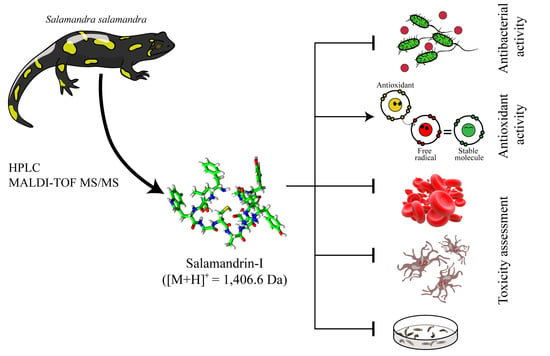
The Antioxidant Peptide Salamandrin-I: First Bioactive Peptide Identified from Skin Secretion of Salamandra Genus (Salamandra salamandra)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biological Samples
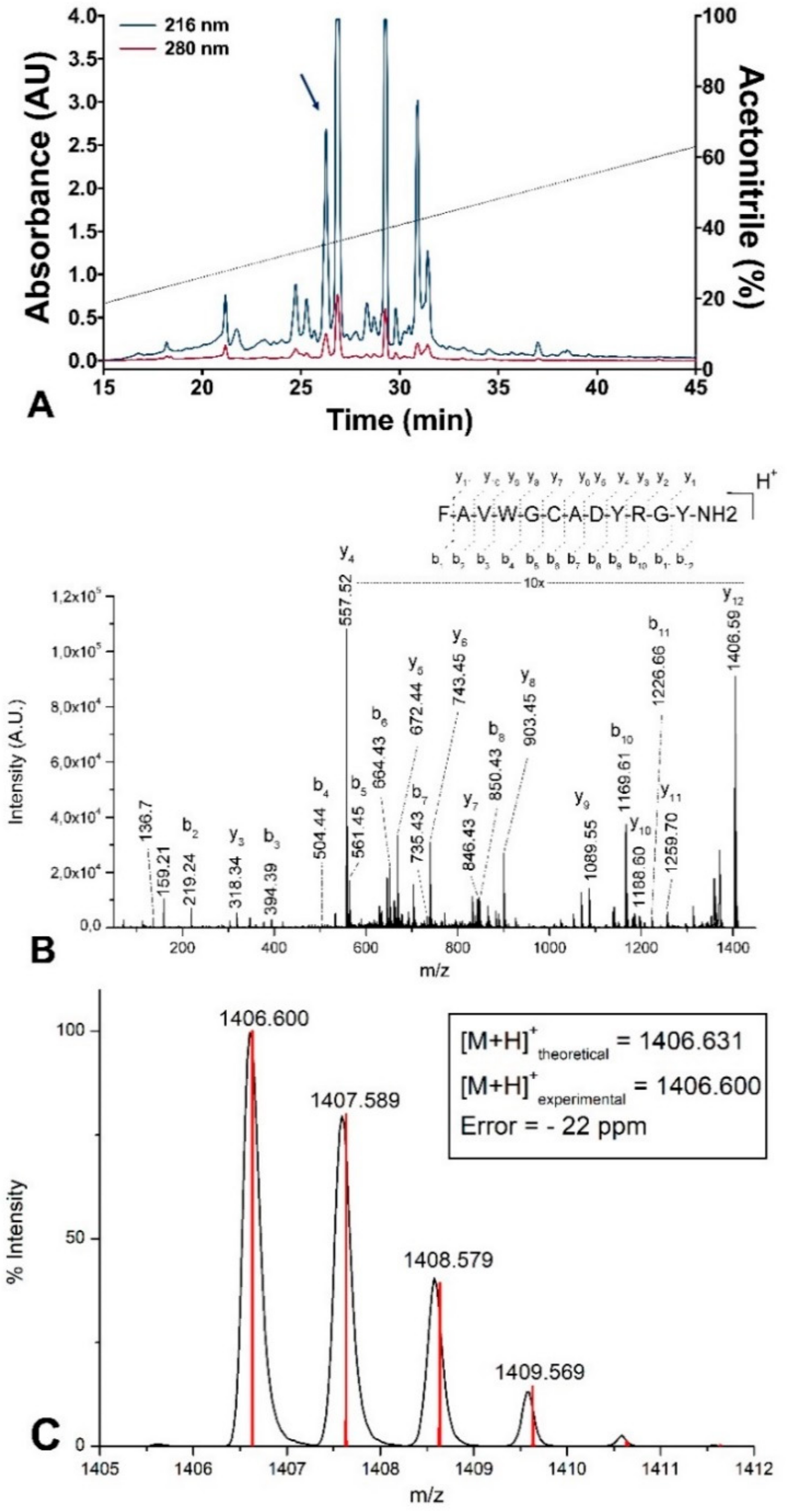
2.2. Purification and Characterization
2.3. Peptide Synthesis
2.4. Structural Studies
2.4.1. Sequence Analysis
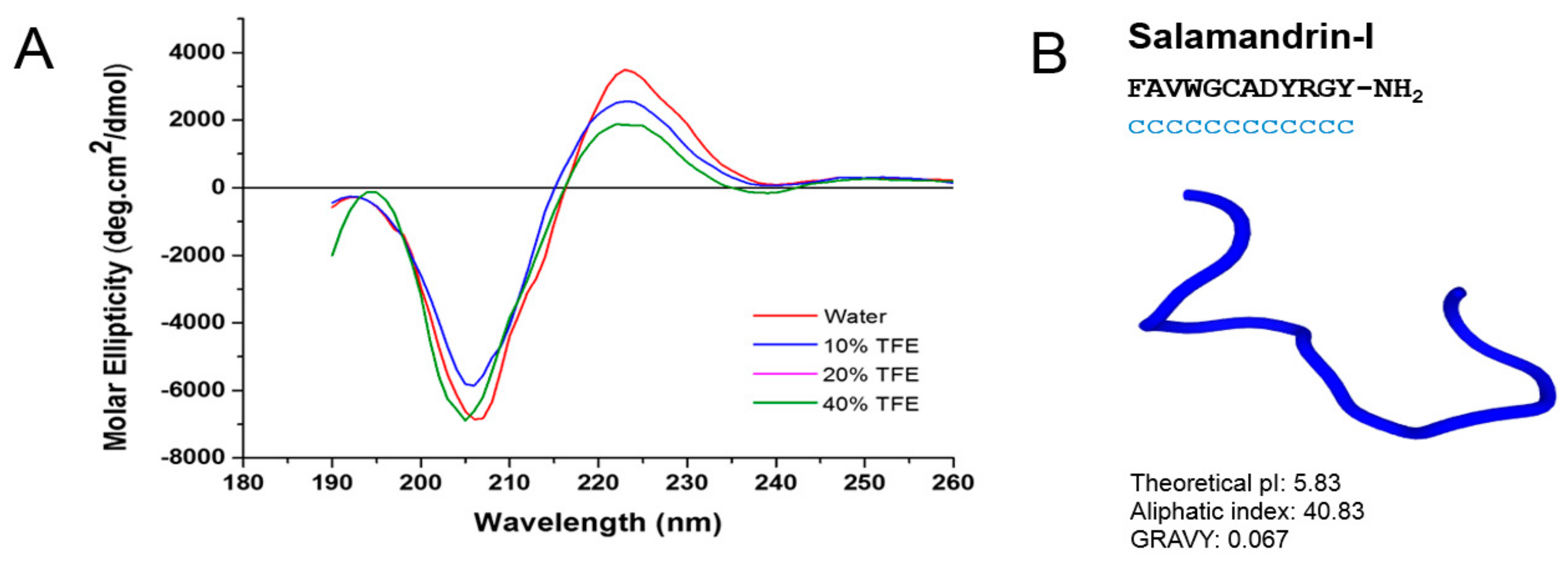
2.4.2. Circular Dichroism
2.5. In Vitro Radical Scavenging Assays
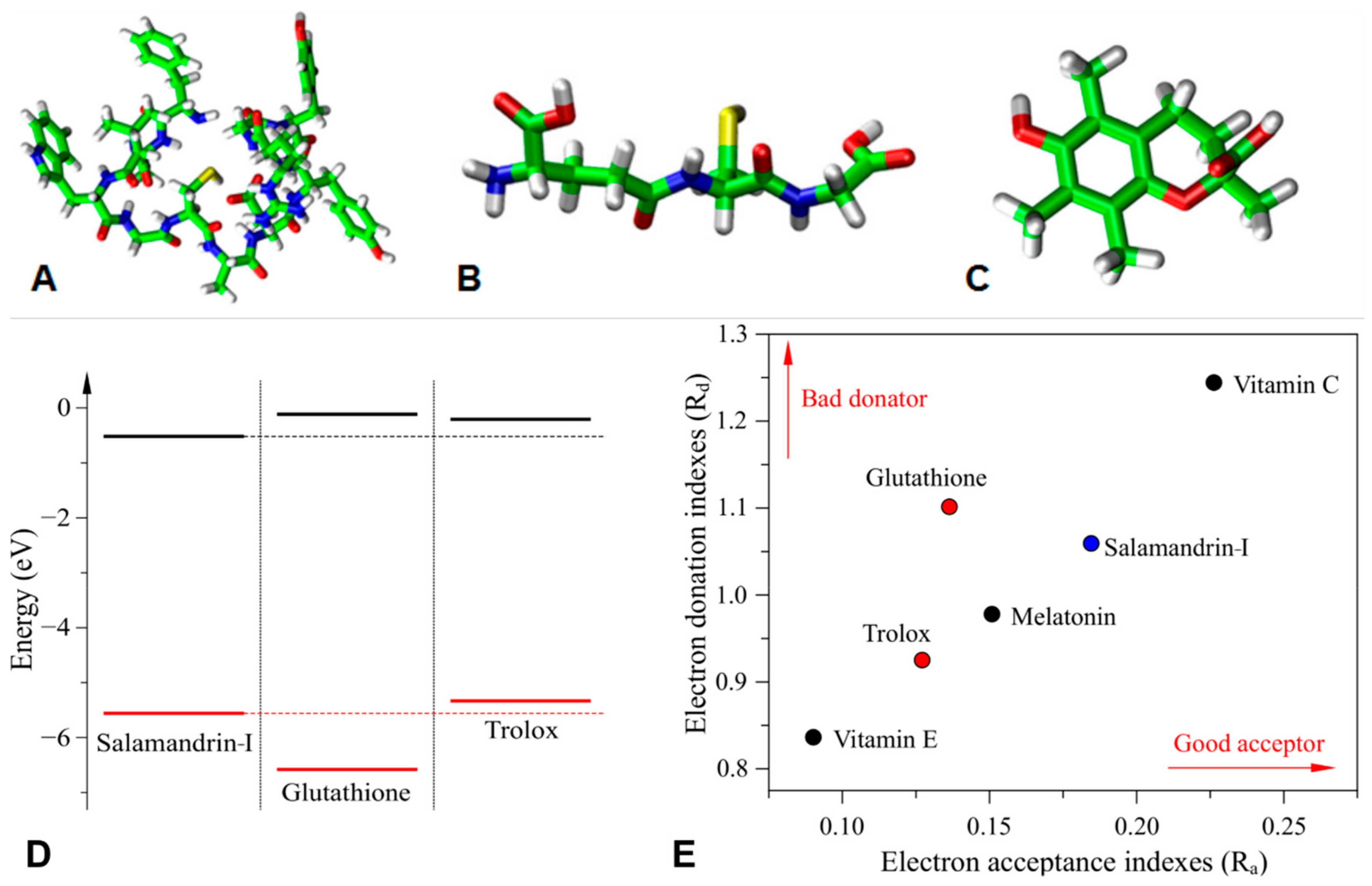
2.6. In Silico Antioxidant Studies
2.7. Antibacterial Assays
2.8. Toxicity Studies
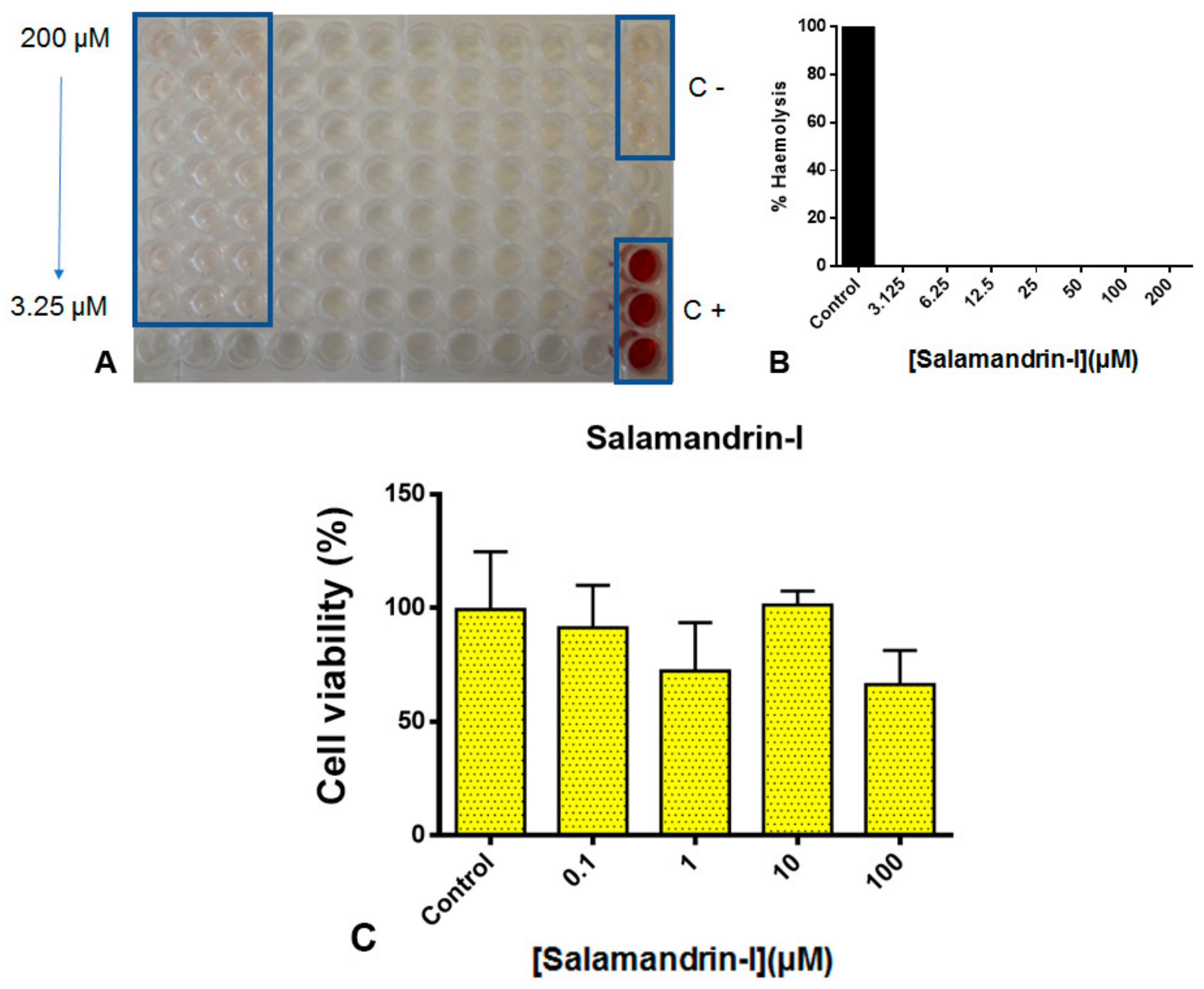
2.8.1. Cytotoxicity Assessment
2.8.2. In Silico Toxicity
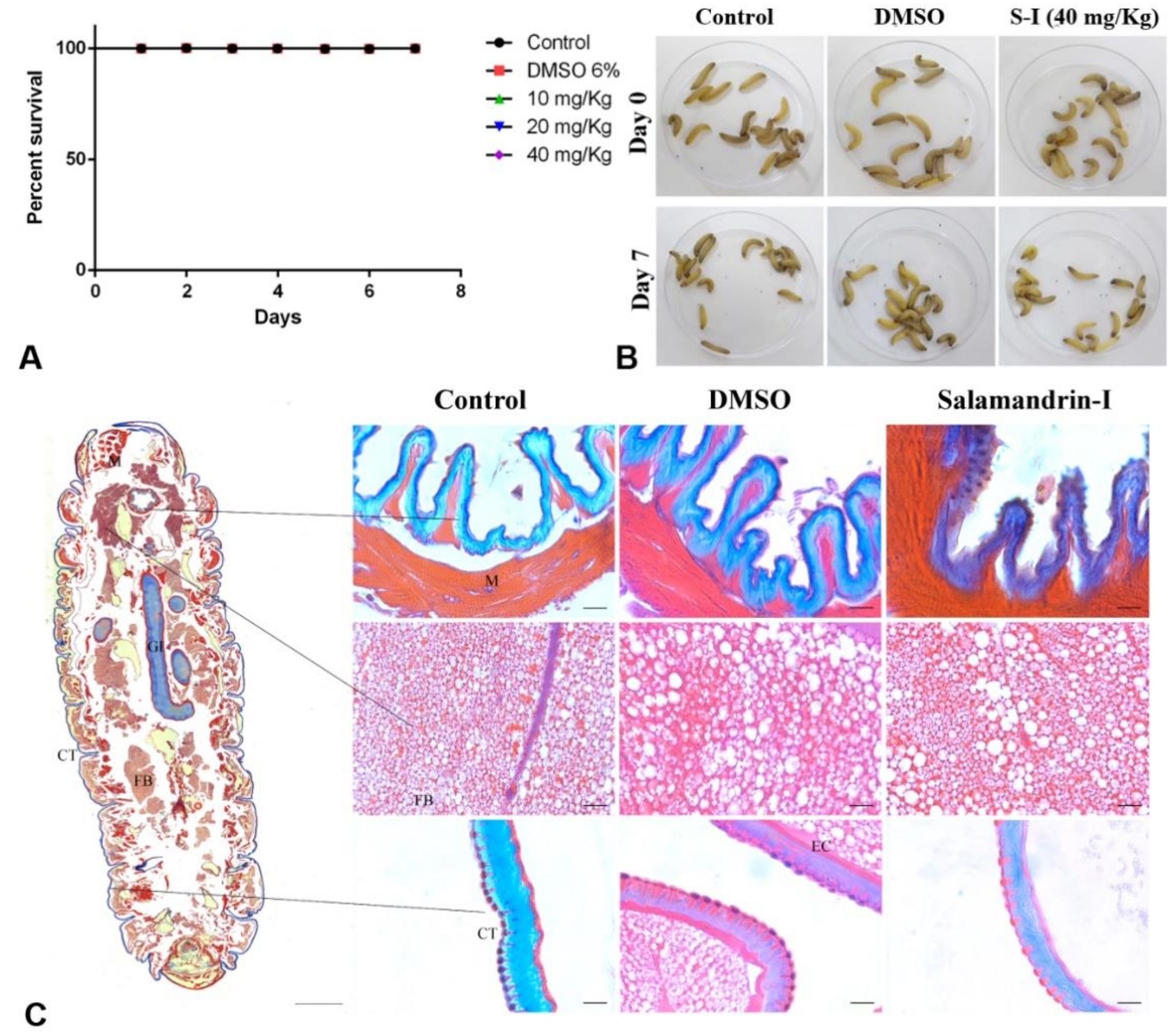
2.8.3. In Vivo Toxicology
3. Results and Discussion
3.1. Purification and Characterization
3.2. Antimicrobial Activity
3.3. In Vitro Radical Scavenging
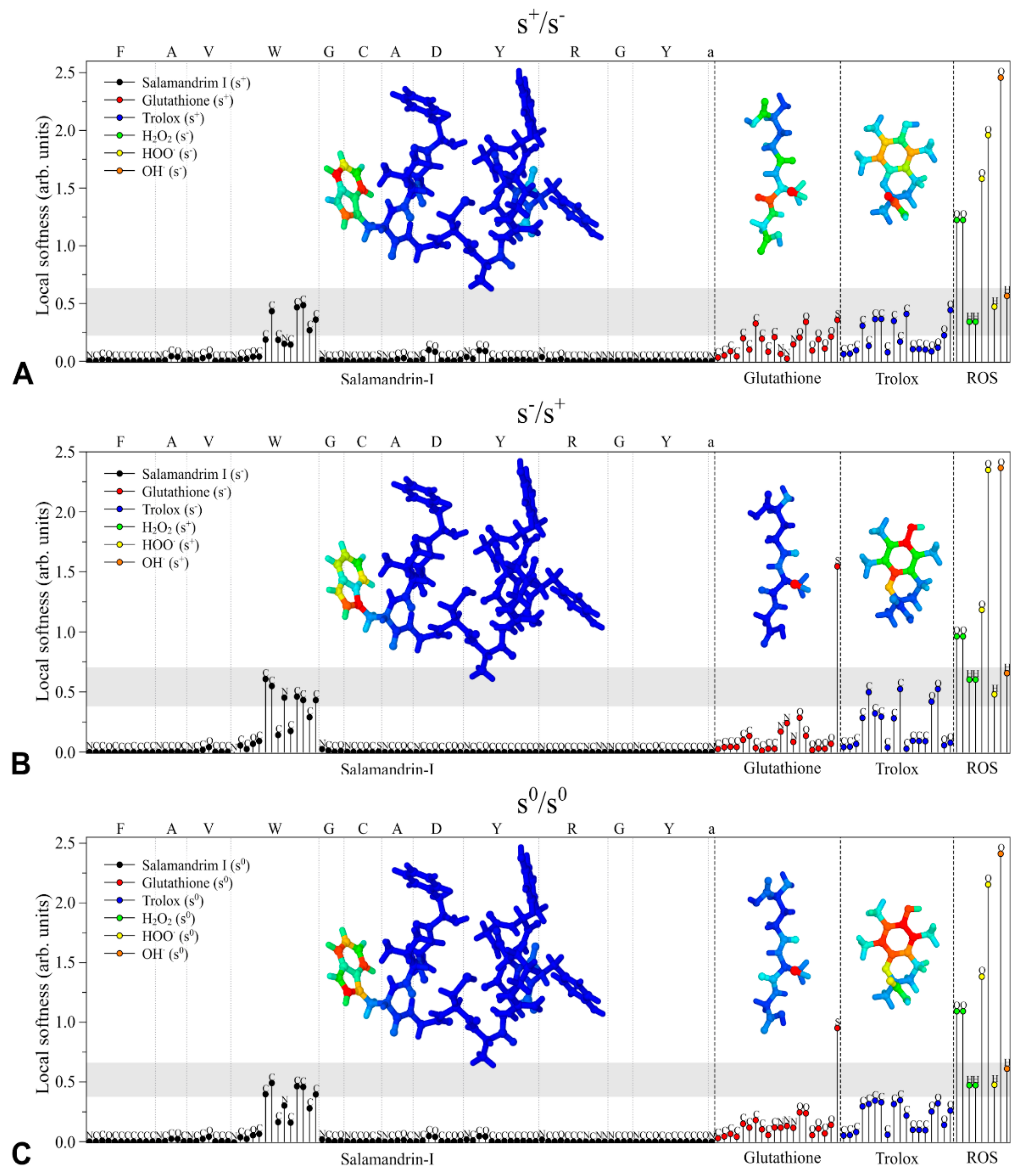
3.4. In Silico Antioxidant Studies
3.5. Toxicity Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Demori, I.; El Rashed, Z.; Corradino, V.; Catalano, A.; Rovegno, L.; Queirolo, L.; Salvidio, S.; Biggi, E.; Zanotti-Russo, M.; Canesi, L.; et al. Peptides for Skin Protection and Healing in Amphibians. Molecules 2019, 24, 347. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, C.; Rollins-Smith, L.; Ibáñez, R.; Durant-Archibold, A.A.; Gutiérrez, M. Toxins and pharmacologically active compounds from species of the family Bufonidae (Amphibia, Anura). J. Ethnopharmacol. 2017, 198, 235–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Sousa, L.Q.; Machado, K.D.; Oliveira, S.F.; Araújo, L.D.; Monção-Filho, E.D.; Melo-Cavalcante, A.A.; Vieira-Júnior, G.M.; Ferreira, P.M.P. Bufadienolides from amphibians: A promising source of anticancer prototypes for radical innovation, apoptosis triggering and Na+/K+-ATPase inhibition. Toxicon 2017, 127, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Marenah, L. Skin secretions of Rana saharica frogs reveal antimicrobial peptides esculentins-1 and -1B and brevinins-1E and -2EC with novel insulin releasing activity. J. Endocrinol. 2006, 188, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.E.; Drucker, D.J. Tissue-specific Expression of Unique mRNAs That Encode Proglucagon-derived Peptides or Exendin 4 in the Lizard. J. Biol. Chem. 1997, 272, 4108–4115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Wang, Y.; Zhang, Y.; Lee, W.-H.; Zhang, Y. Rich diversity and potency of skin antioxidant peptides revealed a novel molecular basis for high-altitude adaptation of amphibians. Sci. Rep. 2016, 6, 19866. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, X.; Liu, X.; Wu, J.; Liu, C.; Gong, W.; Zhao, Z.; Hong, J.; Lin, D.; Wang, Y.; et al. Antioxidant Peptidomics Reveals Novel Skin Antioxidant System. Mol. Cell. Proteomics 2009, 8, 571–583. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Ren, S.; Guo, C.; Zhang, W.; Zhang, X.; Zhang, B.; Li, S.; Ren, J.; Hu, Y.; Wang, H. Identification and functional analyses of novel antioxidant peptides and antimicrobial peptides from skin secretions of four East Asian frog species. Acta Biochim. Biophys. Sin. (Shanghai) 2017, 49, 550–559. [Google Scholar] [CrossRef] [Green Version]
- Guo, C.; Hu, Y.; Li, J.; Liu, Y.; Li, S.; Yan, K.; Wang, X.; Liu, J.; Wang, H. Identification of multiple peptides with antioxidant and antimicrobial activities from skin and its secretions of Hylarana taipehensis, Amolops lifanensis, and Amolops granulosus. Biochimie 2014, 105, 192–201. [Google Scholar] [CrossRef]
- Barbosa, E.A.; Oliveira, A.; Plácido, A.; Socodato, R.; Portugal, C.C.; Mafud, A.C.; Ombredane, A.S.; Moreira, D.C.; Vale, N.; Bessa, L.J.; et al. Structure and function of a novel antioxidant peptide from the skin of tropical frogs. Free Radic. Biol. Med. 2018, 115, 68–79. [Google Scholar] [CrossRef] [Green Version]
- Von Byern, J.; Grunwald, I.; Kosok, M.; Saporito, R.A.; Dicke, U.; Wetjen, O.; Thiel, K.; Borcherding, K.; Kowalik, T.; Marchetti-Deschmann, M. Chemical characterization of the adhesive secretions of the salamander Plethodon shermani (Caudata, Plethodontidae). Sci. Rep. 2017, 7, 6647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manenti, R.; De Bernardi, F.; Ficetola, G.F. Water, stream morphology and landscape: Complex habitat determinants for the fire salamander Salamandra salamandra. Amphib.-Reptil. 2009, 30, 7–15. [Google Scholar] [CrossRef] [Green Version]
- Mebs, D.; Pogoda, W. Variability of alkaloids in the skin secretion of the European fire salamander (Salamandra salamadra terrestris). Toxicon 2005, 45, 603–606. [Google Scholar] [CrossRef] [PubMed]
- Lüddecke, T.; Schulz, S.; Steinfartz, S.; Vences, M. A salamander’s toxic arsenal: Review of skin poison diversity and function in true salamanders, genus Salamandra. Sci. Nat. 2018, 105, 56. [Google Scholar] [CrossRef]
- Brand, G.D.; Magalhães, M.T.Q.; Tinoco, M.L.P.; Aragão, F.J.L.; Nicoli, J.; Kelly, S.M.; Cooper, A.; Bloch, C. Probing Protein Sequences as Sources for Encrypted Antimicrobial Peptides. PLoS ONE 2012, 7, e45848. [Google Scholar] [CrossRef]
- Brand, G.D.; Ramada, M.H.S.; Genaro-Mattos, T.C.; Bloch, C. Towards an experimental classification system for membrane active peptides. Sci. Rep. 2018, 8, 1194. [Google Scholar] [CrossRef] [Green Version]
- Porto, W.F.; Irazazabal, L.; Alves, E.S.F.; Ribeiro, S.M.; Matos, C.O.; Pires, Á.S.; Fensterseifer, I.C.M.; Miranda, V.J.; Haney, E.F.; Humblot, V.; et al. In silico optimization of a guava antimicrobial peptide enables combinatorial exploration for peptide design. Nat. Commun. 2018, 9, 1490. [Google Scholar] [CrossRef]
- Marani, M.M.; Perez, L.O.; de Araujo, A.R.; Plácido, A.; Sousa, C.F.; Quelemes, P.V.; Oliveira, M.; Gomes-Alves, A.G.; Pueta, M.; Gameiro, P.; et al. Thaulin-1: The first antimicrobial peptide isolated from the skin of a Patagonian frog Pleurodema thaul (Anura: Leptodactylidae: Leiuperinae) with activity against Escherichia coli. Gene 2017, 605, 70–80. [Google Scholar] [CrossRef] [Green Version]
- Gill, S.C.; von Hippel, P.H. Calculation of protein extinction coefficients from amino acid sequence data. Anal. Biochem. 1989, 182, 319–326. [Google Scholar] [CrossRef]
- Sreerama, N.; Woody, R.W. Estimation of Protein Secondary Structure from Circular Dichroism Spectra: Comparison of CONTIN, SELCON, and CDSSTR Methods with an Expanded Reference Set. Anal. Biochem. 2000, 287, 252–260. [Google Scholar] [CrossRef]
- Oliveira, M.; Gomes-Alves, A.G.; Sousa, C.; Mirta Marani, M.; Plácido, A.; Vale, N.; Delerue-Matos, C.; Gameiro, P.; Kückelhaus, S.A.S.; Tomas, A.M.; et al. Ocellatin-PT antimicrobial peptides: High-resolution microscopy studies in antileishmania models and interactions with mimetic membrane systems. Biopolymers 2016, 105, 873–886. [Google Scholar] [CrossRef] [Green Version]
- Gião, M.S.; González-Sanjosé, M.L.; Rivero-Pérez, M.D.; Pereira, C.I.; Pintado, M.E.; Malcata, F.X. Infusions of Portuguese medicinal plants: Dependence of final antioxidant capacity and phenol content on extraction features. J. Sci. Food Agric. 2007, 87, 2638–2647. [Google Scholar] [CrossRef]
- Marxen, K.; Vanselow, K.; Lippemeier, S.; Hintze, R.; Ruser, A.; Hansen, U.-P. Determination of DPPH Radical Oxidation Caused by Methanolic Extracts of Some Microalgal Species by Linear Regression Analysis of Spectrophotometric Measurements. Sensors 2007, 7, 2080–2095. [Google Scholar] [CrossRef] [Green Version]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef] [Green Version]
- Batagin-Neto, A.; Oliveira, E.F.; Graeff, C.F.O.; Lavarda, F.C. Modelling polymers with side chains: MEH-PPV and P3HT. Mol. Simul. 2013, 39, 309–321. [Google Scholar] [CrossRef]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and testing of a general amber force field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef]
- Allouche, A.-R. Gabedit-A graphical user interface for computational chemistry softwares. J. Comput. Chem. 2011, 32, 174–182. [Google Scholar] [CrossRef]
- Stewart, J.J.P. Optimization of parameters for semiempirical methods V: Modification of NDDO approximations and application to 70 elements. J. Mol. Model. 2007, 13, 1173–1213. [Google Scholar] [CrossRef] [Green Version]
- Stewart, J.J.P. MOPAC: A semiempirical molecular orbital program. J. Comput. Aided Mol. Des. 1990, 4, 1–105. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilk, L.; Vosko, S.H.; Nusair, M.; Wilk, L.; Nusair, M. Accurate spin-dependent electron liquid correlation energies for local spin density calculations: A critical analysis. Can. J. Phys. 1980, 58, 1200–1211. [Google Scholar]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Pubchem. Available online: https://pubchem.ncbi.nlm.nih.gov (accessed on 18 October 2019).
- Maia, R.A.; Ventorim, G.; Batagin-Neto, A. Reactivity of lignin subunits: The influence of dehydrogenation and formation of dimeric structures. J. Mol. Model. 2019, 25, 228. [Google Scholar] [CrossRef] [PubMed]
- Lewars, E.G. Computational Chemistry; Springer Netherlands: Dordrecht, Netherlands, 2011; ISBN 978-90-481-3860-9. [Google Scholar]
- De Proft, F.; Van Alsenoy, C.; Peeters, A.; Langenaeker, W.; Geerlings, P. Atomic charges, dipole moments, and Fukui functions using the Hirshfeld partitioning of the electron density. J. Comput. Chem. 2002, 23, 1198–1209. [Google Scholar] [CrossRef]
- Roy, R.K.; Pal, S.; Hirao, K. On non-negativity of Fukui function indices. J. Chem. Phys. 1999, 110, 8236–8245. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision A.02; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Martiínez, A.; Rodriíguez-Gironeís, M.A.; Barbosa, A.; Costas, M. Donator Acceptor Map for Carotenoids, Melatonin and Vitamins. J. Phys. Chem. A 2008, 112, 9037–9042. [Google Scholar] [CrossRef]
- Rodrigues de Araújo, A.; Iles, B.; de Melo Nogueira, K.; Dias, J.D.; Plácido, A.; Rodrigues, A.; Albuquerque, P.; Silva-Pereira, I.; Socodatto, R.; Portugal, C.C.; et al. Antifungal and anti-inflammatory potential of eschweilenol C-rich fraction derived from Terminalia fagifolia Mart. J. Ethnopharmacol. 2019, 240, 111941. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, Approved Standard, 10th ed.; CLSI: Wayne, PA, USA, 2015. [Google Scholar]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Disk Susceptibility Tests, Approved Standard, 12th ed.; CLSI: Wayne, PA, USA, 2015. [Google Scholar]
- Bessa, L.J.; Eaton, P.; Dematei, A.; Plácido, A.; Vale, N.; Gomes, P.; Delerue-Matos, C.; SA Leite, J.R.; Gameiro, P. Synergistic and antibiofilm properties of ocellatin peptides against multidrug-resistant Pseudomonas aeruginosa. Future Microbiol. 2018, 13, 151–163. [Google Scholar] [CrossRef] [Green Version]
- Bignami, G.S. A rapid and sensitive hemolysis neutralization assay for palytoxin. Toxicon 1993, 31, 817–820. [Google Scholar] [CrossRef]
- Freitas, J.; Cano, P.; Craig-Veit, C.; Goodson, M.L.; David Furlow, J.; Murk, A.J. Detection of thyroid hormone receptor disruptors by a novel stable in vitro reporter gene assay. Toxicol. Vitr. 2011, 25, 257–266. [Google Scholar] [CrossRef]
- GraphPad Prism, version 6.0 for Windows; GraphPad Software, Inc.: La Jolla, CA, USA, 2013.
- Pires, D.E.V.; Blundell, T.L.; Ascher, D.B. pkCSM: Predicting Small-Molecule Pharmacokinetic and Toxicity Properties Using Graph-Based Signatures. J. Med. Chem. 2015, 58, 4066–4072. [Google Scholar] [CrossRef]
- Arcanjo, D.D.R.; Mafud, A.C.; Vasconcelos, A.G.; da Silva-Filho, J.C.; Amaral, M.P.M.; Brito, L.M.; Bemquerer, M.P.; Kückelhaus, S.A.S.; Plácido, A.; Delerue-Matos, C.; et al. In Silico, In Vitro and In Vivo Toxicological Assessment of BPP-BrachyNH2, A Vasoactive Proline-Rich Oligopeptide from Brachycephalus ephippium. Int. J. Pept. Res. Ther. 2017, 23, 323–331. [Google Scholar] [CrossRef] [Green Version]
- Ignasiak, K.; Maxwell, A. Galleria mellonella (greater wax moth) larvae as a model for antibiotic susceptibility testing and acute toxicity trials. BMC Res. Notes 2017, 10, 428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unsal, S.; Gorer, N. Effects of triflumuron on larval intugement of sixth-instar Galleria mellonella (L.) (Lepidoptera: Pyralidae). In Fresenius Environmental Bulletin; Parlar, H., Parlar, P., Eds.; 5A/2018; Parlar Research & Technology: Freising, Germany, 2019; Volume 27, pp. 3823–3831. [Google Scholar]
- Perdoni, F.; Falleni, M.; Tosi, D.; Cirasola, D.; Romagnoli, S.; Braidotti, P.; Clementi, E.; Bulfamante, G.; Borghi, E. A histological procedure to study fungal infection in the wax moth Galleria mellonella. Eur. J. Histochem. 2014, 58, 2428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, P.; Yang, S.; Shen, C.; Jiang, K.; Rong, M.; Lai, R. The First Salamander Defensin Antimicrobial Peptide. PLoS ONE 2013, 8, e83044. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Maupetit, J.; Derreumaux, P.; Tufféry, P. Improved PEP-FOLD Approach for Peptide and Miniprotein Structure Prediction. J. Chem. Theory Comput. 2014, 10, 4745–4758. [Google Scholar] [CrossRef]
- Martins, L.M.; de Faria Vieira, S.; Baldacim, G.B.; Bregadiolli, B.A.; Caraschi, J.C.; Batagin-Neto, A.; da Silva-Filho, L.C. Improved synthesis of tetraaryl-1,4-dihydropyrrolo[3,2- b ]pyrroles a promising dye for organic electronic devices: An experimental and theoretical approach. Dyes Pigm. 2018, 148, 81–90. [Google Scholar] [CrossRef] [Green Version]
- Fuentes-Lemus, E.; Dorta, E.; Escobar, E.; Aspée, A.; Pino, E.; Abasq, M.L.; Speisky, H.; Silva, E.; Lissi, E.; Davies, M.J.; et al. Oxidation of free, peptide and protein tryptophan residues mediated by AAPH-derived free radicals: Role of alkoxyl and peroxyl radicals. RSC Adv. 2016, 6, 57948–57955. [Google Scholar] [CrossRef]
- Ehrenshaft, M.; Deterding, L.J.; Mason, R.P. Tripping up Trp: Modification of protein tryptophan residues by reactive oxygen species, modes of detection, and biological consequences. Free Radic. Biol. Med. 2015, 89, 220–228. [Google Scholar] [CrossRef] [Green Version]
- Netto, L.E.S.; de Oliveira, M.A.; Monteiro, G.; Demasi, A.P.D.; Cussiol, J.R.R.; Discola, K.F.; Demasi, M.; Silva, G.M.; Alves, S.V.; Faria, V.G.; et al. Reactive cysteine in proteins: Protein folding, antioxidant defense, redox signaling and more. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2007, 146, 180–193. [Google Scholar] [CrossRef] [PubMed]
- Parasuraman, S. Toxicological screening. J. Pharmacol. Pharmacother. 2011, 2, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DiMasi, J.A.; Hansen, R.W.; Grabowski, H.G. The price of innovation: New estimates of drug development costs. J. Health Econ. 2003, 22, 151–185. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Li, Y.; Xu, L.; Li, D.; Hou, T. Recent Developments in Computational Prediction of hERG Blockage. Curr. Top. Med. Chem. 2013, 13, 1317–1326. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, Y.; Wang, J.; Chen, L.; Zhang, L.; Yu, H.; Hou, T. ADMET Evaluation in Drug Discovery. 12. Development of Binary Classification Models for Prediction of hERG Potassium Channel Blockage. Mol. Pharm. 2012, 9, 996–1010. [Google Scholar] [CrossRef]
- Marchese Robinson, R.L.; Glen, R.C.; Mitchell, J.B.O. Development and Comparison of hERG Blocker Classifiers: Assessment on Different Datasets Yields Markedly Different Results. Mol. Inform. 2011, 30, 443–458. [Google Scholar] [CrossRef]
- Cserép, C.; Pósfai, B.; Lénárt, N.; Fekete, R.; László, Z.I.; Lele, Z.; Orsolits, B.; Molnár, G.; Heindl, S.; Schwarcz, A.D.; et al. Microglia monitor and protect neuronal function through specialized somatic purinergic junctions. Science 2020, 367, 528–537. [Google Scholar] [CrossRef]
- Gautam, A.; Chaudhary, K.; Singh, S.; Joshi, A.; Anand, P.; Tuknait, A.; Mathur, D.; Varshney, G.C.; Raghava, G.P.S. Hemolytik: A database of experimentally determined hemolytic and non-hemolytic peptides. Nucleic Acids Res. 2014, 42, D444–D449. [Google Scholar] [CrossRef] [Green Version]
- MacCallum, D.M.; Desbois, A.P.; Coote, P.J. Enhanced efficacy of synergistic combinations of antimicrobial peptides with caspofungin versus Candida albicans in insect and murine models of systemic infection. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 1055–1062. [Google Scholar] [CrossRef]
- Gorr, S.-U.; Flory, C.M.; Schumacher, R.J. In vivo activity and low toxicity of the second-generation antimicrobial peptide DGL13K. PLoS ONE 2019, 14, e0216669. [Google Scholar] [CrossRef] [Green Version]
- McCloskey, A.P.; Lee, M.; Megaw, J.; McEvoy, J.; Coulter, S.M.; Pentlavalli, S.; Laverty, G. Investigating the In Vivo Antimicrobial Activity of a Self-Assembling Peptide Hydrogel Using a Galleria mellonella Infection Model. ACS Omega 2019, 4, 2584–2589. [Google Scholar] [CrossRef]
- Emery, H.; Johnston, R.; Rowley, A.F.; Coates, C.J. Indomethacin-induced gut damage in a surrogate insect model, Galleria mellonella. Arch. Toxicol. 2019, 93, 2347–2360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.; Lai, R. The Chemistry and Biological Activities of Peptides from Amphibian Skin Secretions. Chem. Rev. 2015, 115, 1760–1846. [Google Scholar] [CrossRef]
- Moreira, D.C.; Venancio, L.P.R.; Sabino, M.A.C.T.; Hermes-Lima, M. How widespread is preparation for oxidative stress in the animal kingdom? Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2016, 200, 64–78. [Google Scholar] [CrossRef] [PubMed]

| Peptide | Species | Sequence |
|---|---|---|
| CFBD-1 | C. fudingensi | MAVNGSQGVEFAVWGCADYRGYCRAACFAFEYSLGPKGCTEGYVCCVPNTF |
| Salamandrin-I | S. salamandra | FAVWGCADYRGYa * |
| Peptide | In Vitro Antioxidant Activity (Trolox-eq/mg) | |
|---|---|---|
| ABTS Assay | DPPH Assay | |
| Glutathione | 1.911 ± 0.003 | 0.829 ± 0.005 |
| Salamandrin-I | 0.285 ± 0.003 | 0.081 ± 0.005 |
| Model Name | Salamandrin-I | Glutathione | Trolox |
|---|---|---|---|
| Ames toxicity * | No | Yes | No |
| Max. tolerated dose (human, log mg/kg/day) | 0.438 | 1.104 | 0.800 |
| hERG I inhibitor | No | No | No |
| hERG II inhibitor | Yes | No | No |
| Oral Rat Acute Toxicity (LD50, mol/kg) | 2.482 | 2.468 | 2.382 |
| Oral Rat Chronic Toxicity (LOAEL, log mg/kg bw/day) | 10.773 | 2.919 | 1.857 |
| Hepatotoxicity | Yes | No | No |
| Skin sensitization | No | No | No |
| T. pyriformis toxicity (log µg/L) | 0.285 | 0.285 | 0.197 |
| Minnow toxicity (log mM) $ | 23.468 | 4.569 | 1.674 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plácido, A.; Bueno, J.; Barbosa, E.A.; Moreira, D.C.; Dias, J.d.N.; Cabral, W.F.; Albuquerque, P.; Bessa, L.J.; Freitas, J.; Kuckelhaus, S.A.S.; et al. The Antioxidant Peptide Salamandrin-I: First Bioactive Peptide Identified from Skin Secretion of Salamandra Genus (Salamandra salamandra). Biomolecules 2020, 10, 512. https://doi.org/10.3390/biom10040512
Plácido A, Bueno J, Barbosa EA, Moreira DC, Dias JdN, Cabral WF, Albuquerque P, Bessa LJ, Freitas J, Kuckelhaus SAS, et al. The Antioxidant Peptide Salamandrin-I: First Bioactive Peptide Identified from Skin Secretion of Salamandra Genus (Salamandra salamandra). Biomolecules. 2020; 10(4):512. https://doi.org/10.3390/biom10040512
Chicago/Turabian StylePlácido, Alexandra, João Bueno, Eder A. Barbosa, Daniel C. Moreira, Jhones do Nascimento Dias, Wanessa Felix Cabral, Patrícia Albuquerque, Lucinda J. Bessa, Jaime Freitas, Selma A. S. Kuckelhaus, and et al. 2020. "The Antioxidant Peptide Salamandrin-I: First Bioactive Peptide Identified from Skin Secretion of Salamandra Genus (Salamandra salamandra)" Biomolecules 10, no. 4: 512. https://doi.org/10.3390/biom10040512