the Cu I 515.3 nm, Hα and Hβ lines. While Ne determined from the widths of Cu I 515.3 and Hα line are of the same order of magnitude for all the studied current regimes, values of Ne obtained from the width of Hβ line are by two orders of magnitude lower for cases of I = 660 and 800 A, and by one order of magnitude lower for the case of I = 1000 A.
- It could present a non-negligible self-absorption and it is necessary to evaluate how it affects the line broadening;
- It has a strong broadening by ion dynamics, an effect that can be evaluated by using some computational methods recently developed which allow us to simulate the profiles of the spectral lines [23].
Author Contributions
Conflicts of Interest
Appendix A
- Instrumental broadening;
- Natural broadening;
- Doppler broadening;
- Pressure broadening (resonance broadening, Van der Waals broadening).
Appendix A.1. Instrumental Broadening
Appendix A.2. Natural Broadening
Appendix A.3. Resonance Broadening
Appendix A.4. Van der Waals Broadening
Appendix A.5. Doppler Broadening
Appendix A.6. Stark Broadening
References
- Ternes, T.A.; Hirsch, R. Occurrence and behavior of X-ray contrast media in sewage facilities and the aquatic environment. Environ. Sci. Technol. 2000, 34, 2741–2748. [Google Scholar] [CrossRef]
- Dunnick, J.K.; Melnick, R.L. Assessment of the carcinogenic potential of chlorinated water: Experimental studies of chlorine, chloramine, and trihalomethanes. J. Natl. Cancer Inst. 1993, 85, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Ternes, T.A.; Stueber, J.; Herrmann, N.; McDowell, D.; Ried, A.; Kampmann, M.; Teiser, B. Ozonation: A tool for removal of pharmaceuticals, contrast media and musk fragrances from wastewater. Water Res. 2003, 37, 1976–1982. [Google Scholar] [CrossRef]
- Babaeva, N.Y.; Kushner, M.J. Streamer Branching: The Role of Inhomogeneities and Bubbles. IEEE Trans Plasma Sci. 2008, 36, 892–893. [Google Scholar] [CrossRef]
- Bruggeman, P.; Leys, C. Non-thermal plasmas in and in contact with liquids. Top. Rev. J. Phys. D Appl. Phys. 2009, 42, 1–28. [Google Scholar] [CrossRef]
- Clements, J.S.; Sato, M.; Davis, R.H. Preliminary investigation of prebreakdown phenomena and chemical reactions using a pulsed high-voltage discharge in water. IEEE Trans. Ind. Appl. 1987, 23, 224–235. [Google Scholar] [CrossRef]
- Li, O.L.H.; Chang, J.S.; Guo, Y. Pulsed arc electrohydraulic discharge characteristics, plasma parameters, and optical emission during contaminated pond water treatments. IEEE Electr. Insul. Mag. 2011, 27. [Google Scholar] [CrossRef]
- Sunka, P. Pulse electrical discharges in water and their applications. Phys. Plasmas 2001, 8, 2587–2594. [Google Scholar] [CrossRef]
- Akiyama, H.; Sakugawa, T.; Namihira, T. Industrial applications of pulsed power technology. IEEE Trans. Dielectr. Electr. Insul. 2007, 14, 1051–1064. [Google Scholar] [CrossRef] [Green Version]
- Bluhm, H.; Frey, W.; Giese, H. Application of pulsed HV discharges to material fragmentation and recycling. IEEE Trans. Dielectr. Electr. Insul. 2000, 7, 625–636. [Google Scholar] [CrossRef]
- Locke, B.R.; Sato, M.; Sunka, P.; Hoffmann, M.R.; Chang, J.S. Electrohydraulic discharge and nonthermal plasma for water treatment. Ind. Eng. Chem. Res. 2006, 45, 882–905. [Google Scholar] [CrossRef]
- Chen, Q.; Li, J.; Li, Y. A review of plasma–liquid interactions for nanomaterial synthesis. J. Phys. D Appl. Phys. 2015, 48, 424005. [Google Scholar] [CrossRef]
- Tmenova, T.A.; Veklich, A.N.; Boretskij, V.F.; Cressault, Y.; Valensi, F.; Lopatko, K.G.; Aftandilyants, Y.G. Optical emission spectroscopy of plasma of underwater electric spark discharges between metal granules. Probl. Atomic Sci. Technol. 2017, 1, 132–135. [Google Scholar]
- Cressault, Y.; Teulet, P.; Gleizes, A.; Lopatko, K.; Melnichuk, M.; Aftandilyants, Y.; Gonchar, E.; Boretskij, V.; Veklich, A. Peculiarities of metal nanoparticles generation by underwater discharges for biological applications. In Proceedings of the XXXI International Conference on Phenomena in Ionized Gases, Granada, Spain, 14–19 July 2013. [Google Scholar]
- Lopatko, K.G.; Aftandіlyants, I.G.; Kalenska, S.M. Colloidal Solution of Metal. Ukrainian Patent No. 38459, 12 January 2009. [Google Scholar]
- Xiu, Z.; Zhang, Q.; Puppala, H.L.; Colvin, V.L.; Alvarez, P.J. Negligible Particle-Specific Antibacterial Activity of Silver Nanoparticles. Nano Lett. 2012, 12, 4271. [Google Scholar] [CrossRef] [PubMed]
- Babich, I.L.; Boretskij, V.F.; Veklich, A.N.; Semenyshyn, R.V. Spectroscopic data and Stark broadening of Cu I and Ag I spectral lines: Selection and analysis. Adv. Space Res. 2014, 54, 1254–1263. [Google Scholar] [CrossRef]
- Yang, Y.; Cho, I.Y.; Fridman, A. Plasma Discharge in Liquid: Water Treatment and Applications; CRC Press: Boca Raton, FL, USA, 2012; pp. 10–29. [Google Scholar]
- Konjević, N. Plasma broadening and shifting of non-hydrogenic spectral lines: Present status and applications. Phys. Rep. 1999, 316, 339–401. [Google Scholar] [CrossRef]
- Griem, H.R. Spectral Line Broadening by Plasmas; Academic Press: New York, NY, USA, 1974. [Google Scholar]
- Konjević, N.; Ivković, M.; Sakan, N. Hydrogen Balmer lines for low electron number density plasma diagnostics. Spectrochim. Acta B At. Spectrosc. 2012, 76, 12–26. [Google Scholar] [CrossRef]
- Parkansky, N.; Glikman, L.; Beilis, I.I.; Alterkop, B.; Boxman, R.L.; Gindin, D. W–C electrode erosion in a pulsed arc submerged in liquid. Plasma Chem. Plasma Process. 2007, 27, 789–797. [Google Scholar] [CrossRef]
- Gigosos, M.A.; Cardeñoso, V. New plasma diagnosis tables of hydrogen Stark broadening including ion dynamics. J. Phys. B At. Mol. Opt. Phys. 1996, 29, 4795. [Google Scholar] [CrossRef]
- Luque, J.M.; Calzada, M.D.; Sáez, M. Experimental research into the influence of ion dynamics when measuring the electron density from the Stark broadening of the Hα and Hβ lines. J. Phys. B At. Mol. Opt. Phys. 2003, 36, 1573. [Google Scholar] [CrossRef]
- Kepple, P.; Griem, H.R. Improved Stark Profile Calculations for the Hydrogen Lines Hα, Hβ, Hγ, and Hδ. Phys. Rev. 1968, 173, 317. [Google Scholar] [CrossRef]
- Gigosos, M.A.; Gonzalez, M.A.; Cardenoso, V. Computer simulated Balmer-alpha,-beta and -gamma Stark line profiles for non-equilibrium plasmas diagnostics. Spectrochim. Acta B 2003, 58, 1489–1504. [Google Scholar] [CrossRef]
- Torres, J.; Carabano, O.; Fernández, M.; Rubio, S.; Álvarez, R.; Rodero, A.; Lao, C.; Quintero, M.C.; Gamero, A.; Sola, A. The stark-crossing method for the simultaneous determination of the electron temperature and density in plasmas. J. Phys. Conf. Ser. 2006, 44, 70–79. [Google Scholar] [CrossRef]
- Wiese, W.L.; Smith, M.W.; Glennon, B.M. Atomic Transition Probabilities: Hydrogen through Neon; US Department of Commerce, National Bureau of Standards: Washington, DC, USA, 1996; Voloume 1, pp. 1–8.
- Allen, C.W. Allen’s Astrophysical Quantities, 4th ed.; Cox, A.N., Ed.; Springer: New York, NY, USA, 2015; pp. 27–79. [Google Scholar]
- Nikiforov, A.Y.; Leys, C.; Li, L.; Nemcova, L.; Krcma, F. Physical properties and chemical efficiency of an underwater dc discharge generated in He, Ar, N2 and air bubbles. Plasma Sources Sci. Technol. 2011, 20, 034008. [Google Scholar] [CrossRef]
- Vidal, C.R.; Cooper, J.; Smith, E.W. Hydrogen Stark-broadening tables. Astrophys. J. Suppl. Ser. 1973, 25, 37. [Google Scholar] [CrossRef]
- Kelleher, D.E.; Wiese, W.L. Observation of ion motion in hydrogen Stark profiles. Phys. Rev. Lett. 1973, 31, 1431. [Google Scholar] [CrossRef]
- Wiese, W.L.; Kelleher, D.E.; Helbig, V. Variations in Balmer-line Stark profiles with atom-ion reduced mass. Phys. Rev. A 1975, 11, 1854. [Google Scholar] [CrossRef]
- Seidel, J. Hydrogen Stark broadening by different kinds of model microfields. Z. Naturforsch. A 1980, 35, 679–689. [Google Scholar] [CrossRef]
- Griem, H.R. Plasma Spectroscopy; McGraw-Hill Book Company: New York, NY, USA, 1964. [Google Scholar]
- Samek, O.; Beddows, D.C.; Kaiser, J.; Kukhlevsky, S.V.; Liska, M.; Telle, H.H.; Young, J. Application of laser-induced breakdown spectroscopy to in situ analysis of liquid samples. Opt. Eng. 2000, 39, 2248–2262. [Google Scholar]
- Konjević, R.; Konjević, N. Stark broadening and shift of neutral copper spectral lines. Fizika 1986, 18, 327–335. [Google Scholar]



| Idisch, A 1 | Uc, V 2 | P, kW | τraise, μs 3 | τdisch, μs 4 | τtotal, μs |
|---|---|---|---|---|---|
| 450 | 120 | 54 | 90 | 230 | 320 |
| 660 | 150 | 100 | 95 | 240 | 335 |
| 800 | 180 | 146 | 100 | 240 | 340 |
| 1000 | 220 | 217 | 96 | 240 | 336 |
| Current (I, A) | Number of Consecutive Pulses | Erosion of Anode, g | Erosion of Cathode, g | Total Erosion, g | Erosion Per Pulse (g/Pulse) |
|---|---|---|---|---|---|
| 450 | 15 | 0.0038 | 0.0005 | 0.0043 | 0.00028 |
| 800 | 15 | 0.004 | 0.0012 | 0.0052 | 0.00034 |
| logNe (m−3) | FWHM (nm) for Hα | FWHM (nm) for Hβ |
|---|---|---|
| 20.00 | 0.0142 | 0.0424 |
| 20.33 | 0.0244 | 0.0741 |
| 20.67 | 0.0404 | 0.129 |
| 21.00 | 0.064 | 0.217 |
| 21.33 | 0.102 | 0.361 |
| 21.67 | 0.16 | 0.601 |
| 22.00 | 0.25 | 0.999 |
| 22.33 | 0.393 | 1.67 |
| 22.67 | 0.621 | 2.80 |
| 23.00 | 1.01 | 4.70 |
| 23.33 | 1.68 | 7.77 |
| 23.67 | 2.84 | 1.27 |
| 24.00 | 4.86 | 2.05 |
| 24.33 | 8.31 | 3.25 |
| Line | FWHM (nm) at I = 660 A | FWHM (nm) at I = 800 A | FWHM (nm) at I = 1000 A | Average log Ne (m−3) |
|---|---|---|---|---|
| Hα | 1.13 | 2.60 | 2.40 | 23.36 |
| Hβ | 0.40 | 0.39 | 0.57 | 21.51 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venger, R.; Tmenova, T.; Valensi, F.; Veklich, A.; Cressault, Y.; Boretskij, V. Detailed Investigation of the Electric Discharge Plasma between Copper Electrodes Immersed into Water. Atoms 2017, 5, 40. https://doi.org/10.3390/atoms5040040
Venger R, Tmenova T, Valensi F, Veklich A, Cressault Y, Boretskij V. Detailed Investigation of the Electric Discharge Plasma between Copper Electrodes Immersed into Water. Atoms. 2017; 5(4):40. https://doi.org/10.3390/atoms5040040
Chicago/Turabian StyleVenger, Roman, Tetiana Tmenova, Flavien Valensi, Anatoly Veklich, Yann Cressault, and Viacheslav Boretskij. 2017. "Detailed Investigation of the Electric Discharge Plasma between Copper Electrodes Immersed into Water" Atoms 5, no. 4: 40. https://doi.org/10.3390/atoms5040040





