Temporally and Spatially Resolved Emission Spectroscopy of Hydrogen, Cyanide and Carbon in Laser-Induced Plasma
Abstract
1. Introduction
2. Experimental Details
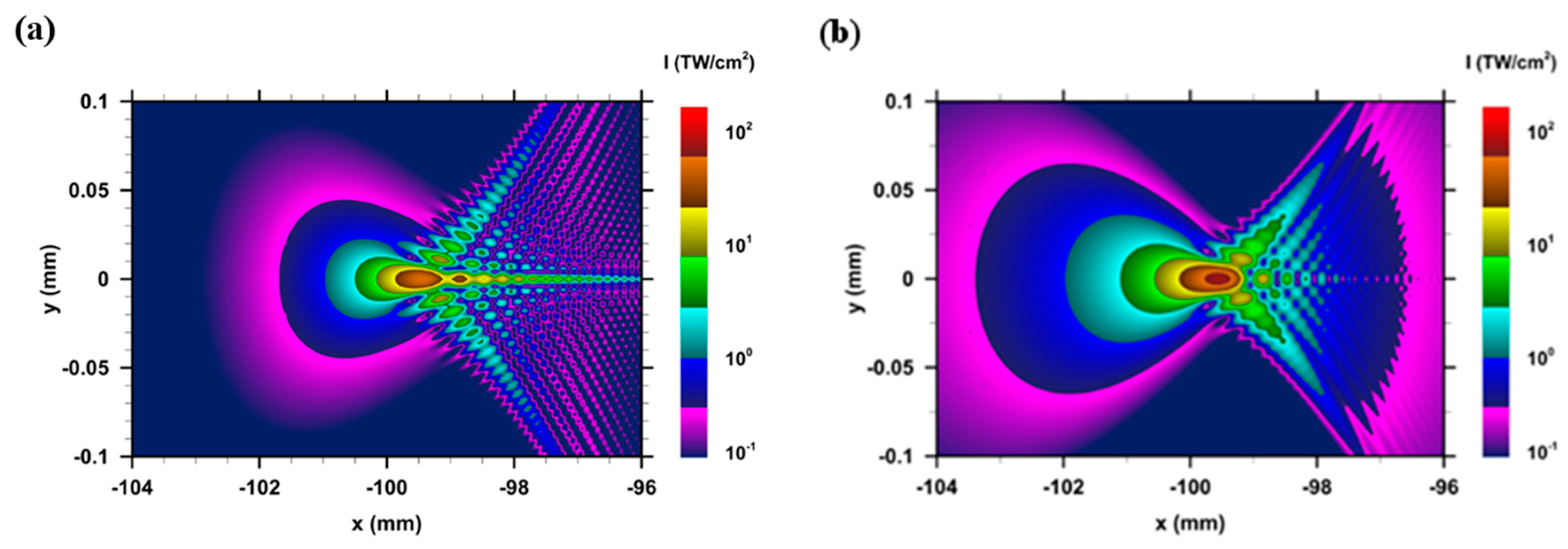
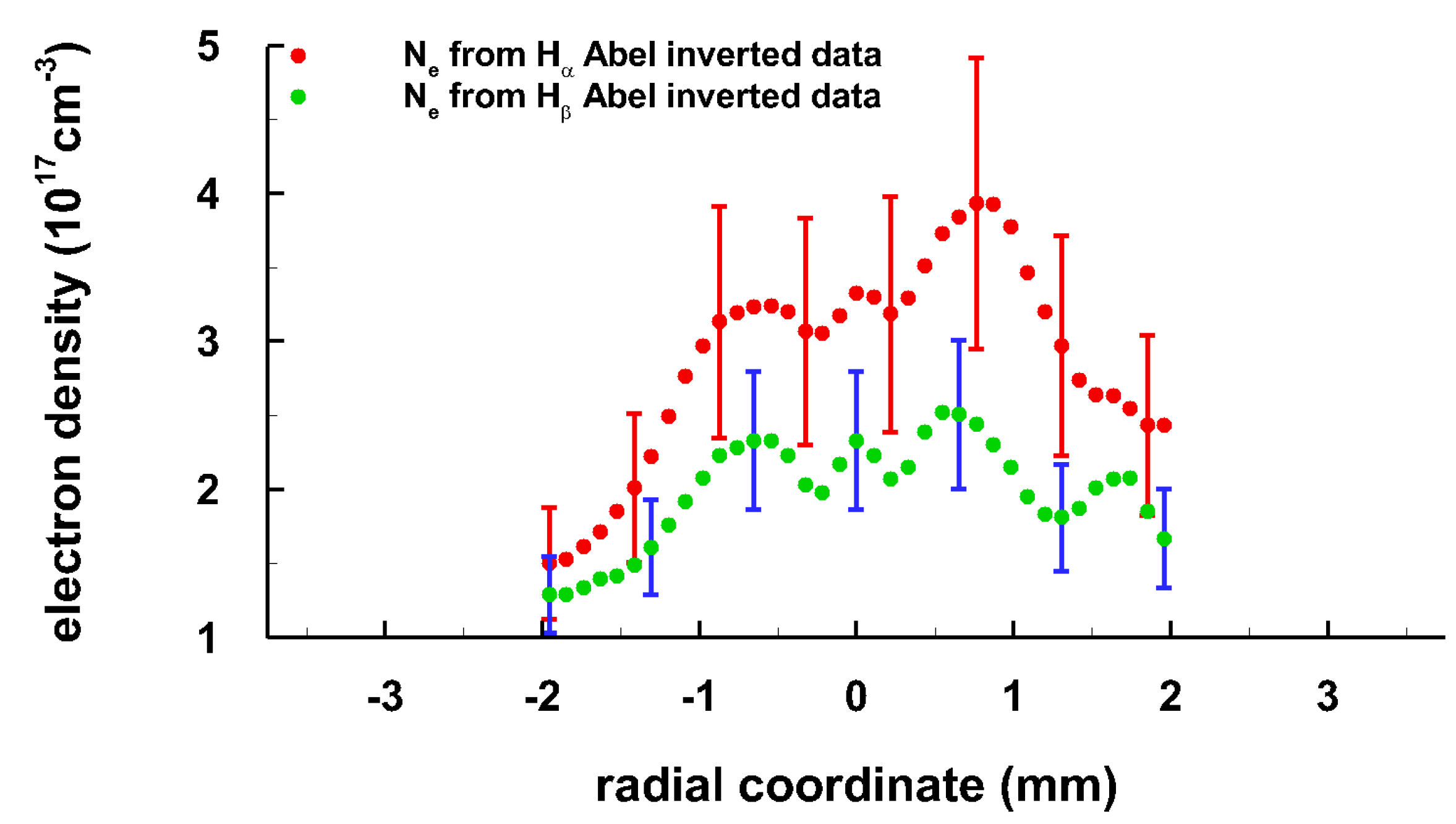
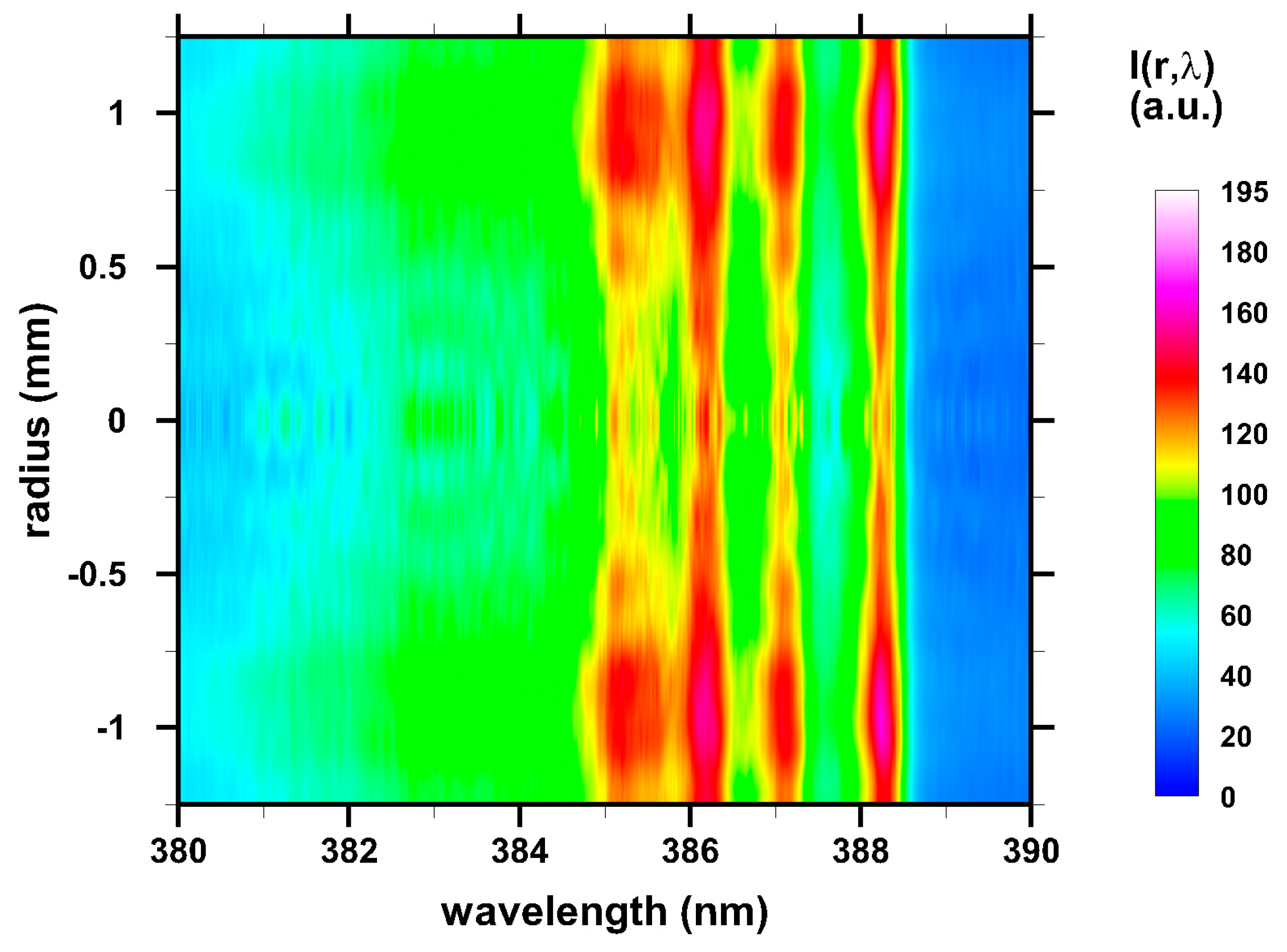
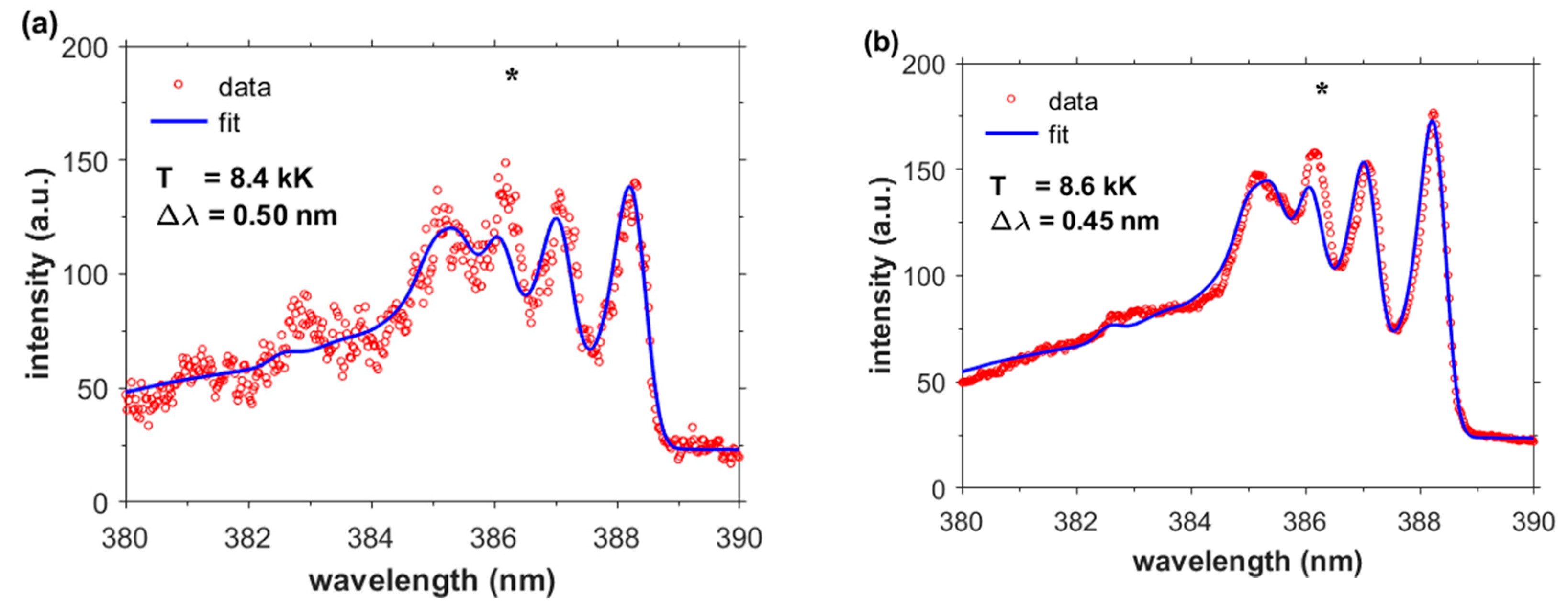
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cremers, D.A.; Radziemski, L.J. Handbook of Laser-Induced Breakdown Spectroscopy; Wiley & Sons Ltd.: Hoboken, NY, USA, 2006. [Google Scholar]
- Singh, J.P.; Thakur, S.N. (Eds.) Laser Induced Breakdown Spectroscopy; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Hahn, D.W.; Omenetto, N. Laser-Induced Breakdown Spectroscopy (LIBS), Part I: Review of Basic Diagnostics and Plasma-Particle Interactions: Still-Challenging Issues within the Analytical Plasma Community. Appl. Spectrosc. 2010, 64, 335A–336A. [Google Scholar] [CrossRef] [PubMed]
- Hahn, D.W.; Omenetto, N. Laser-Induced Breakdown Spectroscopy (LIBS), Part II: Review of Instrumental and Methodological Approaches to Material Analysis and Applications to Different Fields. Appl. Spectrosc. 2012, 66, 347–419. [Google Scholar] [CrossRef] [PubMed]
- Parigger, C.G.; Surmick, D.M.; Gautam, G.; EI Sherbini, A.M. Hydrogen alpha laser ablation plasma diagnostic. Opt. Lett. 2015, 40, 3436–3439. [Google Scholar] [CrossRef] [PubMed]
- Parigger, C.G. Laser-induced breakdown in gases: Experiments and simulation. In Laser Induced Breakdown Spectroscopy; Miziolek, V., Palleschi, I.S., Eds.; Cambridge University Press: Cambridge, MA, USA, 2006. [Google Scholar]
- Dong, M.; Lu, J.; Yao, S.; Zhong, Z.; Li, J.; Li, J.; Lu, W. Experimental study on the characteristics of molecular emission spectroscopy for the analysis of solid materials containing C and N. Opt. Express 2011, 19, 17021–17029. [Google Scholar] [CrossRef] [PubMed]
- Parigger, C.G.; Dackman, M.; Hornkohl, J.O. Time-resolved spectroscopy measurements of hydrogen-alpha, -beta, and -gamma emissions. Appl. Opt. 2008, 47, G1–G6. [Google Scholar] [CrossRef]
- Dong, M.; Chan, G.C.-Y.; Mao, X.; Gonzalez, J.J.; Lu, J.; Russo, R.E. Elucidation of C2 and CN formation mechanisms in laser-induced plasmas through correlation analysis of carbon isotopic ratio. Spectrochim. Acta Part B At. Spectrosc. 2014, 100, 62–69. [Google Scholar] [CrossRef]
- Kotzagianni, M.; Couris, S. Femtosecond laser induced breakdown spectroscopy of air–methane mixtures. Chem. Phys. Lett. 2013, 561, 36–41. [Google Scholar] [CrossRef]
- Minorsky, P.V. On the Inside. Plant Physiol. 2011, 155, 169–170. [Google Scholar] [CrossRef]
- Baudelet, M.; Guyon, L.; Yu, J.; Wolf, J.-P.; Amodeo, T.; Fréjafon, E.; Laloi, P. Spectral signature of native CN bonds for bacterium detection and identification using femtosecond laser-induced breakdown spectroscopy. J. Appl. Phys. 2006, 88, 063901. [Google Scholar] [CrossRef]
- Baudelet, M.; Guyon, L.; Yu, J.; Wolf, J.-P.; Amodeo, T.; Fréjafon, E.; Laloi, P. Femtosecond time-resolved laser-induced breakdown spectroscopy for detection and identification of bacteria: A comparison to the nanosecond regime. J. Appl. Phys. 2006, 99, 084701. [Google Scholar] [CrossRef]
- Ma, J.; Dasgupta, P.L. Recent developments in cyanide detection: A review. Anal. Chim. Acta 2010, 673, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Parigger, C.G.; Surmick, D.M.; Gautam, G. Self-absorption characteristics of measured laser-induced plasma line shapes. J. Phys. Conf. Ser. 2017, 810, 012012. [Google Scholar] [CrossRef]
- Parigger, C.G.; Helstern, C.M.; Gautam, G. Molecular emission spectroscopy of cyanide in laser-induced plasma. Int. Rev. At. Mol. Phys. 2017, 8, 25–35. [Google Scholar]
- Pretzler, G. A New Method for Numerical Abel-Inversion. Z. Naturforschung 1991, 46a, 639–641. [Google Scholar]
- Pretzler, G.; Jäger, H.; Neger, T.; Philipp, H.; Woisetschläger, J. Comparison of Different Methods of Abel Inversion Using Computer Simulated and Experimental Side-On Data. Z. Naturforschung 1992, 47, 955. [Google Scholar]
- Parigger, C.G.; Gautam, G.; Surmick, D.M. Radial electron density measurements in laser-induced plasma from Abel inverted hydrogen Balmer beta line profiles. Int. Rev. At. Mol. Phys. 2015, 6, 43–55. [Google Scholar]
- Helstern, C.M.; Parigger, C.G. Time-resolved plasma spectroscopy of diatomic molecular cyanide. J. Phys. Conf. Ser. 2019, in press. [Google Scholar]
- Parigger, C.G.; Surmick, D.M.; Helstern, C.M.; Gautam, G.; Bol’shakov, A.A. Molecular Laser-Induced Breakdown Spectroscopy. In Laser Induced Breakdown Spectroscopy; Singh, J.P., Thakur, S.N., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; in press. [Google Scholar]
- Eschlböck-Fuchs, S.; Demidov, A.; Gornushkin, I.; Schmid, T.; Rössler, R.; Huber, N.; Panne, U.; Pedarnig, J. Tomography of homogenized laser-induced plasma by Radon transform technique. Spectrochim. Acta Part B At. Spectrosc. 2016, 123, 59–67. [Google Scholar] [CrossRef]
- McBride, B.J.; Gordon, S. Computer Program for Calculating and Fitting Thermodynamic Functions. NASA RP-1271; 1992. Available online: https://cearun.grc.nasa.gov/ (accessed on 26 November 2017).
- Kroll, N.; Watson, K.M. Theoretical Study of Ionization of Air by Intense Laser Pulses. Phys. Rev. A 1972, 5, 1883–1905. [Google Scholar] [CrossRef]
- Thiyagarajan, M.; Thompson, S. Optical breakdown threshold investigation of 1064 nm laser induced air plasmas. J. Appl. Phys. 2012, 111, 073302. [Google Scholar] [CrossRef]
- Gautam, G.; Helstern, C.M.; Drake, K.A.; Parigger, C.G. Imaging of Laser-induced Plasma Expansion Dynamics in Ambient Air. Int. Rev. At. Mol. Phys. 2016, 7, 45–51. [Google Scholar]
- Chen, Y.-L.; Lewis, J.W.L.; Parigger, C. Spatial and Temporal Profiles of Pulsed Laser-Induced Air Plasma Emissions. J. Quant. Spectrosc. Radiat. Transf. 2000, 67, 91–103. [Google Scholar] [CrossRef]
- Taylor, G. The Formation of a Blast Wave by a Very Intense Explosion. II. The Atomic Explosion of 1945. Proc. R. Soc. A 1950, 201, 175–186. [Google Scholar]
- Campanella, B.; Legnaioli, S.; Pagnotta, S.; Poggialini, F.; Palleschi, V. Shock Waves in Laser-Induced Plasmas. Atoms 2019, 7, 57. [Google Scholar] [CrossRef]
- Gautam, G.; Parigger, C.G.; Helstern, C.M.; Drake, K.A. Emission Spectroscopy of Expanding Laser-Induced Gaseous Hydrogen-Nitrogen Plasma. Appl. Opt. 2017, 33, 9277–9284. [Google Scholar] [CrossRef]
- Hornkohl, J.O.; Parigger, C.G.; Lewis, J.W.L. Temperature Measurements from CN Spectra in a Laser-Induced Plasma. J. Quant. Spectrosc. Radiat. Transf. 1991, 46, 405–411. [Google Scholar] [CrossRef]
- Parigger, C.G.; Woods, A.C.; Surmick, D.M.; Gautam, G.; Witte, M.J.; Hornkohl, J.O. Computation of diatomic molecular spectra for selected transitions of aluminum monoxide, cyanide, diatomic carbon, and titanium monoxide. Spectrochim. Acta Part B 2015, 107, 132–138. [Google Scholar] [CrossRef]
- Parigger, C.G. Measurements of Gaseous Hydrogen–Nitrogen Laser-Plasm. Atoms 2019, 7, 61. [Google Scholar] [CrossRef]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parigger, C.G.; Helstern, C.M.; Gautam, G. Temporally and Spatially Resolved Emission Spectroscopy of Hydrogen, Cyanide and Carbon in Laser-Induced Plasma. Atoms 2019, 7, 74. https://doi.org/10.3390/atoms7030074
Parigger CG, Helstern CM, Gautam G. Temporally and Spatially Resolved Emission Spectroscopy of Hydrogen, Cyanide and Carbon in Laser-Induced Plasma. Atoms. 2019; 7(3):74. https://doi.org/10.3390/atoms7030074
Chicago/Turabian StyleParigger, Christian G., Christopher M. Helstern, and Ghaneshwar Gautam. 2019. "Temporally and Spatially Resolved Emission Spectroscopy of Hydrogen, Cyanide and Carbon in Laser-Induced Plasma" Atoms 7, no. 3: 74. https://doi.org/10.3390/atoms7030074
APA StyleParigger, C. G., Helstern, C. M., & Gautam, G. (2019). Temporally and Spatially Resolved Emission Spectroscopy of Hydrogen, Cyanide and Carbon in Laser-Induced Plasma. Atoms, 7(3), 74. https://doi.org/10.3390/atoms7030074





