Analytical Methods for the Determination of Fatty Acid Esters of Hydroxy Fatty Acids (FAHFAs) in Biological Samples, Plants and Foods
Abstract
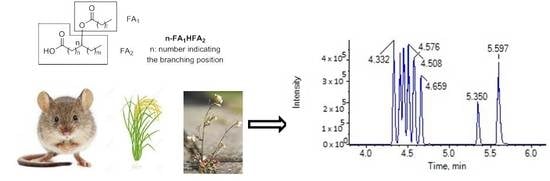
:1. Introduction
2. Diversity of FAHFAs and Elucidation of Their Structures
2.1. Diversity of FAHFAs
2.2. Mass Spectrometry Studies for the Structure Elucidation of FAHFAs
3. Sample Preparation
3.1. Methods for the Extraction of FAHFAS and Solid Phase Extraction (SPE) Protocols
3.2. Derivatization Procedures
4. Analytical Methodologies
4.1. Instrumentation
4.2. Occurrence and Contents of FAHFAs in Biological Samples, Plants and Foods
5. Biological Relevance
6. Conclusion
Author Contributions
Funding
Conflicts of Interest
References
- Kokotos, G.; Nicolaou, A. Bioactive Lipids; The Oily Press: Bridgewater, UK, 2004. [Google Scholar]
- Yore, M.M.; Syed, I.; Moraes-Vieira, P.M.; Zhang, T.; Herman, M.A.; Homan, E.A.; Patel, R.T.; Lee, J.; Chen, S.; Peroni, O.D.; et al. Discovery of a class of endogenous mammalian lipids with anti-diabetic and anti-inflammatory effects. Cell 2014, 159, 318–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LIPID MAPS® Lipidomics Gateway. Available online: https://www.lipidmaps.org/ (accessed on 20 July 2020).
- Nicolaides, N.; Ruth, E.C. Unusual fatty acids in the lipids of steer and human meibomian gland excreta. Curr. Eye Res. 1982, 2, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Chen, S.; Syed, I.; Stahlman, M.; Kolar, M.J.; Homan, E.A.; Chu, Q.; Smith, U.; Boren, J.; Kahn, B.B.; et al. A LC-MS-based workflow for measurement of branched fatty acid esters of hydroxy fatty acids. Nat. Protoc. 2016, 11, 747–763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuda, M.; Brezinova, M.; Rombaldova, B.; Slavikova, M.; Posta, P.; Beier, P.; Janovska, J.; Veleba, J.; Kopecky, J., Jr.; Kudova, E.; et al. Docosahexaenoic acid-derived fatty acid esters of hydroxy fatty acids (FAHFAs) with anti-inflammatory properties. Diabetes 2016, 65, 2580–2590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolar, M.J.; Konduri, S.; Chang, T.; Wang, H.; McNerlin, C.; Ohlsson, L.; Härröd, M.; Siegel, D.; Saghatelian, A. Linoleic acid esters of hydroxy linoleic acids are anti-inflammatory lipids found in plants and mammals. J. Biol. Chem. 2019, 294, 10698–10707. [Google Scholar] [CrossRef] [PubMed]
- Balas, L.; Bertrand-Michel, J.; Viars, F.; Faugere, J.; Lefort, C.; Caspar-Bauguil, S.; Langin, D.; Durand, T. Regiocontrolled syntheses of FAHFAs and LC-MS/MS differentiation of regioisomers. Org. Biomol. Chem. 2016, 14, 9012–9020. [Google Scholar] [CrossRef]
- Nelson, A.T.; Kolar, M.J.; Chu, Q.; Syed, I.; Kahn, B.B.; Saghatelian, A.; Siegel, D. Stereochemistry of endogenous palmitic acid ester of 9-hydroxystearic acid and relevance of absolute configuration to regulation. J. Am. Chem. Soc. 2017, 139, 4943–4947. [Google Scholar] [CrossRef] [Green Version]
- Pflimlin, E.; Bielohuby, M.; Korn, M.; Breitschopf, K.; Löhn, M.; Wohlfart, P.; Konkar, A.; Podeschwa, M.; Bärenz, F.; Pfenninger, A.; et al. Acute and repeated treatment with 5-PAHSA or 9-PAHSA isomers does not improve glucose control in mice. Cell Metab. 2018, 28, 217–227. [Google Scholar] [CrossRef] [Green Version]
- Mountanea, O.G.; Limnios, D.; Kokotou, M.G.; Bourboula, A.; Kokotos, G. Asymmetric synthesis of saturated hydroxy fatty acids and fatty acid esters of hydroxy fatty acids. Eur. J. Org. Chem. 2019, 2010–2019. [Google Scholar] [CrossRef]
- Ma, Y.; Kind, T.; Vaniya, A.; Gennity, I.; Fahrmann, J.F.; Fiehn, O. An in-silico MS/MS library for automatic annotation of novel FAHFA lipids. J. Cheminform. 2015, 7, 53. [Google Scholar] [CrossRef] [Green Version]
- Marshall, D.L.; Saville, J.T.; Maccarone, A.T.; Ailuri, R.; Kelso, M.J.; Mitchell, T.W.; Blanksby, S.J. Determination of ester position in isomeric (O-acyl)-hydroxy fatty acids by ion trap mass spectrometry. Rapid Commun. Mass Spectrom. 2016, 30, 2351–2359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, T.; Lin, M.; Zhang, D.; Li, M.; Zhang, J. A UPLC/MS/MS method for comprehensive profiling and quantification of fatty acid esters of hydroxy fatty acids in white adipose tissue. Anal. Bioanal. Chem. 2018, 410, 7415–7428. [Google Scholar] [CrossRef] [PubMed]
- Matyash, V.; Liebisch, G.; Kurzchalia, T.V.; Shevchenko, A.; Schwudke, D. Lipid extraction by methyl-tert-butyl ether for high-throughput lipidomics. J. Lipid Res. 2008, 49, 1137–1146. [Google Scholar] [CrossRef] [Green Version]
- Kolar, M.J.; Nelson, A.T.; Chang, T.; Ertunc, M.E.; Christy, M.P.; Ohlsson, L.; Härröd, M.; Kahn, B.B.; Siegel, D.; Saghatelian, A. Faster protocol for endogenous fatty acid esters of hydroxy fatty acid (FAHFA) measurements. Anal. Chem. 2018, 90, 5358–5365. [Google Scholar] [CrossRef]
- Lopez-Bascon, M.A.; Calderon-Santiago, M.; Priego-Capote, F. Confirmatory and quantitative analysis of fatty acid esters of hydroxy fatty acids in serum by solid phase extraction coupled to liquid chromatography tandem mass spectrometry. Anal. Chim. Acta 2016, 943, 82–88. [Google Scholar] [CrossRef]
- Brezinova, M.; Kuda, O.; Hansikova, J.; Rombaldova, M.; Balas, L.; Bardova, K.; Durand, T.; Rossmeisl, M.; Cerna, M.; Stranak, Z.; et al. Levels of palmitic acid ester of hydroxystearic acid (PAHSA) are reduced in the breast milk of obese mothers. Bba—Mol. Cell Biol. Lipids 2018, 1863, 126–131. [Google Scholar] [CrossRef]
- Liberati-Čizmek, A.M.; Biluš, M.; Brkić, A.L.; Barić, I.C.; Bakula, M.; Hozić, A.; Cindrić, M. Analysis of fatty acid esters of hydroxyl fatty acid in selected plant food. Plant. Foods Hum. Nutr. 2019, 74, 235–240. [Google Scholar] [CrossRef]
- Hu, C.; Wang, M.; Duan, Q.; Han, X. Sensitive analysis of fatty acid esters of hydroxy fatty acids in biological lipid extracts by shotgun lipidomics after one-step derivatization. Anal. Chim. Acta 2020, 1105, 105–111. [Google Scholar] [CrossRef]
- Zhu, Q.F.; Yana, J.W.; Gao, Y.; Zhang, J.W.; Yuan, B.F.; Feng, Y.Q. Highly sensitive determination of fatty acid esters of hydroxyl fatty acids by liquid chromatography-mass spectrometry. J. Chromatog. B. 2017, 1061, 34–40. [Google Scholar] [CrossRef]
- Zhu, Q.F.; Yan, J.W.; Zhang, T.Y.; Xiao, H.M.; Feng, Y.Q. Comprehensive screening and identification of fatty acid esters of hydroxy fatty acids in plant tissues by chemical isotope labeling-assisted liquid chromatography-mass spectrometry. Anal. Chem. 2018, 90, 10056–10063. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Kind, T.; Zhu, Q.F.; Wang, Y.; Yan, J.W.; Fiehn, O.; Feng, Y.Q. An in-silico generated library for sensitive detection of 2-dimethylaminoethylamine derivatized FAHFA lipids using high-resolution tandem mass spectrometry. Anal. Chem. 2020, 92, 5960–5968. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.C.; Adamec, J.; Regnier, F.E. Enhancement of the LC/MS analysis of fatty acids through derivatization and stable isotope coding. Anal. Chem. 2007, 79, 5150–5157. [Google Scholar] [CrossRef] [PubMed]
- Bollinger, J.G.; Thompson, W.; Lai, Y.; Oslund, R.C.; Hallstrand, T.S.; Sadilek, M.; Turecek, F.; Gelb, M.H. Improved sensitivity mass spectrometric detection of eicosanoids by charge reversal derivatization. Anal. Chem. 2010, 82, 6790–6796. [Google Scholar] [CrossRef] [Green Version]
- Hao, Y.H.; Zhang, Z.; Wang, L.; Liu, C.; Lei, A.W.; Yuan, B.F.; Feng, Y.Q. Stable isotope labeling assisted liquid chromatography–electrospray tandem mass spectrometry for quantitative analysis of endogenous gibberellins. Talanta 2015, 144, 341–348. [Google Scholar] [CrossRef]
- Zhu, Q.F.; Hao, Y.H.; Liu, M.Z.; Yue, J.; Ni, J.; Yuan, B.F.; Feng, Y.Q. Analysis of cytochrome P450 metabolites of arachidonic acid by stable isotope probe labeling coupled with ultra high-performance liquid chromatography/mass spectrometry. J. Chromatogr. A 2015, 1410, 154–163. [Google Scholar] [CrossRef]
- Moraes-Vieira, P.M.; Saghatelian, A.; Kahn, B.B. GLUT4 expression in adipocytes regulates de novo lipogenesis and levels of a novel class of lipids with antidiabetic and anti-inflammatory effects. Diabetes 2016, 65, 1808–1815. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Moraes-Vieira, P.M.; Castoldi, A.; Aryal, P.; Yee, E.U.; Vickers, C.; Parnas, O.; Donaldson, C.J.; Saghatelian, A.; Kahn, B.B. Branched fatty acid esters of hydroxy fatty acids (FAHFAs) protect against colitis by regulating gut innate and adaptive immune responses. J. Biol. Chem. 2016, 291, 22207–22217. [Google Scholar] [CrossRef] [Green Version]
- Syed, I.; Lee, J.; Moraes-Vieira, P.M.; Donaldson, C.J.; Sontheimer, A.; Aryal, P.; Wellenstein, K.; Kolar, M.J.; Nelson, A.T.; Siegel, D.; et al. Palmitic acid hydroxystearic acids activate GPR40, which is involved in their beneficial effects on glucose homeostasis. Cell Metab. 2018, 27, 419–427. [Google Scholar] [CrossRef] [Green Version]
- Syed, I.; Lee, J.; Peroni, O.D.; Yore, M.M.; Moraes-Vieira, P.M.; Santoro, A.; Wellenstein, K.; Smith, U.; McGraw, T.E.; Saghatelian, A.; et al. Methodological issues in studying PAHSA biology: Masking PAHSA effects. Cell Metab. 2018, 28, 543–546. [Google Scholar] [CrossRef] [Green Version]
- Kuda, O. On the complexity of PAHSA research. Cell Metab. 2018, 28, 541–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuda, O.; Brezinova, M.; Silhavy, J.; Landa, V.; Zidek, V.; Dodia, C.; Kreuchwig, F.; Vrbacky, M.; Balas, L.; Durand, T.; et al. Nrf2-mediated antioxidant defense and peroxiredoxin 6 are linked to biosynthesis of palmitic acid ester of 9-hydroxystearic acid. Diabetes 2018, 67, 1190–1199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Syed, I.; Rubin, de Celis, M.F.; Mohan, J.F.; Moraes-Vieira, P.M.; Vijayakumar, A.; Nelson, A.T.; Siegel, D.; Saghatelian, A.; Mathis, D.; Kahn, B.B. PAHSAs attenuate immune responses and promote β-cell survival in autoimmune diabetic mice. J. Clin. Invest. 2019, 129, 3717–3731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brezinova, M.; Cajka, T.; Oseeva, M.; Stepan, M.; Dadova, K.; Rossmeislova, L.; Matous, M.; Siklova, M.; Rossmeisl, M.; Kuda, O. Exercise training induces insulin-sensitizing PAHSAs in adipose tissue of elderly women. Bba—Mol. Cell Biol. Lipids 2020, 1865, 158576. [Google Scholar] [CrossRef] [PubMed]
- Benlebna, M.; Balas, L.; Bonafos, B.; Pessemesse, L.; Vigor, C.; Grober, J.; Bernex, F.; Fouret, G.; Paluchova, V.; Gaillet, S.; et al. Long-term high intake of 9-PAHPA or 9-OAHPA increases basal metabolism and insulin sensitivity but disrupts liver homeostasis in healthy mice. J. Nutr. Biochem. 2020, 79, 108361. [Google Scholar] [CrossRef]
- Paluchova, V.; Vik, A.; Cajka, T.; Brezinova, M.; Brejchova, K.; Bugajev, V.; Draberova, L.; Draber, P.; Buresova, J.; Kroupova, P.; et al. Triacylglycerol-rich oils of marine origin are optimal nutrients for induction of polyunsaturated docosahexaenoic acid ester of hydroxy linoleic acid (13-DHAHLA) with anti-inflammatory properties in mice. Mol. Nutr. Food Res. 2020, 64, 1901238. [Google Scholar] [CrossRef]






| Analytical Technique | Derivatization | Instrumental Analysis | Column/Mobile Phase | Sample | Sample Preparation— Solvent Extraction/ Cartridge-column | Ref. |
|---|---|---|---|---|---|---|
| LC-MS/MS, (-) ESI mode | No | Agilent 6410 (triple quadrupole) combined with HPLC Agilent 1200 (Agilent, Santa Clara, CA, USA). | Luna C18 column (3 μm, 100 Å, 250 × 2.0 mm)/93:7 methanol:water, 0.01% ammonium hydroxide, 5 mM ammonium acetate; flow rate: 0.2 mL/min for 120 min; temperature: 25 °C | Human or mouse serum, subcutaneous WAT, mouse perigonadal WAT, BAT and liver | Bligh–Dyer method/HyperSep silica SPE column (500 mg bed weight, 6 mL column volume, Thermo Scientific) | [5] |
| LC-MS/MS, (-) ESI mode | No | QTRAP 5500/SelexION, (Sciex, Framingham, MA, USA). hybrid triple quadrupole linear ion trap mass spectrometer equipped with an ion mobility cell (Sciex, Framingham, MA, USA) combined with LC Ultimate 3000 RSLC (Thermo) | Kinetex C18 (1.7 μm 2.1 × 150 mm column)/ 70% water, 30% acetonitrile (ACN), 0.01% acetic acid, pH 4 (Solvent A) and 50% ACN, 50% IPA (solvent B); flow rate: 0.2 mL/min for 60 min; temperature: 50 °C | Epididymal WAT, subcutaneous WAT, liver, interscapular BAT and serum | Citric acid buffer, methanol, dichloromethane (1:1:2)/Strata SI-1 silica SPE cartridge (50 μg silica, 70 Å, Sigma-Aldrich) | [6] |
| LC-MS/MS, (-) ESI mode | No | Agilent 6460 (triple quadrupole) combined with HPLC Agilent 1200 | C18 Mediterranea column (10 × 4.6 mm, 3 μm)/5 mM ammonium acetate and 0.01% ammonium hydroxide in 98:2 methanol:water (organic mobile phase) and 5 mM ammonium acetate and 0.01% ammonium hydroxide in 98:2 water:methanol (aqueous phase); flow rate: 0.8 mL/min for 20 min; temperature: 25 °C | Human serum | Deproteinization by addition of methanol/On-line SPE Hysphere C8 cartridges (7 mm, 10 × 2.0 mm, Spark Holland, Emmen, Holland) | [18] |
| UHPLC-MS/MS, (+) ESI mode | DMED and d4-DMED labeling | Shimadzu MS-8040 triple quadrupole combined with Shimadzu LC-30AD UPLC system (Shimadzu, Kyoto, Japan). | Acquity UPLC BEH C18 column (2.1 × 50 mm, 1.7 μm, Waters)/mobile phase (A) formic acid in ACN/water (0.1%, 6/4, v/v) and (B) formic acid in IPA/ACN (0.1%, 9/1, v/v; flow rate: 0.4 mL/min for 22 min; temperature: 40 °C | Rat WAT, lung, kidney, thymus, liver and heart tissues. Human serum from healthy individuals and breast cancer patients | ACN containing 0.1% NH3.H2O/Strong anion exchange solid phase extraction SAX SPE-cartridge (1 mL, 50 mg, Weltech Co) | [22] |
| UPLC-MS/MS, (-) ESI mode | No | QTRAP 5500/SelexION, hybrid triple quadrupole linear ion trap mass spectrometer equipped with an ion mobility cell (Sciex) combined with UPLC Ultimate 3000 RSLC (Thermo) | Kinetex C18 (1.7 μm 2.1 × 150 mm column)/70% water, 30% ACN, 0.01% acetic acid, pH 4 (Solvent A) and 50% ACN, 50% IPA (solvent B); flow rate: 0.2 mL/min for 60 min; temperature: 50 °C | Murine tissues and human breast milk | Citric acid buffer, methanol, dichloromethane (1:1:2)/HyperSep SPE column (500 mg/10 mL, 40–60 μm, 70 Å, Thermo) | [19] |
| UPLC-MS/MS, (-) ESI mode | No | Agilent 6470 triple quadrupole mass spectrometer or Agilent 6550 qTOF combined with an Agilent 1290 UPLC system |
Acquity BEH C18 column (100 mm × 2.1 mm, 1.7 μm)/Solvent A: water containing 5 mM ammonium acetate. Solvent B: acetonitrile/2-propanol (3:1, v/v) containing 2 mM ammonium acetate; flow rate: 0.4 mL/min for 28 min; temperature: 50 °C | WAT from hamsters | Methyl tert-butyl ether, methanol, water (5:1.5:1.5)/HyperSep silica cartridge (Thermo Scientific) | [15] |
| LC-MS, (-) ESI mode | No | TSQ Quantiva LC-MS instrument (Thermo Fisher Scientific) | Acquity UPLC BEH C18 column (1.7 μm, 2.1 mm × 100 mm, Waters)/isocratic 93:7 methanol/water with 5 mM ammonium acetate and 0.03% ammonium hydroxide (v/v); flow rate: 0.2 mL/min for 30 min; temperature: 25 °C | Perigonadal WAT and human plasma | Phospate-buffered saline (PBS), methanol, chloroform (1:1:2)/Strata SI-1 silica SPE cartridge (500 mg silica, 3 mL, Phenomenex) | [17] |
| UHPLC-MS/MS, (+) ESI mode | DMED | Shimadzu MS-8045 mass spectrometer combined with a Shimadzu LC-30AD HPLC system | Acquity UPLC BEH C18 column (2.1 mm × 50 mm, 1.7 μm, Waters)/Formic acid in water (0.1%, v/v, solvent A) and ACN (solvent B); flow rate: 0.4 mL/min for 55 min; temperature: 40 °C | Rice and Arabidopsis thaliana | Bligh-Dyer method/Strong anion exchange solid phase extraction (SAX SPE) cartridge (3 mL, 200 mg, Weltech Co) | [23] |
| LC-MS/MS, (-) ESI mode | No | qTOF Synapt G2-Si mass spectrometer (Waters, Milford, MA, USA) coupled to Waters nanoAcquity UPLC | XTerra MSC18 3.5 μm NanoEase column (75 μm × 150 mm, Waters)/Isocratic elution 93:7 methanol:water phase buffer consisted of 5 mmol/L ammonium acetate and 0.01% ammonium hydroxide; flow rate: 0.7 μL/min for 30 min |
Oat (whole grain, coarse flakes and fine flakes), apple, clementine, lemon, strawberry, blueberry, mango, kiwi, avocado, pineapple, banana, onion, garlic, cherry tomato, carrot, parsley root, pepper and radish | Citric acid buffer, methanol, chloroform (1:1.5:3)/HyperSep silica cartridge (500 mg bed weight, 6 mL, Thermo Scientific) | [20] |
| MS, (+) ESI mode, shotgun lipidomics | AMPP | TSQ Quantiva triple quadrupole mass spectrometer (Thermo Fisher Scientific) equipped with an automated nanospray device (i.e., Nanomate HD, Advion Bioscience, Ithaca, NY, USA) | - | Liver and WAT from homozygous diabetic (db/db) and WT mice, and human plasma | HyperSep silica SPE cartridge (200 mg, 3 mL, Thermo Scientific) | [21] |
| LC-HRMS/MS, (+) ESI mode | DMED | LTQ Orbitrap Elite mass spectrometer (Thermo Fisher Scientific) combined with UltiMate 3000 UHPLC System (Thermo Fisher Scientific). | Acquity UPLC BEH C18 column (2.1 mm × 50 mm, 1.7 μm, Waters)/A ACN/water (6/4, v/v) containing 0.1% formic acid and B IPA/ACN (9/1, v/v) containing 0.1% formic acid; flow rate: 0.4 mL/min for 37 min; temperature: 40 °C | Arabidopsis thaliana | Methanol, chloroform, water (1:2:1)/Strong anion exchange solid phase extraction SAX-SPE cartridge (200 mg, 3 mL, Weltech Co) | [24] |
| Analyte | Quantitative Transition (m/z) | Qualitative Ions (m/z) | Ref. |
|---|---|---|---|
| PAHSA | 537 → 255 | 299, 281 | [2,5,6,15,17,18] |
| SAHSA | 565 → 283 | 299, 281 | [2,5,15,18] |
| OAHSA | 563 → 281 | 299, 281 | [2,5,15,17,18] |
| POHSA | 535 → 253 | 299, 281 | [2,5,15,18] |
| DHAHLA | 605 → 327 | 295, 277 | [6] |
| DHAHDHA | 653 → 327 | 343, 325 | [6] |
| Source | 5-PAHSA (pmol/g) | 9-PAHSA (pmol/g) | 13/12-PAHSA (pmol/g) | Total PAHSA (pmol/g) |
|---|---|---|---|---|
| Serum | 0.2–0.5 * | 1–4 * | 2–3 * | 7–10 * |
| WAT | 40 | 100 | 25 | 150–200 |
| BAT | 180 | 120 | 30 | 250–300 |
| Liver | 0 | 20 | 10 | 30 |
| Kidney | 5 | 20 | 2 | - |
| Pancreas | 0 | 5 | 4 | - |
| Apple | 0.1 | 0.4 | 0.8 | - |
| Broccoli | - | 1.7 | 1.3 | - |
| Beef | - | 4 | 6 | - |
| Chicken | 0.25 | 1.2 | 2 | - |
| Egg yolk | - | 4 | 7 | - |
| Egg white | - | 0.3 | 0.5 | - |
| FAHFA | Rat WAT (pg/g) |
|---|---|
| 13-PAHSA | 84.6 |
| 12-PAHSA | 22.1 |
| 9-PAHSA | 89.8 |
| 9-OAHSA | 13.9 |
| 13-SAHSA | 81.9 |
| 12-SAHSA | 132.1 |
| 9-SAHSA | 51.7 |
| Source | FAHFAs (10−7 g/g Fresh Weight) |
|---|---|
| Whole grain oat | 3.20 |
| Clementine | 2.51 |
| Garlic | 2.43 |
| Pineapple | 2.16 |
| Strawberries | 1.59 |
| Mango | 1.51 |
| Carrot | 1.40 |
| Parsley root | 1.14 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kokotou, M.G. Analytical Methods for the Determination of Fatty Acid Esters of Hydroxy Fatty Acids (FAHFAs) in Biological Samples, Plants and Foods. Biomolecules 2020, 10, 1092. https://doi.org/10.3390/biom10081092
Kokotou MG. Analytical Methods for the Determination of Fatty Acid Esters of Hydroxy Fatty Acids (FAHFAs) in Biological Samples, Plants and Foods. Biomolecules. 2020; 10(8):1092. https://doi.org/10.3390/biom10081092
Chicago/Turabian StyleKokotou, Maroula G. 2020. "Analytical Methods for the Determination of Fatty Acid Esters of Hydroxy Fatty Acids (FAHFAs) in Biological Samples, Plants and Foods" Biomolecules 10, no. 8: 1092. https://doi.org/10.3390/biom10081092