Biologically Active Compounds from Goji (Lycium Barbarum L.) Leaves Aqueous Extracts: Purification and Concentration by Membrane Processes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
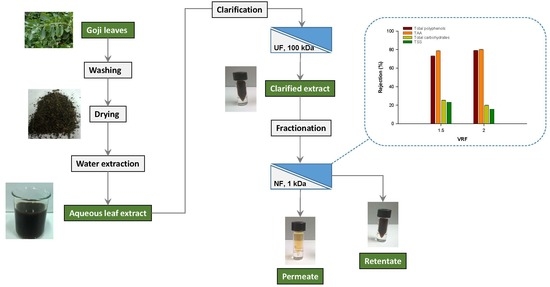
2.2. Aqueous Extraction and Pre-Treatment
2.3. Fractionation of the Clarified Extract
2.4. Measurement of Total Soluble Solids, Total Suspended Solids, and pH
2.5. Total Carbohydrates
2.6. Total Polyphenols
2.7. In Vitro Total Antioxidant Activity
2.8. Statistical Analysis
3. Results and Discussion
3.1. Optimization Extraction of Antioxidant Compounds from Goji Leaves
3.1.1. Effect of Temperature on Total Polyphenols and TSS Yields
3.1.2. Effect of L/S Ratio on Total Polyphenol, TAA, and TSS Yields
3.1.3. Effect of pH on Total Polyphenols and TSS Yields
3.2. Clarification of Goji Leaf Aqueous Extract
3.3. Treatment of Clarified Extract with UF and NF Membranes: Effect of TMP on Permeate Flux and Selectivity
3.4. Concentration of Clarified Extract with 1 kDa Membrane
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Teixeira, S.; Luís, I.M.; Oliveira, M.M.; Abreu, I.A.; Batista, R. Goji berries superfood—Contributions for the characterisation of proteome and IgE-binding proteins. Food Agric. Immunol. 2019, 30, 262–280. [Google Scholar] [CrossRef] [Green Version]
- Potterat, O. Goji (Lycium barbarum and L. chinense): Phytochemistry, pharmacology and safety in the perspective of traditional uses and recent popularity. Planta Med. 2010, 76, 7–19. [Google Scholar] [CrossRef] [Green Version]
- Rotar, A.M.; Vodnar, D.C.; Bunghez, F.; Catunescu, G.M.; Pop, C.R.; Jimborean, M.; Semeniuc, C.A. Effect of Goji Berries and honey on lactic acid bacteria viability and shelf life stability of yoghurt. Not. Bot. Horti Agrobot. Cluj Napoca 2015, 43, 196–203. [Google Scholar] [CrossRef] [Green Version]
- Mocan, A.; Zengin, G.; Simirgiotis, M.; Schafberg, M.; Mollica, A.; Vodnar, D.C.; Crisan, G.; Rohn, S. Functional constituents of wild and cultivated Goji (L. barbarum L.) leaves: Phytochemical characterization, biological profile, and computational studies. J. Enzym. Inhib. Med. Chem. 2017, 32, 153–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeh, Y.C.; Hahm, T.S.; Sabliov, C.M.; Lo, Y.M. Effects of Chinese wolfberry (Lycium chinense P. Mill.) leaf hydrolysates on the growth of Pediococcus acidilactici. Bioresour. Technol. 2008, 99, 1383–1393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.H.; Wang, Y.Y.; Gong, G.L.; Li, F.; Ren, H.T.; Liu, Y. Adsorption and desorption properties of macroporous resins for flavonoids from the extract of Chinese wolfberry (Lycium barbarum L.). Food Bioprod. Process. 2015, 93, 148–155. [Google Scholar] [CrossRef]
- Dong, J.Z.; Lu, D.Y.; Wang, Y. Analysis of flavonoids from leaves of cultivated Lycium barbarum L. Plant Food Hum. Nutr. 2009, 64, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Peng, Z.; Zhang, X.; Yang, J.; Lai, X.; Guowu, Y. Determination of polyphenols in Lycium barbarum leaves by high-performance liquid chromatography-tandem mass spectrometry. Anal. Lett. 2017, 50, 761–776. [Google Scholar] [CrossRef]
- Mocan, A.; Vlase, L.; Vodnar, D.C.; Bischin, C.; Hanganu, D.; Gheldiu, A.-M.; Oprean, R.; Silaghi-Dumitrescu, R.; Crișan, G. Polyphenolic content, antioxidant and antimicrobial activities of Lycium barbarum L. and Lycium chinense Mill. Leaves. Molecules 2014, 19, 10056–10073. [Google Scholar] [CrossRef] [PubMed]
- Osman, N.I.; Awal, A.; Sidik, N.J.; Abdullah, S. In vitro regeneration and antioxidant properties of Lycium Barbarum L. (Goji). J. Teknol. 2013, 62, 35–38. [Google Scholar]
- Ren, L.; Li, J.; Xiao, Y.; Zhang, Y.; Fan, J.; Zhang, B.; Wang, L.; Shen, X. Polysaccharide from Lycium barbarum L. leaves enhances absorption of endogenous calcium, and elevates cecal calcium transport protein levels and serum cytokine levels in rats. J. Funct. Food. 2017, 33, 227–234. [Google Scholar] [CrossRef]
- Dong, J.Z.; Gao, W.S.; Lu, D.Y.; Wang, Y. Simultaneous extraction extraction and analysis of four polyphenols from leaves of Lycium barbarum L. J. Food Biochem. 2011, 35, 914–931. [Google Scholar] [CrossRef]
- Anunciato, T.P.; da Rocha Filho, P.A. Carotenoids and polyphenols in nutricosmetics, nutraceuticals, and cosmeceuticals. J. Cosmet. Dermatol. 2012, 11, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Xu, Z.; Xiang, C.; Liu, J.; Zhou, L.; Li, T.; Yang, Z.; Ding, C. Comparative evaluation of maceration and ultrasonic-assisted extraction of phenolic compounds from fresh olives. Ultrason. Sonochem. 2017, 37, 328–334. [Google Scholar] [CrossRef]
- Bengardino, M.B.; Fernandez, M.V.; Nutter, J.; Jagus, R.J.; Agüero, M.V. Recovery of bioactive compounds from beet leaves through simultaneous extraction: Modelling and process optimization. Food Bioprod. Process. 2019, 118, 227–236. [Google Scholar] [CrossRef]
- Xynos, N.; Papaefstathioua, G.; Gikasb, E.; Argyropouloua, A.; Aligiannisa, N.; Skaltsounisa, A.L. Design optimization study of the extraction of olive leaves performed with pressurized liquid extraction using response surface methodology. Sep. Purif. Technol. 2014, 122, 323–330. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Cassano, A.; Conidi, C. Membrane-based technologies for meeting the recovery of biologically active compounds from foods and their by-products. Crit. Rev. Food Sci. Nutr. 2019, 59, 2927–2948. [Google Scholar] [CrossRef]
- Nazir, A.; Khan, K.; Maan, A.; Zia, R.; Giorno, L.; Schroën, K. Membrane separation technology for the recovery of nutraceuticals from food industrial streams. Trends Food Sci. Technol. 2019, 86, 426–438. [Google Scholar] [CrossRef]
- Cassano, A.; Conidi, C.; Ruby-Figueroa, R.; Castro-Muñoz, R. Nanofiltration and tight ultrafiltration membranes for the recovery of polyphenols from agro-food by-products. Int. J. Mol. Sci. 2018, 19, 351. [Google Scholar] [CrossRef] [Green Version]
- Galanakis, C.M. Separation of functional macromolecules and micromolecules: From ultrafiltration to the border of nanofiltration. Trends Food Sci. Technol. 2015, 42, 44–63. [Google Scholar] [CrossRef]
- Bazinet, L.; Doyen, A. Antioxidants, mechanisms, and recovery by membrane processes. Crit. Rev. Food Sci. Nutr. 2017, 57, 677–700. [Google Scholar] [CrossRef] [PubMed]
- Conidi, C.; Drioli, E.; Cassano, A. Membrane-based agro-food production processes for polyphenol separation, purification and concentration. Curr. Opin. Food Sci. 2018, 23, 149–164. [Google Scholar] [CrossRef]
- Van der Bruggen, B.; Vandecasteele, C.; Van Gestel, T.; Doyen, W.; Leysen, R. A review of pressure-driven membrane processes in wastewater treatment and drinking water production. Environ. Prog. 2003, 22, 46–56. [Google Scholar] [CrossRef]
- Esmaeili, M.; Virtanen, T.; Lahti, J.; Mänttäri, M.; Kallioinen, M. Vanillin as an antifouling and hydrophilicity promoter agent in surface modification of polyethersulfone membrane. Membranes 2019, 9, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boussu, K.; Van der Bruggen, B.; Volodin, A.; Van Haesendonck, C.; Delcour, J.A.; Van der Meerend, P.; Vandecasteele, C. Characterization of commercial nanofiltration membranes and comparison with self-made polyethersulfone membranes. Desalination 2006, 191, 245–253. [Google Scholar] [CrossRef]
- Lin, J.; Ye, W.; Baltaru, M.C.; Tang, Y.P.; Bernstein, N.J.; Gao, P.; Balta, S.; Vlad, M.; Volodin, A.; Sotto, A.; et al. Tight ultrafiltration membranes for enhanced separation of dyes and Na2SO4 during textile wastewater treatment. J. Membr. Sci. 2016, 514, 217–228. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.; Hamilton, J.; Rebers, P.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzimol. 1999, 299, 152–178. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C.A. Antioxidant activity applying and improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Mellinas, A.C.; Jiménez, A.; Garrigós, M.C. Optimization of microwave-assisted extraction of cocoa bean shell waste and evaluation of its antioxidant, physicochemical and functional properties. LWT Food Sci. Technol. 2020, 127, 109361. [Google Scholar] [CrossRef]
- Wissam, Z.; Ghada, B.; Wassim, A.; Warid, K. Effective extraction of polyphenols and proanthocyanidins from pomegranate’s peel. Int. J. Pharm. Pharm. Sci. 2012, 4, 675–682. [Google Scholar]
- Jovanović, A.A.; Ðordević, V.B.; Zdunić, G.M.; Pljevljakušić, D.S.; Šavikin, K.P.; Godevac, D.M.; Bugarsk, B.M. Optimization of the extraction process of polyphenols from Thymus serpyllum L. herb using maceration, heat- and ultrasound-assisted techniques. Sep. Purif. Technol. 2017, 179, 369–380. [Google Scholar] [CrossRef] [Green Version]
- Dent, M.; Dragović-Uzelac, V.; Penić, M.; Brnčić, M.; Bosiljkov, T.; Levaj, B. The effect of extraction solvents, temperature and time on the composition and mass fraction of polyphenols in Dalmatian wild sage (Salvia officinalis L.) extracts. Food Technol. Biotechnol. 2013, 51, 84–91. [Google Scholar]
- Perva-Unzunalić, A.; Škerget, M.; Želijko Knez, Z.; Weinreich, B.; Otto, F.; Grüner, S. Extraction of active ingredients from green tea (Camellia sinensis): Extraction efficiency of major catechins and caffeine. Food Chem. 2006, 96, 597–605. [Google Scholar] [CrossRef]
- Vuong, Q.V.; Golding, J.B.; Stathopoulos, C.E.; Nguyen, M.H.; Roach, P.D. Optimizing conditions for the extraction of catechins from green tea using hot water. J. Sep. Sci. 2011, 34, 3099–3106. [Google Scholar] [CrossRef]
- Kumar, A.; Thakur, B.K.; De, S. Selective extraction of (−)epigallocatechin gallate from green tea leaves using two-stage infusion coupled with membrane separation. Food Bioprocess Technol. 2012, 5, 2568–2577. [Google Scholar] [CrossRef]
- Bucić-Kojić, A.; Planinić, M.; Tomas, S.; Bilić, M.; Velić, D. Study of solid-liquid extraction kinetics of total polyphenols from grape seeds. J. Food Eng. 2007, 81, 236–242. [Google Scholar]
- Stamatopoulos, K.; Chatzilazarou, A.; Katsoyannos, E. Optimization of multistage extraction of olive leaves for recovery of phenolic compounds at moderated temperatures and short extraction times. Foods 2014, 3, 66–81. [Google Scholar] [CrossRef] [Green Version]
- Bindes, M.M.M.; Cardoso, V.L.; Reis, M.H.M.; Boffito, D.C. Maximisation of the polyphenols extraction yield from green tea leaves and sequential clarification. J. Food Eng. 2019, 241, 97–104. [Google Scholar] [CrossRef]
- Pinelo, M.; Rubilar, M.; Jerez, M.; Sineiro, J.; Núñez, M.J. Effect of solvent, temperature, and solvent-to solid ratio on the total phenolic content and antiradical activity of extracts from different components of grape pomace. J. Agric. Food Chem. 2005, 53, 2111–2117. [Google Scholar] [CrossRef]
- Boussetta, N.; Soichi, E.; Lanoisellé, J.L.; Vorobiev, E. Valorization of oilseed residues: Extraction of polyphenols from flaxseed hulls by pulsed electric fields. Ind. Crop. Prod. 2014, 52, 347–353. [Google Scholar] [CrossRef]
- Rajha, H.N.; Boussetta, N.; Louka, N.; Maroun, R.G.; Vorobiev, E.A. comparative study of physical pretreatments for the extraction of polyphenols and proteins from vine shoots. Food Res. Int. 2014, 65, 462–468. [Google Scholar] [CrossRef]
- Mondal, S.; Majumdar, G.C.; De, S. Clarifications of stevia extract using cross flow ultrafiltration and concentration by nanofiltration. Sep. Purif. Technol. 2012, 89, 125–134. [Google Scholar] [CrossRef]
- Cassano, A.; Donato, L.; Conidi, C.; Drioli, E. Recovery of bioactive compounds in kiwifruit juice by ultrafiltration. Innov. Food Sci. Emerg. Technol. 2008, 9, 556–562. [Google Scholar] [CrossRef]
- Conidi, C.; Cassano, A.; Garcia-Castello, E. Valorization of artichoke wastewaters by integrated membrane process. Water Res. 2014, 48, 363–374. [Google Scholar] [CrossRef]
- Zhang, H.; Tsao, R. Dietary polyphenols, oxidative stress, antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Liu, S.C.; Lin, J.T.; Hu, C.C.; Shen, B.Y.; Chen, T.Y.; Chang, Y.L.; Shih, C.H.; Yang, D.J. Phenolic compositions and antioxidant attributes of leaves and stems from three inbred varieties of Lycium chinense Miller harvested at various times. Food Chem. 2017, 215, 284–291. [Google Scholar] [CrossRef]
- Ulbricht, M.; Ansorge, W.; Danielzik, I.; König, M.; Schuster, O. Fouling in microfiltration of wine: The influence of the membrane polymer on adsorption of polyphenols and polysaccharides. Sep. Purif. Technol. 2009, 68, 335–342. [Google Scholar] [CrossRef]
- Cissé, M.; Vaillant, F.; Pallet, D.; Dornier, M. Selecting ultrafiltration and nanofiltration membranes to concentrate anthocyanins from roselle extract (Hibiscus sabdariffa L.). Food Res. Int. 2011, 44, 2607–2614. [Google Scholar] [CrossRef]
- Conidi, C.; Cassano, A. Recovery of phenolic compounds from bergamot juice by nanofiltration membranes. Desalin. Water Treat. 2015, 56, 3510–3518. [Google Scholar] [CrossRef]
- Benítez, J.; Acero, J.L.; Leal, A.I.; González, M. The use of ultrafiltration and nanofiltration membranes for the purification of cork processing wastewater. J. Hazard. Mater. 2009, 162, 1438–1445. [Google Scholar] [CrossRef] [PubMed]
- Boussu, K.; Vandecasteele, C.; Van der Bruggen, B. Relation between membrane characteristics and performance in nanofiltration. J. Membr. Sci. 2008, 310, 51–65. [Google Scholar] [CrossRef]
- Conidi, C.; Fucà, L.; Drioli, E.; Cassano, A. A membrane-based process for the recovery of glycyrrhizin and phenolic compounds from licorice wastewaters. Molecules 2019, 24, 2279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Díaz-Reinoso, B.; Moure, A.; Domínguez, H.; Parajó, J.C. Ultra- and nanofiltration of aqueous extracts from distilled fermented grape pomace. J. Food Eng. 2009, 91, 587–593. [Google Scholar] [CrossRef]
- Conidi, C.; Cassano, A.; Drioli, E. Recovery of phenolic compounds from orange press liquor by nanofiltration. Food Bioprod. Process. 2012, 90, 867–874. [Google Scholar] [CrossRef]
- Aguiar Prudêncio, A.P.; Prudêncio, E.S.; Castanho Amboni, R.D.M.; Negrão Murakami, A.N.; Maraschin, M.; Cunha Petrus, J.C.; Ogliari, P.J.; Leite, R.S. Phenolic composition and antioxidant activity of the aqueous extract of bark from residues from mate tree (Ilex paraguariensis St. Hil.) bark harvesting concentrated by nanofiltration. Food Bioprod. Process. 2012, 90, 399–405. [Google Scholar] [CrossRef]
- Cassano, A.; Conidi, C.; Ruby Figueroa, R.; Castro Muñoz, R. A two-step nanofiltration process for the production of phenolic-rich fractions from artichoke aqueous extracts. Int. J. Mol. Sci. 2015, 16, 8968–8987. [Google Scholar] [CrossRef] [Green Version]












| Membrane Type | UH004P | NP010 | NP030 |
|---|---|---|---|
| Membrane material | PES | PES | PES |
| Nominal MWCO (kDa) | 4.0 | 1.0 | 0.3 |
| Configuration | Flat-sheet | Flat-sheet | Flat-sheet |
| Operating temperature (°C) | 5–95 | 5–95 | 5–95 |
| pH range | 0–14 | 0–14 | 0–14 |
| Water permeability at 25 °C (L/m2h bar) | 17.12 a | 16.0 a | 4.53 a |
| Contact angle (°) | 42 (pH 3.8) b | 72 (pH 6) c | 88 (pH 6) c |
| Zeta potential at pH 7 (mV) | −7.2 d | −12 c | −15 c |
| Roughness (Å) | - | 13 c | 25 c |
| Parameters | Feed | Permeate | Retentate |
|---|---|---|---|
| Total suspended solids | 5.2 ± 2.3 | n.d. | 6.2 ± 0.1 |
| pH | 7.2 ± 0.1 | 7.1 ± 0.2 | 7.2 ± 0.8 |
| Total soluble solids (°Brix) | 10.5 ± 0.2 | 9.5 ± 0.12 | 12 ± 1.2 |
| Total polyphenols (mg GAE/L) | 1870.7 ± 4.5 | 1530.2 ± 2.8 | 2570 ± 2.6 |
| TAA (mM Trolox) | 13.0 ± 1.2 | 11.0 ± 2.6 | 14.0 ± 1.6 |
| Total carbohydrates (g glucose/L) | 6.33 ± 1.6 | 5.43 ± 0.12 | 9.65 ± 2.8 |
| Parameter | Membrane Type | ||
|---|---|---|---|
| PES 4 kDa | PES 1 kDa | PES 0.3 kDa | |
| WP0 (L/m2h bar) | 17.12 | 16.0 | 4.53 |
| WP1 (L/m2h bar) | 7.85 | 12.4 | 2.53 |
| WP2 (L/m2h bar) | 10.77 | 16.45 | 4.45 |
| FI (%) | 54.0 | 22.5 | 44.0 |
| CE (%) | 63.0 | 100 | 97.6 |
| Sample | VRF | Total Polyphenols (mg GAE/L) | TAA (mM Trolox) | Total Carbohydrates (g Glucose/L) | Total Soluble Solids (°Brix) |
|---|---|---|---|---|---|
| Feed | 1520.0 ± 1.5 | 9.5 ± 1.4 | 5.6 ± 0.1 | 7.2 ± 0.2 | |
| Permeate | 1.5 | 525.6 ± 0.9 | 2.5 ± 0.2 | 4.3 ± 0.1 | 5.6 ± 0.1 |
| Permeate | 2 | 534.1 ± 0.2 | 3.0 ± 0.7 | 4.8 ± 0.1 | 6.1 ± 0.4 |
| Retentate | 1.5 | 1961.7 ± 1.1 | 11.5 ± 2.2 | 5.6 ± 0.8 | 7.5 ± 0.3 |
| Retentate | 2 | 2563.6 ± 0.4 | 15.3 ± 1.0 | 5.7 ± 0.2 | 7.6 ± 0.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conidi, C.; Drioli, E.; Cassano, A. Biologically Active Compounds from Goji (Lycium Barbarum L.) Leaves Aqueous Extracts: Purification and Concentration by Membrane Processes. Biomolecules 2020, 10, 935. https://doi.org/10.3390/biom10060935
Conidi C, Drioli E, Cassano A. Biologically Active Compounds from Goji (Lycium Barbarum L.) Leaves Aqueous Extracts: Purification and Concentration by Membrane Processes. Biomolecules. 2020; 10(6):935. https://doi.org/10.3390/biom10060935
Chicago/Turabian StyleConidi, Carmela, Enrico Drioli, and Alfredo Cassano. 2020. "Biologically Active Compounds from Goji (Lycium Barbarum L.) Leaves Aqueous Extracts: Purification and Concentration by Membrane Processes" Biomolecules 10, no. 6: 935. https://doi.org/10.3390/biom10060935