Plasma Spectroscopy of Various Types of Gypsum: An Ideal Terrestrial Analogue
Abstract
1. Introduction
2. Mineralogy and Geochemistry of Gypsum
3. Materials and Methods
3.1. Sample Description
3.2. Powder X-ray Diffraction (XRD)
3.3. Experimental Set-Up
3.4. Multivariate Analysis
4. Results and Discussion
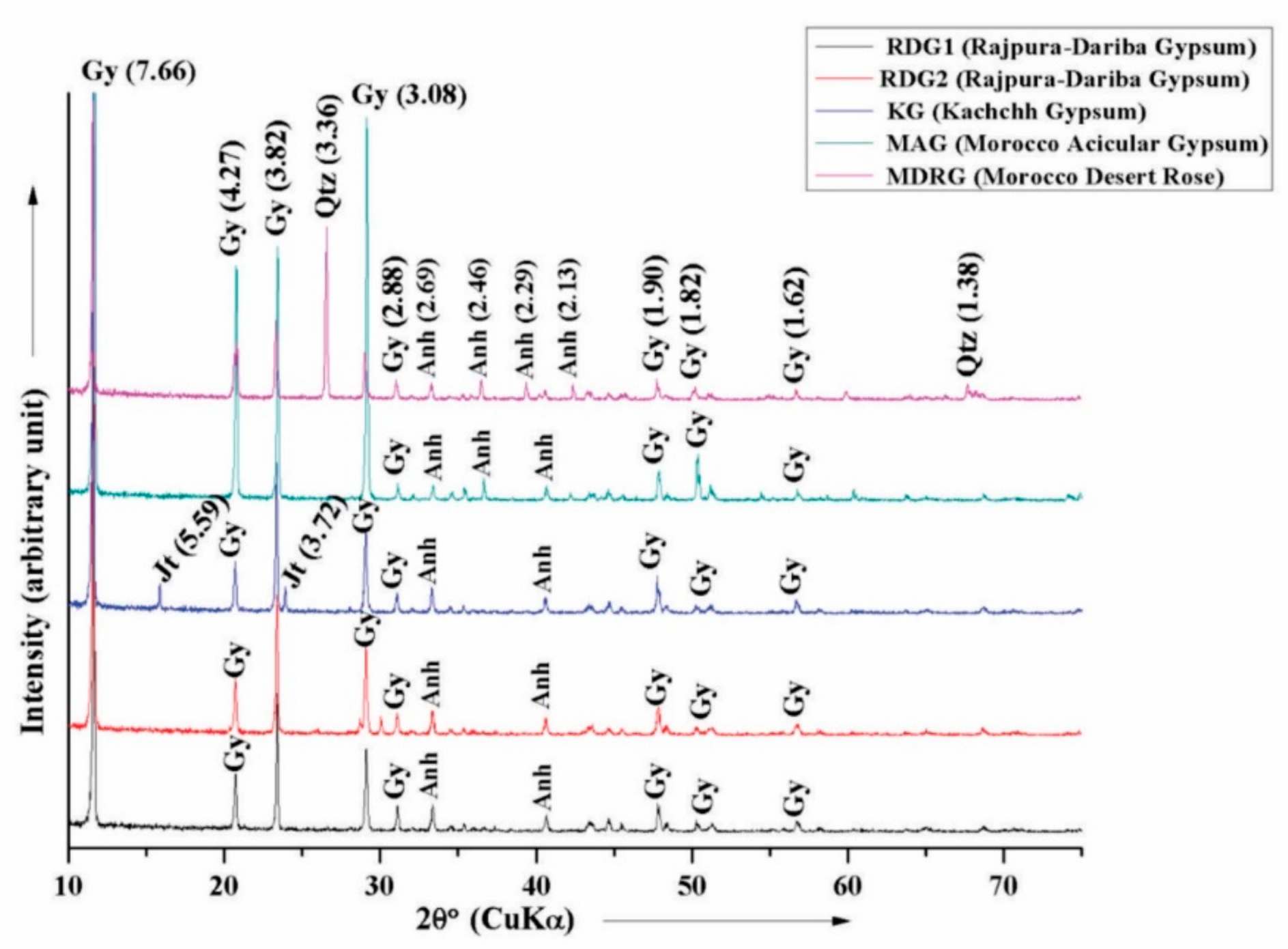
4.1. X-ray Diffraction Analysis
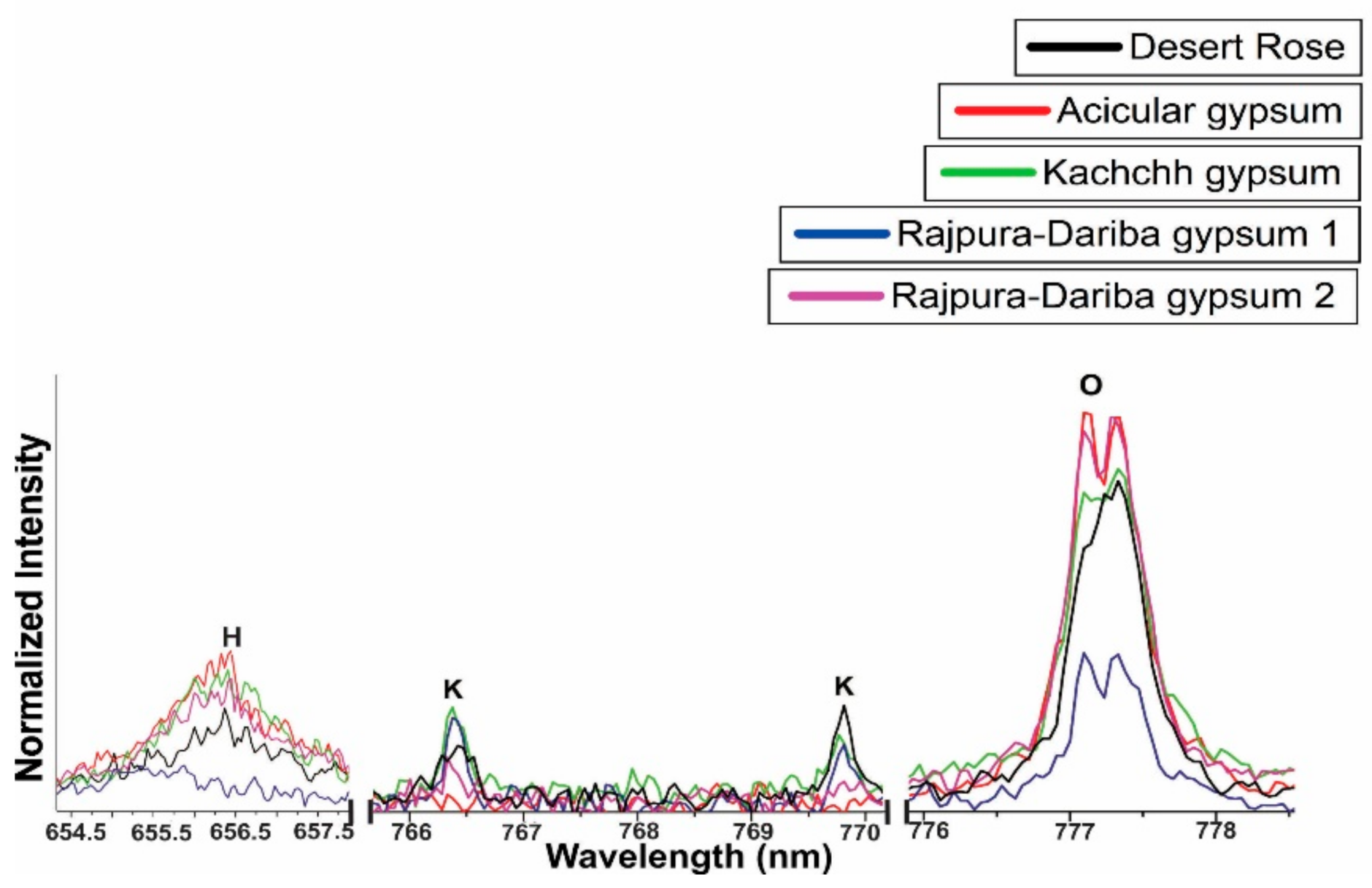
4.2. LIBS Analysis
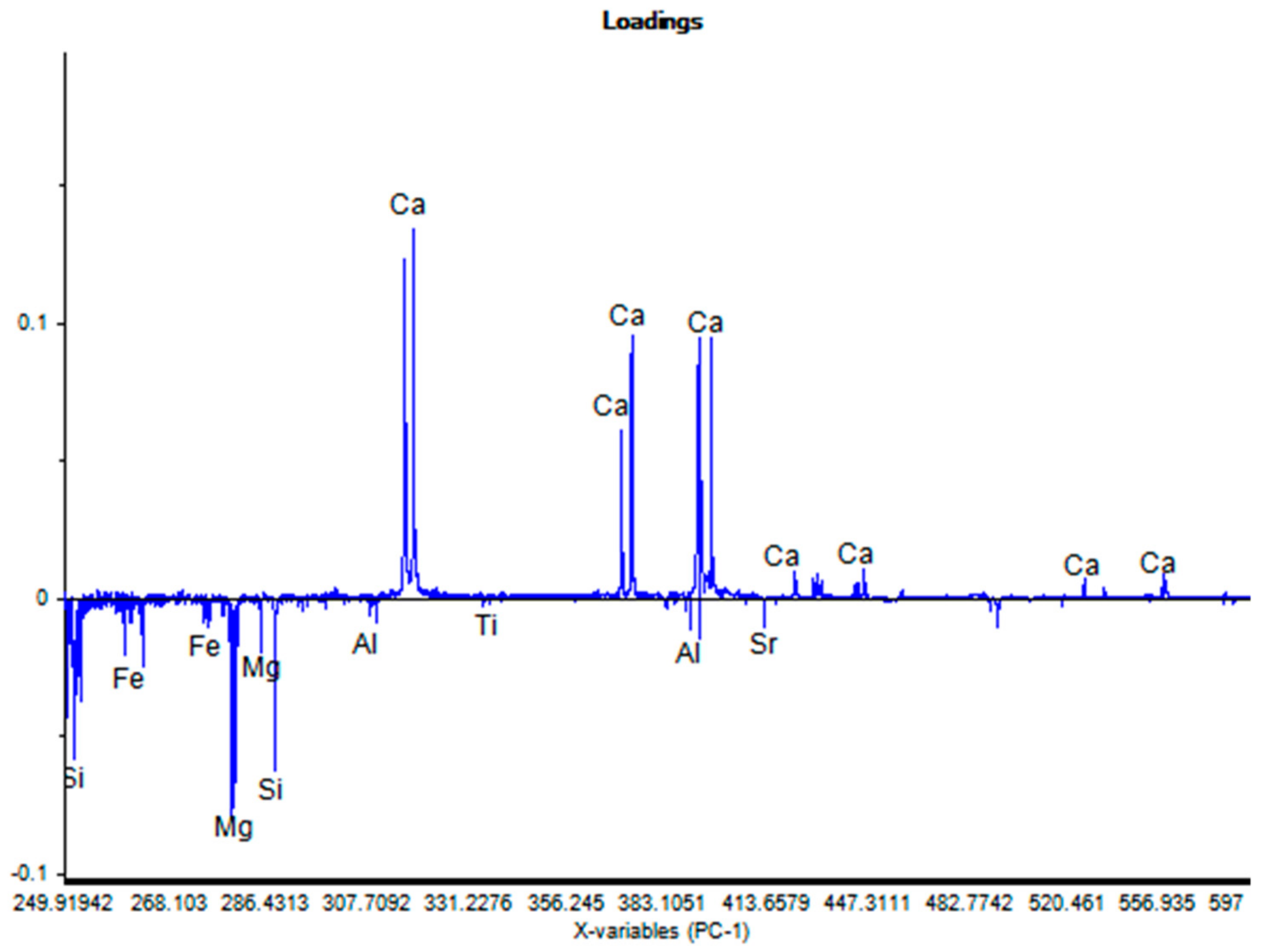
4.3. Principal Component Analysis (PCA)
4.4. Partial Least-Square Regression (PLSR)
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gaft, M.; Sapir-Sofer, I.; Modiano, H.; Stana, R. Laser induced breakdown spectroscopy for bulk minerals online analyses. Spectrochim. Acta Part B At. Spectrosc. 2007, 62, 1496–1503. [Google Scholar] [CrossRef]
- Díaz Pace, D.M.; Gabriele, N.A.; Garcimuño, M.; D’Angelo, C.A.; Bertuccelli, G.; Bertuccelli, D. Analysis of Minerals and Rocks by Laser-Induced Breakdown Spectroscopy. Spectrosc. Lett. 2011, 44, 399–411. [Google Scholar] [CrossRef]
- Wiens, R.C.; Maurice, S.; Barraclough, B.; Saccoccio, M.; Barkley, W.C.; Bell, J.F., III; Bender, S.; Bernardin, J.; Blaney, D.; Blank, J.; et al. The ChemCam Instrument Suite on the Mars Science Laboratory (MSL) Rover: Body Unit and Combined System Tests. Space Sci. Rev. 2012, 170, 167–227. [Google Scholar] [CrossRef]
- Grotzinger, J.P.; Arvidson, R.E.; Bell, J.; Calvin, W.; Clark, B.C.; Fike, D.A.; Golombek, M.; Greeley, R.; Haldemann, A.; Herkenhoff, K.E.; et al. Stratigraphy and sedimentology of a dry to wet eolian depositional system, Burns formation, Meridiani Planum, Mars. Earth Planet. Sci. Lett. 2005, 240, 11–72. [Google Scholar] [CrossRef]
- Knoll, A.H.; Squyres, S.W.; Arvidson, R.E.; Bell, J.F., III; Calef, F., III; Clark, B.C.; Cohen, B.A.; Crumpler, L.A.; de Souza, P.A., Jr.; Farrand, W.H.; et al. Ancient Impact and Aqueous Processes at Endeavour Crater, Mars. Science 2012, 336, 570–576. [Google Scholar] [CrossRef]
- Squyres, S.W.; Knoll, A.H. Sedimentary rocks at Meridiani Planum: Origin, diagenesis, and implications for life on Mars. Earth Planet. Sci. Lett. 2005, 240, 1–10. [Google Scholar] [CrossRef]
- Elwood Madden, M.E.; Bodnar, R.J.; Rimstidt, J.D. Jarosite as an indicator of water-limited chemical weathering on Mars. Nature 2004, 431, 821–823. [Google Scholar] [CrossRef]
- Nachon, M.; Clegg, S.M.; Mangold, N.; Schröder, S.; Kah, L.C.; Dromart, G.; Ollila, A.; Johnson, J.R.; Oehler, D.C.; Bridges, J.C.; et al. Calcium sulfate vein observations at Yellowknife Bay using ChemCam on the Curiosity Rover. In Proceedings of the American Geophysics Union, Fall Meeting, San Francisco, CA, USA, 9–13 December 2013. Abstract P23C–1797. [Google Scholar]
- Crumpler, L.S.; Arvidson, R.E.; Bell, J.; Clark, B.C.; Cohen, B.A.; Farrand, W.H.; Gellert, R.; Golombek, M.; Grant, J.A.; Guinness, E.; et al. Context of ancient aqueous environments on Mars from in situ geologic mapping at Endeavour Crater. J. Geophys. Res. 2015, 120, 538–569. [Google Scholar] [CrossRef]
- Anderson, D.E.; Ehlmann, B.L.; Forni, O.; Clegg, S.M.; Cousin, A.; Thomas, N.H.; Lasue, J.; Delapp, D.M.; McInroy, R.E.; Gasnault, O.; et al. Characterization of LIBS emission lines for the identification of chlorides, carbonates, and sulfates in salt/basalt mixtures for the application to MSL ChemCam data. J. Geophys. Res. 2017, 122, 744–770. [Google Scholar] [CrossRef]
- Ziolkowski, L.A.; Mykytczuk, N.C.S.; Omelon, C.R.; Johnson, H.; Whyte, L.G.; Slater, G.F. Arctic Gypsum Endoliths: A biogeochemical characterization of a viable and active microbial community. Biogeosci. Discuss. 2013, 10, 2269–2304. [Google Scholar] [CrossRef]
- Bonny, S.; Jones, B. Microbes and mineral precipitation, Miette Hot Springs, Jasper National Park, Alberta, Canada. Can. J. Earth Sci. 2003, 40, 1483–1500. [Google Scholar] [CrossRef]
- Parnell, J.; Lee, P.; Cockell, C.S.; Osinski, G.R. Microbial colonization in impact-generated hydrothermal sulphate deposits, Haughton impact structure, and implications for sulphates on Mars. Int. J. Astrobiol. 2004, 3, 247–256. [Google Scholar] [CrossRef]
- Tang, M.; Ehreiser, A.; Li, Y.-L. Gypsum in modern Kamchatka volcanic hot springs and the Lower Cambrian black shale: Applied to the microbial-mediated precipitation of sulfates on Mars. Am. Mineral. 2014, 99, 2126–2137. [Google Scholar] [CrossRef]
- Rasuk, M.C.; Kurth, D.; Flores, M.R.; Contreras, M.; Novoa, F.; Poire, D.; Farias, M.E. Microbial Characterization of Microbial Ecosystems Associated to Evaporites Domes of Gypsum in Salar de Llamara in Atacama Desert. Microb. Ecol. 2014, 68, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Pati, J.K.; Reimold, W.U. Impact cratering -fundamental process in geoscience and planetary science. J. Earth Syst. Sci. 2007, 116, 81–98. [Google Scholar] [CrossRef]
- Aubrey, A.; Cleaves, H.J.; Chalmers, J.H.; Skelley, A.M.; Mathies, R.A.; Grunthaner, F.J.; Ehrenfreund, P.; Bada, J.L. Sulfate minerals and organic compounds on Mars. Geology 2006, 34, 357–360. [Google Scholar] [CrossRef]
- Benison, K.C.; Karmanocky, F.J. Could microorganisms be preserved in Mars gypsum? Insights from terrestrial examples. Geology 2014, 42, 61–618. [Google Scholar] [CrossRef]
- Ritsema, C.J.; Groenenberg, J.E. Pyrite Oxidation, Carbonate Weathering, and Gypsum Formation in a Drained Potential Acid Sulfate Soil. Soil Sci. Soc. Am. J. 1993, 57, 968–976. [Google Scholar] [CrossRef]
- Langevin, Y.; Poulet, F.; Bibring, J.-P.; Gondet, B. Sulfates in the North Polar Region of Mars Detected by OMEGA/Mars Express. Science 2005, 307, 1584–1586. [Google Scholar] [CrossRef]
- Massé, M.; Le Mouélic, S.; Bourgeois, O.; Combe, J.-P.; Le Deit, L.; Sotin, C.; Bibring, J.-P.; Gondet, B.; Langevin, Y. Mineralogical composition, structure, morphology, and geological history of Aram Chaos crater fill on Mars derived from OMEGA Mars Express data. J. Geophys. Res. 2008, 113. [Google Scholar] [CrossRef]
- Milliken, R.E.; Grotzinger, J.P.; Thomson, B.J. Paleoclimate of Mars as captured by the stratigraphic record in Gale Crater. Geophys. Res. Lett. 2010, 37. [Google Scholar] [CrossRef]
- Bibring, J.-P. Mars Surface Diversity as Revealed by the OMEGA/Mars Express Observations. Science 2005, 307, 1576–1581. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.L. Mars’ north polar gypsum: Possible origin related to early Amazonian magmatism at Alba Patera and aeolian mining. In Proceedings of the Fourth International Conference on Mars Polar Science and Exploration, Davos, Switzerland, 2–6 October 2006. No. 8024. [Google Scholar]
- Fishbaugh, K.E.; Poulet, F.; Chevrier, V.; Langevin, Y.; Bibring, J.-P. On the origin of gypsum in the Mars north polar region. J. Geophys. Res. 2007, 112. [Google Scholar] [CrossRef]
- Squyres, S.W.; Aharonson, O.; Clark, B.C.; Cohen, B.A.; Crumpler, L.; de Souza, P.A.; Farrand, W.H.; Gellert, R.; Grant, J.; Grotzinger, J.P.; et al. Pyroclastic Activity at Home Plate in Gusev Crater, Mars. Science 2007, 316, 738–742. [Google Scholar] [CrossRef] [PubMed]
- Szynkiewicz, A.; Ewing, R.C.; Moore, C.H.; Glamoclija, M.; Bustos, D.; Pratt, L.M. Origin of terrestrial gypsum dunes-Implications for Martian gypsum-rich dunes of Olympia Undae. Geomorphology 2010, 121, 69–83. [Google Scholar] [CrossRef]
- Dehouck, E.; Chevrier, V.; Gaudin, A.; Mangold, N.; Mathé, P.-E.; Rochette, P. Evaluating the role of sulfide-weathering in the formation of sulfates or carbonates on Mars. Geochim. Cosmochim. Acta 2012, 90, 47–63. [Google Scholar] [CrossRef]
- Squyres, S.W.; Arvidson, R.E.; Bell, J.F., III; Brückner, J.; Cabrol, N.A.; Calvin, W.; Carr, M.H.; Christensen, P.R.; Clark, B.C.; Crumpler, L.; et al. The Opportunity Rover’s Athena Science Investigation at Meridiani Planum, Mars. Science 2004, 306, 1698–1703. [Google Scholar] [CrossRef]
- Young, B.W.; Chan, M.A. Gypsum veins in Triassic Moenkopi mudrocks of southern Utah: Analogs to calcium sulfate veins on Mars. J. Geophys. Res. 2017, 122, 150–171. [Google Scholar] [CrossRef]
- Kasprzyk, A. Sedimentological and diagenetic patterns of anhydrite deposits in the Badenian evaporite basin of the Carpathian Foredeep, southern Poland. Sed. Geol. 2003, 158, 167–194. [Google Scholar] [CrossRef]
- Laxmiprasad, A.S.; Sridhar Raja, V.L.N.; Menon, S.; Goswami, A.; Rao, M.V.H.; Lohar, K.A. An in situ laser induced breakdown spectroscope (LIBS) for Chandrayaan-2 rover: Ablation kinetics and emissivity estimations. Adv. Space Res. 2013, 52, 332–341. [Google Scholar] [CrossRef]
- Deer, W.A.; Howie, R.A.; Zussman, J. An Introduction to the Rock Forming Minerals; Longman: London, UK, 1966; p. 469. [Google Scholar]
- Mandal, P.K.; Mandal, T.K. Anion water in gypsum (CaSO4·2H2O) and hemihydrate (CaSO4·1/2H2O). Cem. Concr. Res. 2002, 32, 313–316. [Google Scholar] [CrossRef]
- Heijnen, W.M.M.; Hartman, P. Structural morphology of gypsum (CaSO4·2H2O), brushite (CaHPO4·2H2O) and pharmacolite (CaHAsO4·2H2O). J. Cryst. Growth 1991, 108, 290–300. [Google Scholar] [CrossRef]
- Yen, A.; Ming, D.; Vaniman, D.; Gellert, R.; Blake, D.; Morris, R.; Morrison, S.; Bristow, T.; Chipera, S.; Edgett, K.; et al. Multiple stages of aqueous alteration along fractures in mudstone and sandstone strata in Gale Crater, Mars. Earth Planet. Sci. Lett. 2017, 471, 186–198. [Google Scholar] [CrossRef]
- Liao, X.; Zhang, J.; Li, J.; Chigira, M.; Wu, X. Study on the chemical weathering of black shale in northern Guangxi, China. In Proceedings of the 10 Asian Reginal Confrence of IAEG, Kyoto, Japan, 26–29 September 2015. [Google Scholar]
- Al-Youssef, M. Gypsum Crystals Formation and Habits, Dukhan Sabkha, Qatar. J. Earth Sci. Clim. Chang. 2015, 6, 321. [Google Scholar] [CrossRef]
- Kuhn, T.; Herzig, P.; Hannington, M.; Garbe-Schönberg, D.; Stoffers, P. Origin of fluids and anhydrite precipitation in the sediment-hosted Grimsey hydrothermal field north of Iceland. Chem. Geol. 2003, 202, 5–21. [Google Scholar] [CrossRef]
- Stawski, T.M.; van Driessche, A.E.S.; Ossorio, M.; Diego Rodriguez-Blanco, J.; Besselink, R.; Benning, L.G. Formation of calcium sulfate through the aggregation of sub-3 nanometre primary species. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef]
- Palacio, S.; Azorín, J.; Montserrat-Martí, G.; Ferrio, J.P. The crystallization water of gypsum rocks is a relevant water source for plants. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef]
- Joanna, J. Crystallization, Alternation and Recrystallization of Sulphates. In Advances in Crystallization Processes; InTech: London, UK, 2012. [Google Scholar]
- Freyer, D.; Voigt, W. Crystallization and Phase Stability of CaSO4 and CaSO4—Based Salts. Mon. Chem. 2003, 134, 693–719. [Google Scholar] [CrossRef]
- Pirlet, H.; Wehrmann, L.M.; Brunner, B.; Frank, N.; DeWanckele, J.; Van Rooij, D.; Foubert, A.; Swennen, R.; Naudts, L.; Boone, M.; et al. Diagenetic formation of gypsum and dolomite in a cold-water coral mound in the Porcupine Seabight, off Ireland. Sedimentology 2009, 57, 786–805. [Google Scholar] [CrossRef]
- Whitlock, H.P. Desert Roses. Natural. History 1930, 3, 421–425. [Google Scholar]
- Macfadyen, W.A. Sandy Gypsum Crystals from Berbera, British Somaliland. Geol. Mag. 1950, 87, 409–420. [Google Scholar] [CrossRef]
- Eswaran, H.; Zi-Tong, G. Properties, genesis, classification, and distribution of soils with gypsum. In Occurrence, Characteristics, and Genesis of Carbonate, Gypsum, and Silica Accumulations in Soils; Nettleton, W.D., Ed.; Soil Science Society of America Special Publication; SSSA: Madison, WI, USA, 1991; Volume 26, pp. 89–119. [Google Scholar]
- Watson, A. Structure, chemistry and origins of gypsum crusts in southern Tunisia and the central Namib Desert. Sedimentology 1985, 32, 855–875. [Google Scholar] [CrossRef]
- Chitale, D.V. Natroalunite in a Laterite Profile over Deccan Trap Basalts at Matanumad, Kutch, India. Clays Clay Miner. 1987, 35, 196–202. [Google Scholar] [CrossRef]
- Greenberger, R.N.; Mustard, J.F.; Kumar, P.S.; Dyar, M.D.; Breves, E.A.; Sklute, E.C. Low temperature aqueous alteration of basalt: Mineral assemblages of Deccan basalts and implications for Mars. J. Geophys. Res. Planets 2012, 117. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Mitra, S.; Gupta, S.; Jain, N.; Chauhan, P.; Parthasarathy, G.; Ajai. Jarosite occurrence in the Deccan Volcanic Province of Kachchh, western India: Spectroscopic studies on a Martian analog locality. J. Geophys. Res. Planets 2016, 121, 402–431. [Google Scholar] [CrossRef]
- Halder, S.K.; Deb, M.; Goodfellow, W.D. Geology and mineralization of Rajpura-Dariba Lead-Zinc belt, Rajasthan. In International Workshop Proceedings; University of Delhi: Delhi, India, 2001; pp. 177–187. [Google Scholar]
- National Institute of Standards and Technology, Electronic Database. Available online: http://physics.nist.gov/PhysRefData/ASD/lines form.html (accessed on 11 June 2019).
- Rai, A.K.; Maurya, G.S.; Kumar, R.; Pathak, A.K.; Pati, J.K.; Rai, A.K. Analysis and Discrimination of Sedimentary, Metamorphic, and Igneous Rocks Using Laser-Induced Breakdown Spectroscopy. J. Appl. Spectrosc. 2017, 83, 1089–1095. [Google Scholar] [CrossRef]
- Gottfried, J.L.; Harmon, R.S.; De Lucia, F.C.; Miziolek, A.W. Multivariate analysis of laser-induced breakdown spectroscopy chemical signatures for geomaterial classification. Spectrochim. Acta Part B At. Spectrosc. 2009, 64, 1009–1019. [Google Scholar] [CrossRef]
- Rai, A.K.; Pati, J.K.; Kumar, R. Spectro-chemical study of moldavites from Ries impact structure (Germany) using LIBS. Opt. Laser Technol. 2019, 117, 146–157. [Google Scholar] [CrossRef]
- Rai, A.K.; Singh, A.K.; Pati, J.K.; Gupta, S.; Chakarvorty, M.; Niyogi, A.; Pandey, A.; Dwivedi, M.M.; Pandey, K.; Prakash, K. Assessment of topsoil contamination in an urbanized interfluve region of Indo-Gangetic Plains (IGP) using magnetic measurements and spectroscopic techniques. Environ. Monit. Assess. 2019, 191, 403. [Google Scholar] [CrossRef]
- Forni, O.; Maurice, S.; Gasnault, O.; Wiens, R.C.; Cousin, A.; Clegg, S.M.; Sirven, J.-B.; Lasue, J. Independent component analysis classification of laser induced breakdown spectroscopy spectra. Spectrochim. Acta Part B At. Spectrosc. 2013, 86, 31–41. [Google Scholar] [CrossRef]
- Forni, O.; Gaft, M.; Toplis, M.; Clegg, S.M.; Ollila, A.; Sautter, V.; Nachon, M.; Gasnault, O.; Mangold, N.; Maurice, S.; et al. First fluorine detection on Mars with ChemCam on-board MSL. In Proceedings of the 45th Lunar and Planetary Science Conference, The Woodlands, TX, USA, 17–21 March 2014; p. 1328. [Google Scholar]
- Cremers, D.A.; Radziemski, L.J. Handbook of Laser Induced Breakdown Spectroscopy; JohnWiley: Chichester, UK, 2006. [Google Scholar]
- Rinaudo, C.; Franchini-Angela, M.; Boistelle, R. Curvature of gypsum crystals induced by growth in the presence of impurities. Mineral. Mag. 1989, 53, 479–482. [Google Scholar] [CrossRef]
- Moore, G.W. Origin and Chemical Composition of Evaporite Deposits; Open-File Rep.174; U.S. Geological Survey: Reston, VA, USA, 1960.
- Dean, W.E.; Tung, A.L. Trace and minor elements in anhydrite and halite, Supai Formation (Permian), East central Arizona. 4th International Symposium on Salt, Cleveland, Northern Ohio. Geol. Soc. 1974, 1, 75–285. [Google Scholar]
- Kushnir, J. The coprecipitation of strontium, magnesium sodium, potassium and chloride ions with gypsum: A1n experiment study. Geochim. Cosmochim. Acta 1980, 44, 1471–1482. [Google Scholar] [CrossRef]
- Kushnir, J. The composition and origin of brines during the Messinian desiccation event in the Meditterranean Basin as deduced from concentrations of ions coprecipitated with gypsum and anhydrite. Chem. Geol. 1982, 35, 333–350. [Google Scholar] [CrossRef]
- Warren, J. Evaporites: Their Evolution and Economics; Blackwell Science: Oxford, UK, 1999. [Google Scholar]
- Rapin, W.; Meslin, P.-Y.; Maurice, S.; Vaniman, C.; Nachon, M.; Mangold, N.; Schröder, S.; Gasnault, O.; Forni, O.; Wiens, R.C.; et al. Hydration state of calcium sulfates in Gale crater, Mars: Identification of bassanite veins. Earth Planet. Sci. Lett. 2016, 452, 197–205. [Google Scholar] [CrossRef]








| Element | Acicular Gypsum | Desert Rose | Kachchh Gypsum | RajpuraDariba Gypsum 1 | RajpuraDariba Gypsum 2 |
|---|---|---|---|---|---|
| H (1) | 656.3 (I) | 656.3 (I) | 656.3 (I) | 656.3 (I) | 656.3 (I) |
| N (7) | 744.2(I), 746.8(I), 868.3(I) | 744.2(I), 746.8(I), 868.3(I) | 744.2(I), 746.8(I), 868.3(I) | 744.2(I), 746.8(I), 868.3(I) | 744.2(I), 746.8(I), 868.3(I) |
| O(8) | 777.4(I), 844.6(I), 926.6(I) | 777.4(I), 844.6(I), 926.6(I) | 777.4(I), 844.6(I), 926.6(I) | 777.4(I), 844.6(I), 926.6(I) | 777.4(I), 844.6(I), 926.6(I) |
| Na (11) | 588.9(I), 589.5(I) | 588.9(I), 589.5(I) | 588.9(I), 589.5(I) | 588.9(I), 589.5(I) | 588.9(I), 589.5(I) |
| Mg (12) | - | 279.0(II), 279.5(II), 280.2(II), 285.2(I), | 279.0(II), 279.5(II), 280.2(II), 285.2(I), | 279.0(II), 279.5(II), 280.2(II), 285.2(I), | 279.0(II), 279.5(II), 280.2(II), 285.2(I), |
| Al (13) | - | 308.2(I), 309.2(I), 394.3(I), 396.1(I) | 308.2(I), 309.2(I), 394.3(I), 396.1(I) | - | - |
| Si (14) | - | 220.7(I), 221.0(I), 221.6(I), 250.6(I), 251.4(I), 251.6(I), 251.9(I), 252.8(I), 288.1(I), 390.5(I), 413.1(II), 557.6(II), 634.7(II), 716.5(I), 741.5(I) | 220.7(I), 221.0(I), 221.6(I), 250.6(I), 251.4(I), 251.6(I), 251.9(I), 252.8(I), 288.1(I), 390.5(I), 413.1(II), 557.6(II), 634.7(II) | 252.8(I), 288.1(I) | - |
| Element | Acicular Gypsum | Desert Rose | Kachchh Gypsum | Rajpura-Dariba Gypsum 1 | Rajpura-Dariba Gypsum 2 |
|---|---|---|---|---|---|
| S (16) | 542.8(I), 543.2(I), 545.3(I) | 542.8(I), 543.2(I), 545.3(I) | 542.8(I), 543.2(I), 545.3(I) | 542.8(I), 543.2(I), 545.3(I) | 542.8(I), 543.2(I), 545.3(I) |
| K (19) | 766.4(I), 769.8(I) | 766.4(I), 769.8(I) | 766.4(I), 769.8(I) | 766.4(I), 769.8(I) | 766.4(I), 769.8(I) |
| Ti (22) | - | - | 334.9(II), 336.1(II) | - | - |
| Mn (25) | - | - | 257.6(II), 403.0(I) | - | - |
| Fe (26) | - | 238.2(II), 239.5(II), 240.4(II), 249.3(II), 252.3(I), 259.9(II), 258.5(II), 259.8(II), 261.1(II)263.1(II), 271.9(I), 273.9(II), 274.9(II), 275.5(II), 344.0(I), 358.1(I), 373.7(I) | 238.2(II), 239.5(II), 240.4(II), 249.3(II), 252.3(I), 273.9(II), 274.9(II), 275.5(II), 344.0(I) | - | - |
| Sr (38) | - | - | - | - | 407 (I) |
| Ba (56) | - | - | - | - | 455.4(II), 493.3(II) |
| Sample | Calcium Concentration | Sulfur Concentration | ||||
|---|---|---|---|---|---|---|
| EPMA Reference Value | PLSR Predicted Value | Accuracy * | EPMA Reference Value | PLSR Predicted Value | Accuracy * | |
| (a) | 24.82 | 24.115 | 101.09 | 10.76 | 11.290 | 104.92 |
| (b) | 26.45 | 25.190 | 95.23 | 13.23 | 12.113 | 91.55 |
| (c) | 25.48 | 26.878 | 105.48 | 10.91 | 10.197 | 93.46 |
| (d) | 28.99 | 28.695 | 98.98 | 14.82 | 15.406 | 103.95 |
| (e) | 23.71 | 23.41 | 98.83 | 11.23 | 10.865 | 96.74 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rai, A.K.; Pati, J.K.; Parigger, C.G.; Rai, A.K. Plasma Spectroscopy of Various Types of Gypsum: An Ideal Terrestrial Analogue. Atoms 2019, 7, 72. https://doi.org/10.3390/atoms7030072
Rai AK, Pati JK, Parigger CG, Rai AK. Plasma Spectroscopy of Various Types of Gypsum: An Ideal Terrestrial Analogue. Atoms. 2019; 7(3):72. https://doi.org/10.3390/atoms7030072
Chicago/Turabian StyleRai, Abhishek K., Jayanta K. Pati, Christian G. Parigger, and Awadhesh K. Rai. 2019. "Plasma Spectroscopy of Various Types of Gypsum: An Ideal Terrestrial Analogue" Atoms 7, no. 3: 72. https://doi.org/10.3390/atoms7030072
APA StyleRai, A. K., Pati, J. K., Parigger, C. G., & Rai, A. K. (2019). Plasma Spectroscopy of Various Types of Gypsum: An Ideal Terrestrial Analogue. Atoms, 7(3), 72. https://doi.org/10.3390/atoms7030072






