1. Introduction
Nicotinic acetylcholine receptors (nAChRs) play important physiological roles in the body, particularly in the brain, autonomic nervous system, and at neuromuscular junctions [
1]. Seventeen known nAChR subunits co-assemble as pentamers in various combinations to form multiple receptor subtypes [
2] and the process of how these receptors fold and assemble is not well understood. To date, several proteins have been identified as chaperones during nAChR assembly and are required for the surface expression of many nAChR receptor subtypes. At least two chaperones, Resistance to Inhibitors of Cholinesterase 3 (RIC3) and TMEM35A/Nicotinic Acetylcholine Regulator (NACHO) participate in folding, assembly and surface expression of the α7nAChR subtype as measured by the ability of cell surface receptors to bind alpha-bungarotoxin [
3,
4,
5,
6]. RIC3 was originally identified in a screen for mutations that allow the nematode C. elegans to survive after exposure to aldicarb, an acetylcholinesterase inhibitor [
7]. RIC3 is highly conserved across animal species [
8] and plays a critical role in regulating the assembly of the α7nAChR subtype as well as related serotonin 5HT3 receptor subtypes [
2,
6]. TMEM35A protein (Transmembrane protein 35A) was originally called TUF1 (for The Unknown Factor-1) [
9]. Kennedy et al. prepared antibodies against TMEM35A (available as Sigma cat. # HPA048583) and generated a knockout (KO) animal [
10]. These proved useful when David Bredt’s lab used an unbiased calcium influx screen, showing that TMEM35A is an important chaperone for α7nAChR functional expression [
3,
4]. Although TMEM35A is still the official gene name, Bredt’s group has renamed the protein NACHO. Hereafter,
tmem35a and NACHO refers to the encoding gene and polypeptide, respectively. Similarly, RIC3 refers to the protein and
ric3 to the gene, while
chrna7 refers to the gene for the α7nAChR subunit. The
tmem35a gene is located on the X chromosome of both mice and humans and is unrelated to
tmem35b, a gene with little sequence homology found on other chromosomes (#1 in human, #4 in mice).
Unlike antibodies available for NACHO, we previously demonstrated that no suitable antibodies for Western blot (WB) analysis of mouse, rat and human RIC3 were available in 2013 [
11]. Such a lack of working antibodies limits the investigation into the precise role of RIC3 in facilitating α7nAChR expression in mammalian species. More importantly, a lack of reliable antibodies can lead to inconsistent and non-reproducible results, which pose a serious problem in biological research. However, several new antibodies are now available on the market. We sought to determine their specificity across several species including human and mouse. We also investigated whether RIC3 splice variants and single nucleotide polymorphisms (SNPs) affect the ability of these antibodies to bind using in vitro systems. Additionally, a major hindrance to studying RIC3 effects on α7nAChR expression was the lack of a
ric3 KO animal model. We report here preliminary results using such a knockout.
Finally, many publications have shown that available antibodies for α7nAChRs are not acceptable for reliable Western blots [
12,
13,
14,
15]. Following our recent publication confirming these results [
16], Synaptic Systems contacted us to evaluate a polyclonal rabbit-anti mouse α7nAChR antibody directed against amino acids 491-502, which are identical in human and rat α7nAChRs. This paper is a progress report on the usefulness of these molecular tools for determining the respective roles of NACHO and RIC3 in promoting receptor folding, assembly and cell surface expression of α7nAChRs. While addressing these specific objectives, our findings generate new questions about the interactions between NACHO and RIC3.
3. Results
We tested whether NACHO is required for surface expression of α7nAChR by correlating the presence of NACHO in various primary and transformed cell types with their ability to bind
125I-αBGT when
chrna7 is present. We previously reported both
chrna7 mRNA and αBGT binding in primary peritoneal macrophage cells from C57Bl/6 mice and this binding is absent in macrophages derived from
chrna7 KO mice [
19]. We find that macrophages isolated from wild type animals do not express NACHO (
Figure 1A), and yet express surface α7nAChR evident by
125I-αBGT binding (
Figure 1B). We tested other cell lines (
Figure 1A,
Figure S1A) and found that endogenous NACHO is detectable only in human SH-SY5Y (
Figure S1A), rat GH3, and rat GH4C1 cells (
Figure 1A,
Figure S1A). Together, these data suggest that NACHO is not a
sine qua non for specific cell lines to fold, assemble, and traffic α7nAChRs to the cell surface.
We next tested the Synaptic Systems antibody against the mouse α7nAChR C-terminal using both human and mouse α7nAChRs expressed in HEK-293 cells (
Figure 2). The antibody showed a smear at high molecular weights (~ 80 kD) and two bands between 50 and 40 kD. The two lower bands were not present in non-transfected cells (control) or RFP-transfected cells (RFP). Cells co-transfected with
tmem35a and
hchrna7 at a 1:1 ratio showed a similar WB band pattern, but with decreased intensity, which was likely due to the decreased
chrna7 DNA concentration (
Figure 2,
hα7+tmem35a). The results showed that Synaptic Systems antibody is useful for WB analysis of human, mouse, and rat α7nAChRs in cultured cells.
A RIC3 sequence comparison among human, rat, mouse and xenopus showed the sequences that were used as antigens for six commercially available anti-RIC3 antibodies, as well as the location of the SNPs and splice variants present in our expression constructs (
Figure 3). In addition,
ric3 gene exons are highly conserved across the same four species, with an ambiguous splice site between exons 4 and 5 that leads to the presence or absence of a single serine residue (denoted S+ or S-). Our DNA constructs (with the exception of rat
ric3[S-]) were tagged with a DDK (FLAG) at the C-terminal, which was used to control for differential expression efficiency between different species (
Figure 4A).
Table 1 summarizes the properties of the six antibodies tested. All six antibodies recognized human RIC3, but only two showed staining for mouse and rat RIC3s. Thermo-Fisher anti-hRIC3 (PA5-64196) recognized both splice variants of human and mouse RIC3, and weakly rat RIC3, with a major band at approximately 40 kD (
Figure 4B). This antibody did not recognize xenopus RIC3. Mouse RIC3 (both S+ and S-) showed additional smaller bands, suggesting proteolysis despite the presence of protease inhibitors. Alomone Laboratories ANC-020 anti-RIC3 antibody showed a similar band pattern and recognized both splice variants of human and mouse RIC3, and rat RIC3 (
Figure 4C). Alomone Laboratories antibody also detected a high molecular weight non-specific band around 100 kD. Novus Biologicals H00079608-B01P anti-RIC3 weakly stained human, mouse and rat RIC3, and quickly lost its activity when stored with the other antibodies (
Figure S3).
Figure S4 shows the other three antibodies tested that recognized human RIC3, but not mouse or rat RIC3. Both Thermo-Fischer and Alomone antibodies that recognize mouse RIC3 showed reactivity to all SNPs in human
ric3 gene (
Figure S5).
ric3 KO mice showed no overt phenotype.
Figure 5A shows the results of a preliminary autoradiographic analysis of
125I-αBGT binding to brain slices from wild type,
tmem35a KO and
ric3 KO (N = 2/genotype) animals. In one experiment, hippocampal and cortical (coronal) sections of
ric3 KO mice showed a significant decrease of specific αBGT binding compared to wild type mice. Other smaller brain structures might have reduced toxin binding in
ric3 KO mice (
Figure 5A arrows). Analysis of these structures will be pursued in future studies using high-resolution images. In this experiment, the examination of pixel intensity using ImageJ indicated that
ric3 KO brain slices have approximately 50% less toxin binding in hippocampus and a significant loss of binding in the cortex compared to wild type animals (
Figure 5B). However, these results were not replicated in a second experiment (
Figure S6), suggesting that the effects of RIC3 on α7nAChR expression in brain may be subtle. In contrast,
tmem35a KOs (
Figure 5 and
Figure S6) showed a complete loss of
125I-αBGT binding in any brain regions, confirming previous findings by Gu et al. [
3,
4]. Collectively,
ric3 KO showed little effect compared to
tmem35a KO on α7nAChR expression in mouse brain.
These in vivo results contrast sharply with previous in vitro results. Gu et al. [
3,
4] report that RIC3 and NACHO act synergistically in HEK cells to promote surface α7nAChR expression as measured by electrophysiological recording and fluorescent αBGT binding. However, they did not demonstrate the effect of varying
ric3 and
tmem35a cDNA ratios on surface α7nAChR expression. Alexander et al. [
23] demonstrated that different ratios between
ric3 alone and
chrna7 sufficiently induce differential surface α7nAChR expression, with high
ric3 to
chrna7 ratios causing internal receptor aggregation and retention, resulting in an inverted U-shaped expression curve. Similarly, Ben-David et al. [
24] noted an inverted U-shaped expression curve with varying amounts of mouse
ric3 cRNA injected with a fixed amount of
chrna7 cRNA into oocytes. These data suggest that the ratios between the three genes (
ric3,
tmem35a, and
chrna7) will be important factors determining total surface receptor expression. We investigated the effects of maintaining
chrna7 level equal to the sum of the two chaperones in HEK cells (
Figure 6). RIC3 promoted surface α7nAChR expression in HEK cells as evident by αBGT binding even with the absence of NACHO expression (
Figure 6). The effects were synergistic as a 3:1
tmem35a to
ric3 ratio produced a threefold greater response than either gene alone.
We next tried to show the presence of NACHO, RIC3 and α7nAChR in hippocampal lysates of wild type,
tmem35a KO and
ric3 KO animals. In wild type brain lysates, Sigma anti-NACHO shows appropriate bands as described [
10], but not RIC3 (Thermofisher PA5-64196) or α7nAChR antibodies. Despite attempts using different lysis buffers, including RIPA, RIPA enhanced with 1% triton X100 and the buffer used by Kennedy et al. [
10] for NACHO (10 mM Tris, pH 7.4, 100 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM NaF, 20 mM Na
4P
2O
7, 2 mM Na
3VO
4, 1% Triton X-100, 10% glycerol, 0.1% SDS, 0.5% deoxycholate), none has worked so far for RIC3 or α7nAChR. These results indicate technical or biological challenges (e.g., post-translational modifications, too low expression levels). Based on the detection of the endoplasmic reticulum marker calnexin and NACHO (
Figure S7) using an extraction protocol from Gu et al. [
3], it is unlikely that technical difficulty was the source of the negative results. The available in situ hybridization data in the Allen Mouse Brain Atlas [
21] suggests the rank order for mRNA concentrations in mouse cortex is calnexin >>
tmem35a >
chrna7 >
ric3. These data suggest that low levels of α7nAChR and RIC3 in mouse brain are likely limiting factors for antibody detection.
4. Discussion
Gu et al. and Matta et al. [
3,
4] proposed that NACHO is a master regulator required for α7nAChR folding, assembly and expression on cell surfaces. However, we find the presence or absence of NACHO in various cell lines does not necessarily correlate with the ability of the cells to support surface α7nAChR expression (
Figure 1,
Figure S1). In particular, primary mouse peritoneal macrophages do not require NACHO to express surface α7nAChR evident by αBGT binding (
Figure 1), which is not detected in macrophages from
chrna7 KO animals [
19]. Previous publications report difficulties showing functional α7nAChR ion channels in macrophages or similar cell types (reviewed in [
19] but see [
25]). As such, it has been proposed that these cells express metabotropic rather than ionotropic α7nAChRs [
26]. We also tested other cell lines (
Figure S1A) and found that SH-SY5Y, not SH-EP1, cells express NACHO. While some studies showed that SH-SY5Y cells endogenously express surface α7nAChR, we do not find this (see
Figure S1A for further discussion). Mulcahy et al. [
27] report the SH-EP1 cell line lacks RIC3, but is capable of αBGT binding when transfected with
chrna7, and this binding does not change much when transfected with both
chrna7 and
ric3. Here, we show that this cell line also lacks NACHO (
Figure S1A). Taken together, these findings suggest additional factors regulating surface α7nAChR expression in these cells.
Others (and we) have previously demonstrated the problematic nature of available antibodies against α7nAChR [
12,
13,
14,
15,
16]. These antibodies failed to generate reproducible results, which is a serious problem in biological research [
28]. An ideal antibody raised against a protein will specifically recognizes its epitope on the target protein and nothing else. On this basis, the results with the Synaptic Systems antibody are encouraging. This antibody showed little non-specific binding in Western blots and the antibody recognizes receptors across multiple species using cell culture models. However, to date, we have not been able to establish its utility for α7nAChR immunohistochemistry in mouse brain.
In terms of RIC3 antibodies, antibodies that recognize homologous proteins between species are useful to detect differential protein expression across anatomical locations and cell types, while those that fail to recognize protein splice variants or SNPs may give an incomplete expression profile of a target protein. The production of antibodies often involves the selection of specific peptides as antigens. Linear protein epitopes normally consist of sequences of six to nine amino acids and conformational epitopes are often considered as linear epitopes brought together by the three-dimensional structure of proteins [
29]. However, the actual binding contacts between an antibody and its protein epitope may involve only a small subset of amino acids within linear epitope sequences. Most of the RIC3 antibodies used in this study were raised against peptides, which suggests the possibility of multiple linear epitopes distributed over the length of the peptide (e.g., all polyclonal antibodies, except the Santa Cruz monoclonal antibody). In addition, besides the alpha helices in the transmembrane domain(s) and the coiled-coiled domain, RIC3 is a largely disordered protein [
30], suggesting that antibody recognition should be based primarily on the linear amino acid sequences. However, Koperniak et al. [
11] found that antibodies against human and rat RIC3 available in 2013 were highly dependent on protein conformation and that heat denaturation destroyed all binding. Therefore, we cannot rule out a contribution of protein conformation to recognition of RIC3 epitopes by antibodies.
In order to normalize for differences in transfection efficiencies or codon usage artifacts for RIC3 expression, we primarily used C-terminal Myc-FLAG tagged versions of human, mouse and xenopus. Presumably, the tag would not interfere with antibody binding. Wang et al. [
31] demonstrated that C-terminal Myc or GFP tagging does not interfere with full-length mouse RIC3 activity. RIC3 exists in various isoforms due to alternate splicing. Alternate splicing is commonly believed to expand the number of functional proteins from a fixed genome [
32], but considerable controversy exists whether many splice variants are truly functional or instead represent splicing errors (e.g., [
33]). Antibodies that discriminate between splice variants would be highly useful to help settle this question. NCBI reference sequences (RefSeq) lists nine isoforms (
a, c, e, f, g, h, i, j, k) of human
ric3 (Gene ID: 79608) based on the lettering scheme of Seredenina et al. [
34]. Isoform
a corresponds to full-length
hric3S+ and isoform
c corresponds to
hricS-. All the other isoforms are missing various exons, parts of exons, or contained insertions. These differences make it difficult to detect all splice variants with a single antibody. For instance, the first twelve amino acids (EKLINRVGPNGE) of the fourteen amino acid peptide used to produce the Alomone Laboratories antibody are present only in isoforms
a, c, and
i, even though the coding sequence is exclusively in exon 4. In contrast, the sequence of the 29 amino acid antigen from exon 6 used to produce ThermoFisher PA5-48432 is represented in seven of the nine human isoforms. However, the first twelve amino acids in the Alomone immunogen are represented in all three isoforms listed in RefSeq in mouse RIC3 (Gene ID: 320360). Ben-David et al. report a fourth mouse isoform (RIC3-TM, [
35]) consisting of only exons 1 and 2 with an alternate C terminus and this isoform has no overlap with the shorter peptides used to produce the antibodies studied here. Based on sequences, the only antibodies we tested that could recognize RIC3-TM were the Santa Cruz monoclonal and the Novus Biologicals polyclonal antibodies. We found that the former did not recognize full-length mouse RIC3 that includes exons 1 and 2. Further work will be required to determine if the Novus antibody recognizes the mouse RIC3-TM isoform. Ben-David et al. [
35] found mRNA for RIC3-TM in mouse brain and suggest it may have different functional effects from the full-length RIC3. These findings underscore the importance finding an antibody that recognizes mouse RIC3-TM. Nevertheless, Thermofisher/Invitrogen PA5-64196 and Alomone Labs ANC-020 should be useful for detecting the major mouse, rat and human RIC3 isoforms.
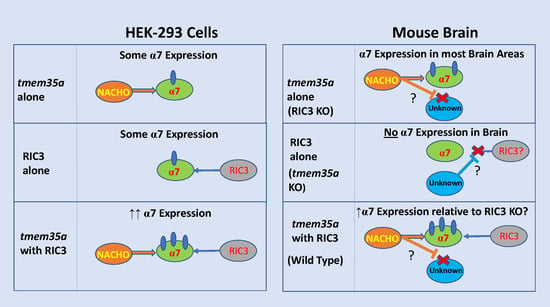
Gu et al. [
3] and Matta et al. [
4] propose that NACHO acts as a master regulator for folding and assembly of many types of nicotinic receptors including α7nAChRs. Several observations form the basis of this hypothesis for α7nAChRs: (1)
tmem35a KO completely loses αBGT binding in mouse brain (which we confirmed in
Figure 5 and
Figure S6) and the results were similar to those seen for
chrna7 knockouts [
36]; (2) RIC3 works synergistically with NACHO in heterologous expression systems such as HEK cells (which we confirmed in
Figure 6); (3)
tmem35a KO does not change
ric3 mRNA expression (Matta et al.
Figure S1B [
4]). Similarly, we found that hippocampal transcriptome of
tmem35a KO mice showed no difference in
ric3 mRNA expression (data not shown). However, these results leave unanswered whether NACHO loss of function affects the ability of brain cells to express α7nAChR subunits or RIC3 proteins. Also, if RIC3 works synergistically with NACHO in vitro, why does knocking out
tmem35a prevent RIC3 from being a functional chaperone in vivo? An alternative hypothesis to explain the data is that NACHO inhibits the action of an unidentified factor or that the lack of NACHO blocks the action of RIC3 through a mechanism, which is not found in heterologous in vitro expression systems. In this regard, as illustrated in the graphical abstract, NACHO is needed for functional RIC3 in brain cells. Using the new tools we are developing, we can start to address these issues. However, it is critically important to show that RIC3 protein is present in the brains of
tmem35a KO and wild type animals.
Preliminary autoradiographic analysis of
125I-αBGT binding to mouse brain sections confirms that knocking out
tmem35a lost virtually all αBGT binding as previously shown by Gu and Matta et al. [
3,
4]. In contrast, knocking out
ric3 causes variable effects depending on brain regions, but exact analysis of this phenomenon requires overcoming several technical hurdles. For example, the phosphor imaging sheets saturate and show a non-linear response to radioactivity. This tends to emphasize non-specific binding relative to specific binding, leading to high backgrounds. A standard curve of varying amounts of known radioactivity is necessary to convert differences in average pixel intensity to fmoles of bound αBGT per mm
3 tissue. Because of this and other factors, such as differences in
125I-αBGT specific activity and age at the time of exposure, it is not possible to compare results exactly between different autoradiographic experiments at present. Also, we need better techniques to study small brain structures, to register brain regions between different animals, and to align the histological images of the brain sections with autoradiograms. Whiteaker et al. found many small brain structures in their autoradiographic analysis of
125I-αBGT and tritiated methyllycaconitine binding in mouse brain [
37]. It may be necessary to use serial cryosectioning to make sure that small brain structures are present across several sections in comparisons between wild type and KO animals. None of these problems are insurmountable. In this study, we concentrated on large brain structures such as hippocampus and cortex due to their accessibility. However, even with this small sample size it is clear that knocking out
ric3 has less effects on α7nAChR expression than knocking out
tmem35a. These data raise new questions about the interaction of these two chaperones. Measuring the relative amounts of mRNA and respective polypeptides for the two chaperones will be essential for determining why specific chaperone loss-of-function results in differential effects in brain.
Overall, the present data supports the conclusions of Gu et al. [
3] and Matta et al. [
4] that NACHO is a master regulator of α7nAChR folding, assembly and expression in mouse brain. What remains perplexing is the striking difference between the actions of NACHO and RIC3 in vivo vs. in vitro. Is the in vitro data an artifact of ectopic chaperone overexpression in HEK cells? Or, does NACHO in vivo regulate other unknown proteins or intracellular processes that impact receptor expression in ways not yet appreciated?