In Vitro and In Vivo Evaluation of 99mTc-Polymyxin B for Specific Targeting of Gram-Bacteria
Abstract
:1. Introduction
2. Materials and Methods
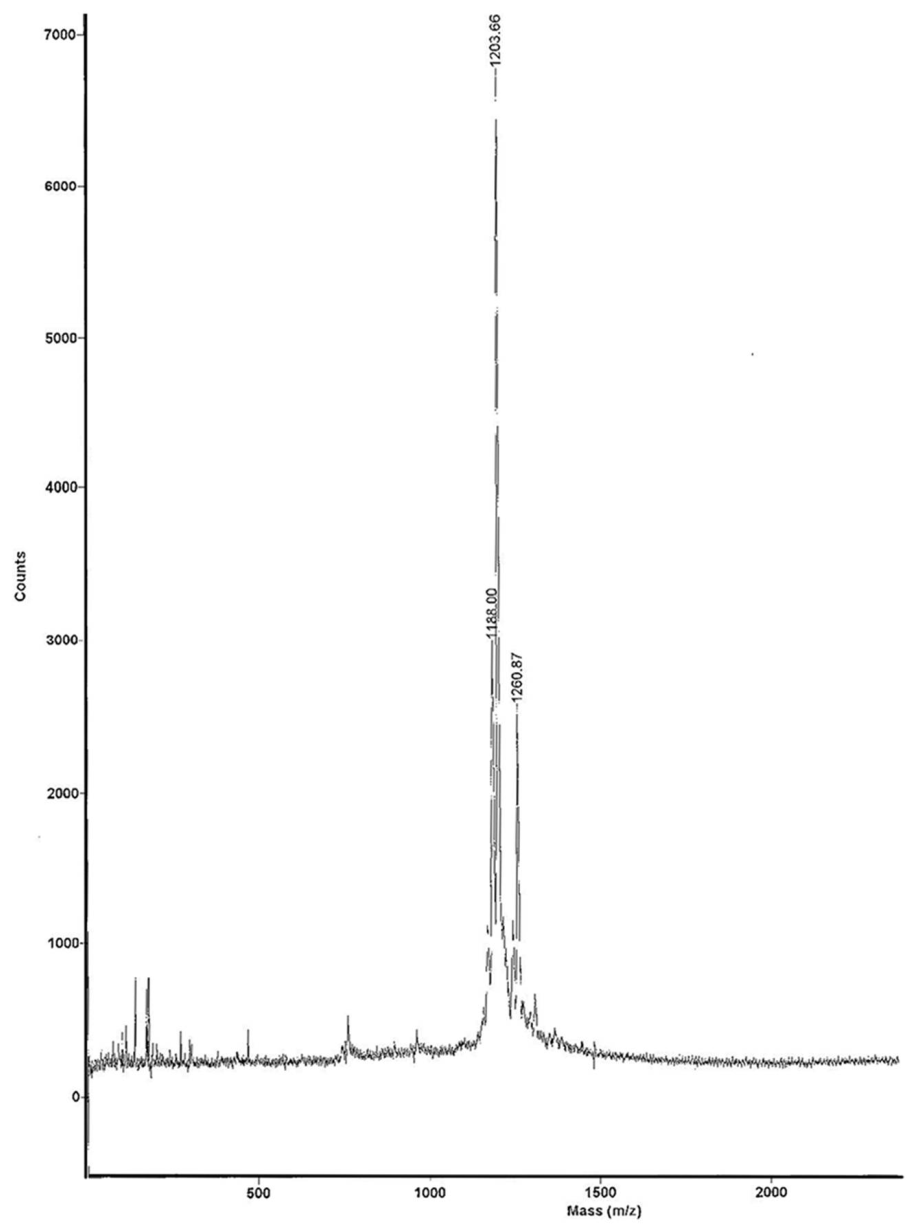
2.1. Conjugation
2.2. Radiolabeling Procedure
2.3. Quality Controls
2.4. Micro-Organisms
2.5. In Vitro Binding Studies
2.6. Biodistribution Studies
2.7. Targeting Studies
2.8. Statistical Analysis
3. Results
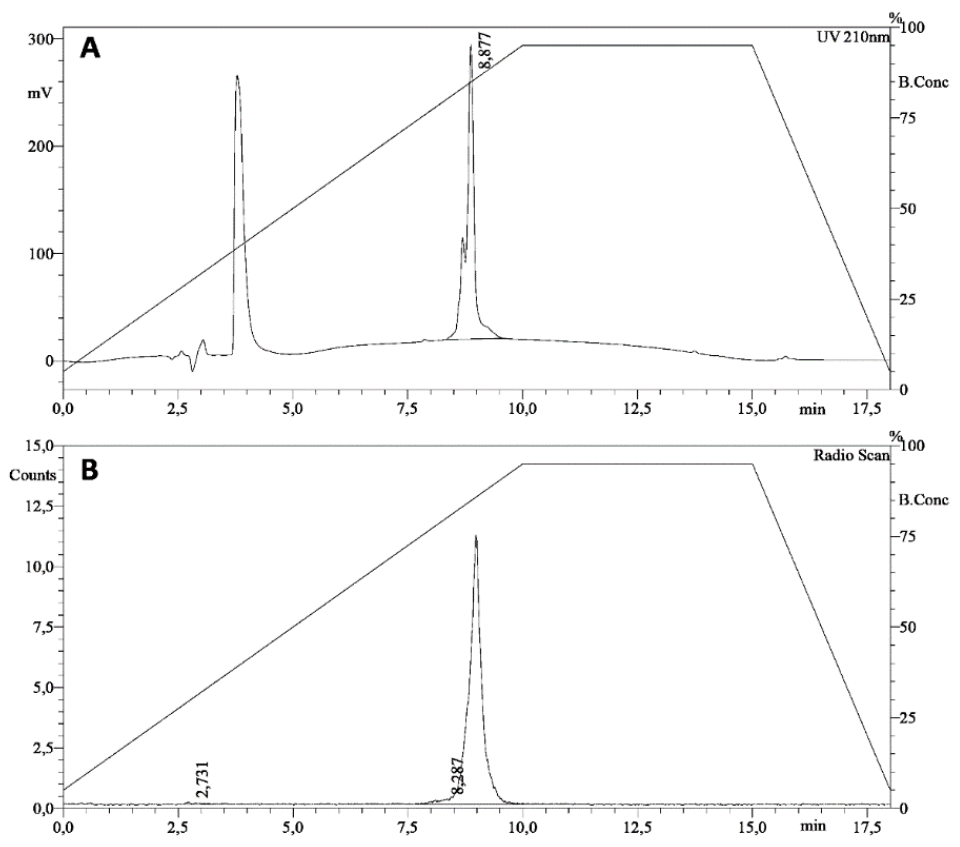
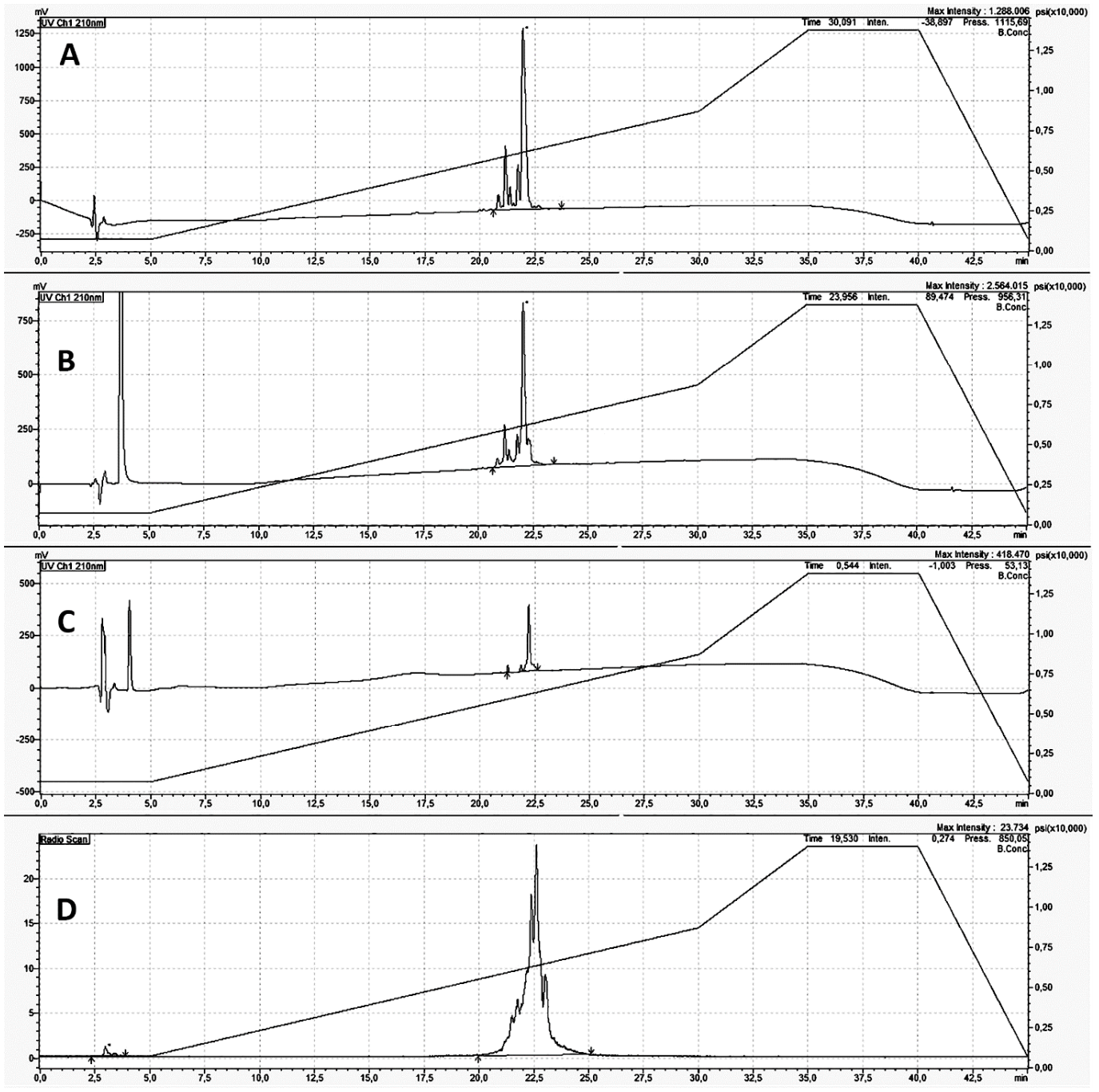
3.1. Radiolabeling
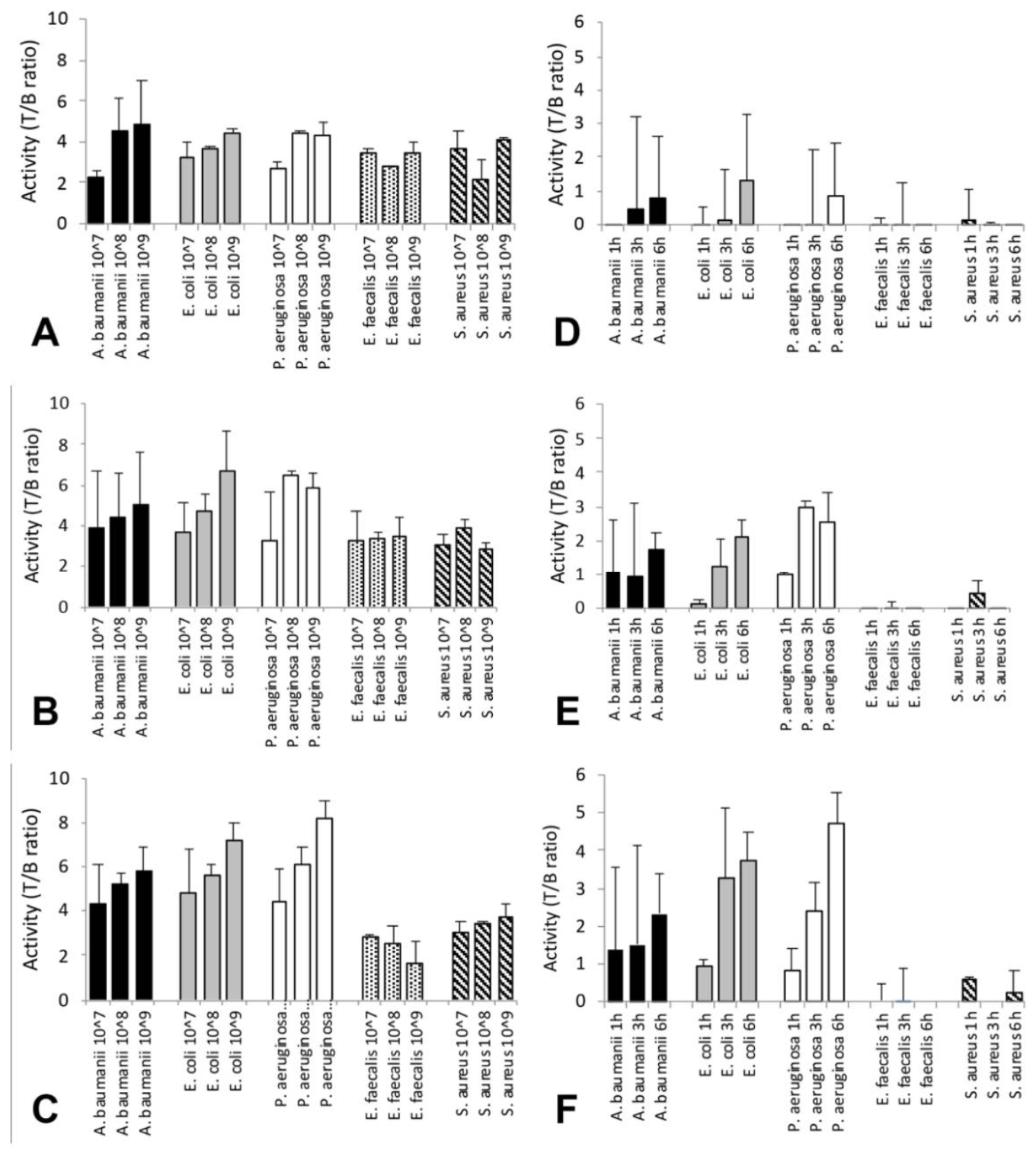
3.2. In Vitro Binding Studies
3.3. Biodistribution Studies
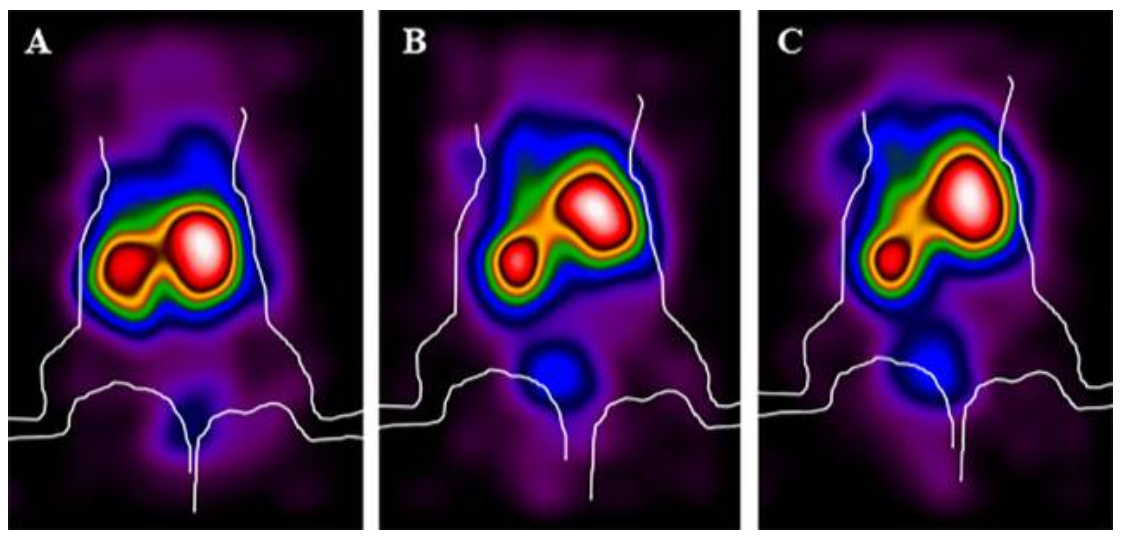
3.4. Targeting Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Auletta, S.; Varani, M.; Horvat, R.; Galli, F.; Signore, A.; Hess, S. PET radiopharmaceuticals for specific bacteria imaging: A systematic review. J. Clin. Med. 2019, 8, 197. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Jiang, N.; Zhu, L. Experimental studies on imaging of infected site with 99mTc-labeled ciprofloxacin in mice. Chin. Med. J. 2009, 122, 1907–1909. [Google Scholar] [PubMed]
- Langer, O.; Brunner, M.; Zeitlinger, M.; Ziegler, S.; Muller, U.; Dobrozemsky, G.; Lackner, E.; Joukhadar, C.; Mitterhauser, M.; Wadsak, W.; et al. In vitro and in vivo evaluation of [18F]ciprofloxacin for the imaging of bacterial infections with PET. Eur. J. Nucl. Med. Mol. Imaging 2005, 32, 143–150. [Google Scholar] [CrossRef]
- Li, J.; Zheng, H.; Fodah, R.; Warawa, J.M.; Ng, C.K. Validation of 2-18F-Fluorodeoxysorbitol as a potential radiopharmaceutical for imaging bacterial infection in the lung. J. Nucl. Med. 2018, 59, 134–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vilche, M.; Reyes, A.L.; Vasilskid, E.; Oliver, P.; Balter, H.; Engler, H. 68Ga-NOTA-UBI-29-41 as a PET tracer for detection of bacterial infection. J. Nucl. Med. 2016, 57, 622–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Vries, E.F.J.; Roca, M.; Jamar, F.; Israel, O.; Signore, A. Guidelines for the labelling of leukocytes with 99mTc-HMPAO. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 842–848. [Google Scholar] [CrossRef] [Green Version]
- Roca, M.; De Vries, E.F.J.; Jamar, F.; Israel, O.; Signore, A. Guidelines for the labelling of leukocytes with 111In-oxine. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 835–841. [Google Scholar] [CrossRef] [Green Version]
- Glaudemans, A.W.; Prandini, N.; Di Girolamo, M.; Argento, G.; Lauri, C.; Lazzeri, E.; Muto, M.; Sconfienza, L.M.; Signore, A. Hybrid imaging of musculoskeletal infections. Q. J. Nucl. Med. Mol. Imaging 2018, 62, 3–13. [Google Scholar]
- Eisler, T.; Svensson, O.; Engström, C.F.; Reinholt, F.P.; Lundberg, C.; Wejkner, B.; Schmalholz, A.; Elmstedt, E. Ultrasound for diagnosis of infection in revision total hip arthroplasty. J. Arthroplast. 2001, 16, 1010–1017. [Google Scholar] [CrossRef]
- Battaglia, M.; Vannini, F.; Guaraldi, F.; Rossi, G.; Biondi, F.; Sudanese, A. Validity of preoperative ultrasound-guided aspiration in the revision of hip prosthesis. Ultrasound Med. Biol. 2011, 37, 1977–1983. [Google Scholar] [CrossRef]
- Tomas, X.; Bori, G.; Garcia, S.; Garcia-Diez, A.I.; Pomes, J.; Soriano, A.; Ríos, J.; Almela, M.; Mensa, J.; Gallart, X.; et al. Accuracy of CT-guided joint aspiration in patients with suspected infection status post-total hip arthroplasty. Skeletal Radiol. 2011, 40, 57–64. [Google Scholar] [CrossRef]
- Meermans, G.; Haddad, F.S. Is there a role for tissue biopsy in the diagnosis of periprosthetic infection? Clin. Orthop. Relat. Res. 2010, 468, 1410–1417. [Google Scholar] [CrossRef] [Green Version]
- Jordan, R.W.; Smith, N.A.; Saithna, A.; Sprowson, A.P.; Foguet, P. Sensitivities, specificities and predictive values of microbiological culture techniques for the diagnosis of prosthetic joint infection. BioMed Res. Int. 2014, 2014, 180416. [Google Scholar] [CrossRef] [Green Version]
- Ainsworth, G.C.; Brown, A.M.; Brownlee, G. Aerosporin, an antibiotic produced by Bacillus aerosporus Greer. Nature 1947, 160, 263. [Google Scholar] [CrossRef]
- Stansly, P.G.; Shepherd, R.G.; White, H.J. Polymyxin: A new chemotherapeutic Agent. Bull. Johns Hopkins Hosp. 1947, 81, 43–54. [Google Scholar] [PubMed]
- Benedict, R.G.; Langlykke, A.F. Antibiotic activity of Bacillus polymyxa. J. Bacteriol. 1947, 54, 24–25. [Google Scholar]
- Newton, B.A. The properties and mode of action of the polymyxins. Bacteriol. Rev. 1956, 20, 14–27. [Google Scholar] [CrossRef]
- Falagas, M.E.; Kasiakou, S.K. Toxicity of polymyxins: A systematic review of the evidence from old and recent studies. Crit. Care 2006, 10, R27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velkov, T.; Thompson, P.E.; Nation, R.L.; Li, J. Structure-activity relationships of polymyxin antibiotics. J. Med. Chem. 2010, 53, 1898–1916. [Google Scholar] [CrossRef] [Green Version]
- Falagas, M.E.; Rafailidis, P.I.; Matthaou, D.K. Resistance to polymyxins: Mechanisms, frequency and treatment options. Drug Resist. Updates 2010, 13, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Orwa, J.A.; Govaerts, C.; Busson, R.; Roets, E.; Van Schepdael, A.; Hoogmartens, J. Isolation and structural characterization of polymyxin B components. J. Chromatogr. A 2001, 912, 369–373. [Google Scholar] [CrossRef]
- El-Sayed, A.M.A.E.; Zhong, L.L.; Shen, C.; Yang, Y.; Doi, Y.; Tian, G.B. Colistin and its role in the Era of antibiotic resistance: An extended review (2000–2019). Emerg. Microbes Infect. 2020, 9, 868–885. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Nation, R.L.; Turnidge, J.D.; Milne, R.W.; Coulthard, K.; Rayner, C.R.; Paterson, D.L. Colistin: The reemerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect. Dis. 2006, 6, 589–601. [Google Scholar] [CrossRef]
- Zavascki, A.P.; Goldani, L.Z.; Li, J.; Nation, R.L. Polymyxin B for the treatment of multidrug-resistant pathogens: A critical review. J. Antimicrob. Chemother. 2007, 60, 1206–1215. [Google Scholar] [CrossRef] [Green Version]
- Vaara, M. New polymyxin derivatives that display improved efficacy in animal infection models as compared to polymyxin B and colistin. Med. Res. Rev. 2018, 38, 1661–1673. [Google Scholar] [CrossRef] [PubMed]
- Rennen, H.J.; Boerman, O.C.; Koenders, E.B.; Oyen, W.J.; Corstens, F.H. Labeling proteins with Tc-99m via hydrazinonicotinamide (HYNIC): Optimization of the conjugation reaction. Nucl. Med. Biol. 2000, 27, 599–604. [Google Scholar] [CrossRef]
- Scopinaro, F.; Pani, R.; De Vincentis, G.; Soluri, A.; Pellegrini, R.; Porfiri, L.M. High-resolution scintimammography improves the accuracy of technetium-99m methoxyisobutylisonitrile scintimammography: Use of a new dedicated gamma camera. Eur. J. Nucl. Med. 1999, 26, 1279–1288. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Zhao, L.; Li, Y.; Liu, Y.; Xiao, W.; Song, Y.; Luo, L.; Huang, D.; Yancopoulos, J.D.; Wiegand, S.J.; et al. A subretinal Matrigel rat choroidal neovascularization (CNV) model and inhibition of CNV and associated inflammation and fibrosis by VEGF trap. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6009–6017. [Google Scholar] [CrossRef] [PubMed]
- Auletta, S.; Galli, F.; Lauri, C.; Martinelli, D.; Santino, I.; Signore, A. Imaging bacteria with radiolabelled quinolones, cephalosporins and siderophores for imaging infection: A systematic review. Clin. Transl. Imaging 2016, 4, 229–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Auletta, S.; Baldoni, D.; Varani, M.; Galli, F.; Hajar, I.A.; Duatti, A.; Ferro-Flores, G.; Trampuz, A.; Signore, A. Comparison of 99mTc-UBI 29-41, 99mTc-ciprofloxacin, 99mTc-ciprofloxacin dithiocarbamate and 111In-biotin for targeting experimental Staphylococcus aureus and Escherichia coli foreign-body infections: An ex-vivo study. Q. J. Nucl. Med. Mol. Imaging 2019, 63, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Ferro-Flores, G.; Arteaga de Murphy, C.; Pedraza-López, M.; Meléndez-Alafort, L.; Zhang, Y.M.; Rusckowski, M.; Hnatowich, D.J. In vitro and in vivo assessment of 99mTc-UBI specificity for bacteria. Nucl. Med. Biol. 2003, 30, 597–603. [Google Scholar] [CrossRef]
- Meléndez-Alafort, L.; Nadali, A.; Pasut, G.; Zangoni, E.; De Caro, R.; Cariolato, L.; Giron, M.C.; Castagliuolo, I.; Veronese, F.M.; Mazzi, U. Detection of sites of infection in mice using 99mTc-labeled PN(2)S-PEG conjugated to UBI and 99mTc-UBI: A comparative biodistribution study. Nucl. Med. Biol. 2009, 36, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Welling, M.M.; Mongera, S.; Lupetti, A.; Balter, H.S.; Bonetto, V.; Mazzi, U.; Pauwels, E.K.; Nibbering, P.H. Radiochemical and biological characteristics of 99mTc-UBI 29-41 for imaging of bacterial infections. Nucl. Med. Biol. 2002, 29, 413–422. [Google Scholar] [CrossRef]
- Akhtar, M.S.; Iqbal, J.; Khan, M.A.; Irfanullah, J.; Jehangir, M.; Khan, B.; Ul-Haq, I.; Muhammad, G.; Nadeem, M.A.; Afzal, M.S.; et al. 99mTc-labeled antimicrobial peptide ubiquicidin (29-41) accumulates less in Escherichia coli infection than in Staphlococcus aureus infection. J. Nucl. Med. 2004, 45, 849–856. [Google Scholar]
- Sarda-Mantel, L.; Saleh-Mghir, A.; Welling, M.M.; Meulemans, A.; Vrigneaud, J.M.; Raguin, O.; Hervatin, F.; Martet, G.; Chau, F.; Lebtahi, R.; et al. Evaluation of 99mTc-UBI 29-41 scintigraphy for specific detection of experimental Staphylococcus aureus prosthetic joint infections. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 1302–1309. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.S.; Qaisar, A.; Irfanullah, J.; Iqbal, J.; Khan, B.; Jehangir, M.; Nadeem, M.A.; Khan, M.A.; Afzal, M.S.; Ul-Haq, I.; et al. Antimicrobial peptide 99mTc-ubiquicidin 29-41 as human infection imaging agent: Clinical trial. J. Nucl. Med. 2005, 46, 567–573. [Google Scholar]
- Meléndez-Alafort, L.; Rodríguez-Cortés, J.; Ferro-Flores, G.; Arteaga De Murphy, C.; Herrera-Rodríguez, R.; Mitsoura, E.; Duncker, C.M. Biokinetics of (99m)Tc-UBI 29-41 in humans. Nucl. Med. Biol. 2004, 31, 373–379. [Google Scholar] [CrossRef]
- Gandomkar, M.; Najafi, R.; Shafiei, M.; Mazidi, M.; Goudarzi, M.; Mirfallah, S.H.; Ebrahimi, F.; Heydarpor, H.R.; Abdie, N. Clinical evaluation of antimicrobial peptide [(99m)Tc/Tricine/HYNIC(0)]ubiquicidin 29-41 as a human-specific infection imaging agent. Nucl. Med. Biol. 2009, 36, 199–205. [Google Scholar] [CrossRef]
- Sathekge, M.; Garcia-Perez, O.; Paez, D.; El-Haj, N.; Kain-Godoy, T.; Lawal, I.; Estrada-Lobato, E. Molecular imaging in musculoskeletal infections with 99mTc-UBI 29-41 SPECT/CT. Ann. Nucl. Med. 2018, 32, 54–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weinstein, E.A.; Ordonez, A.A.; De Marco, V.P.; Murawski, A.M.; Pokkali, S.; MacDonald, E.M.; Klunk, M.; Mease, R.C.; Pomper, M.G.; Jain, S.K. Imaging Enterobacteriaceae infection in vivo with 18F-fluorodeoxysorbitol positron emission tomography. Sci. Transl. Med. 2014, 6, 259ra146. [Google Scholar] [CrossRef] [Green Version]
- Yeaman, M.R.; Yount, N.Y. Mechanisms of antimicrobial peptide action and resistance. Pharmacol. Rev. 2003, 55, 27–55. [Google Scholar] [CrossRef] [Green Version]
- Abdelraouf, K.; He, J.; Ledesma, K.R.; Hu, M.; Tam, V.H. Pharmacokinetics and renal disposition of polymyxin B in an animal model. Antimicrob. Agents Chemother. 2012, 56, 5724–5727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, J.M.; Thorek, D.L.J.; Miller, L.S. Mouse model of Gram-negative prosthetic joint infection reveals therapeutic targets. JCI Insight 2018, 3, e121737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Signore, A.; Artiko, V.; Conserva, M.; Ferro-Flores, G.; Welling, M.M.; Jain, S.K.; Hess, S.; Sathekge, M. Imaging bacteria with radiolabelled probes: Is it feasible? J. Clin. Med. 2020, 9, 2372. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Bhatt, H.; Shinto, A.; Korde, A.; Kumar, M.; Kamaleshwaran, K.; Joseph, J.; Sarma, H.D.; Dash, A. 68Ga-NOTA-ubiquicidin fragment for PET imaging of infection: From bench to bedside. J. Pharm. Biomed. Anal. 2018, 159, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Ebenhan, T.; Sathekge, M.M.; Lwngana, T.; Koole, M.; Gheysens, O.; Govender, T.; Zeevaart, J.R. 68Ga-NOTA-functionalized Ubiquicidin: Cytotoxicity, biodistribution, radiation dosimetry, and first-in-human PET/CT imaging of infections. J. Nucl. Med. 2018, 59, 334–339. [Google Scholar] [CrossRef] [Green Version]
- Bhatt, J.; Mukherjee, A.; Korde, A.; Kumar, M.; Sarma, H.D.; Dash, A. Radiolabeling and preliminary evaluation of Ga-68 labeled NODAGA-Ubiquicidin fragments for prospective infection imaging. Mol. Imaging Biol. 2017, 19, 59–67. [Google Scholar] [CrossRef]


| 0.9% NaCl | Human Serum | ||||
|---|---|---|---|---|---|
| 1 h | 3 h | 6 h | 1 h | 3 h | 6 h |
| 99 ± 1.3% | 99 ± 1.5% | 98 ± 1.8% | 97 ± 1.6% | 96 ± 1.8% | 96 ± 2.1% |
| Bacterial Strain | 37 °C | 4 °C | ||
|---|---|---|---|---|
| HOT | +100× cold | HOT | +100× cold | |
| E. coli | 36.2 ± 12.5 | 9.9 ± 1.7 | 24.2 ± 8.6 | 10.4 ± 2.7 |
| P. aeruginosa | 31.5 ± 7.6 * | 12.5 ± 5.3 | 32.7 ± 11.6 | 17.7 ± 9.8 |
| A. baumanii | 37.4 ± 0.9 ** | 5.4 ± 8.3 | 28 ± 4.3 ** | 7.3 ± 8.9 |
| K. pneumoniae | 45 ± 5.7 *** | 20.6 ± 6.4 | 23.8 ± 2.3 | 20.2 ± 4.7 |
| S. aureus | 15.9 ± 9.2 | 12.6 ± 1.9 | 15.4 ± 7.8 | 12.6 ± 4.3 |
| E. faecalis | 18.5 ± 8.3 | 19.5 ± 4 | 14.8 ± 7.9 | 13 ± 1.3 |
| Organ | 1 h | 3 h | 6 h |
|---|---|---|---|
| Blood | 3.34 ± 0.51 | 1.87 ± 0.19 | 1.57 ± 0.18 |
| Small Bowel | 2.88 ± 0.21 | 2.01 ± 0.36 | 1.20 ± 0.01 |
| Large Bowel | 1.22 ± 0.22 | 1.62 ± 0.81 | 2.09 ± 0.41 |
| Kidneys | 163.92 ± 11.63 | 163.96 ± 31.41 | 154.42 ± 38.78 |
| Spleen | 11.95 ± 2.27 | 8.52 ± 8.28 | 12.95 ± 4.26 |
| Stomach | 1.87 ± 0.15 | 1.05 ± 0.30 | 0.65 ± 0.28 |
| Liver | 14.50 ± 0.64 | 10.89 ± 4.84 | 12.67 ± 0.71 |
| Muscle | 1.48 ± 0.45 | 0.63 ± 0.13 | 0.67 ± 0.07 |
| Bone | 3.02 ± 0.30 | 1.55 ± 0.41 | 1.38 ± 0.32 |
| Lungs | 5.16 ± 0.57 | 3.35 ± 0.73 | 3.46 ± 1.05 |
| Salivary Glands | 2.27 ± 0.31 | 1.43 ± 0.27 | 1.19 ± 0.11 |
| Organ | 1 h | 3 h | 6 h |
| Blood | 3.87 ± 0.55 | 2.18 ± 0.20 | 1.87 ± 0.17 |
| Small Bowel | 2.34 ± 0.19 | 1.67 ± 0.45 | 1.21 ± 0.03 |
| Large Bowel | 0.66 ± 0.07 | 0.96 ± 0.45 | 1.05 ± 0.23 |
| Kidneys | 32.5 ± 1.78 | 32.06 ± 6.97 | 30.39 ± 5.82 |
| Spleen | 0.64 ± 0.12 | 0.41 ± 0.41 | 0.57 ± 0.10 |
| Stomach | 0.50 ± 0.10 | 0.41 ± 0.16 | 0.39 ± 0.03 |
| Liver | 11 ± 0.73 | 8.22 ± 2.92 | 8.54 ± 1.35 |
| Muscle | 0.45 ± 0.05 | 0.24 ± 0.11 | 0.22 ± 0.07 |
| Bone | 0.21 ± 0.01 | 0.12 ± 0.04 | 0.13 ± 0.06 |
| Lungs | 0.67 ± 0.05 | 0.38 ± 0.09 | 0.39 ± 0.15 |
| Salivary Glands | 0.27 ± 0.08 | 0.14 ± 0.01 | 0.12 ± 0.01 |
| 1 h | ||||||
| Bacterial Strain | E. faecalis | S. aureus | E. faecalis | S. aureus | E. faecalis | S. aureus |
| 107 | 108 | 109 | ||||
| A. baumanii | ns | ns | ns | ns | ns | ns |
| P. aeruginosa | ns | ns | <0.0001 | 0.04 | ns | ns |
| E. coli | ns | ns | 0.0008 | ns | ns | ns |
| 3 h | ||||||
| Bacterial Strain | E. faecalis | S. aureus | E. faecalis | S. aureus | E. faecalis | S. aureus |
| 107 | 108 | 109 | ||||
| A. baumanii | ns | ns | ns | ns | ns | ns |
| P. aeruginosa | ns | ns | 0.0002 | 0.0007 | 0.02 | 0.006 |
| E. coli | ns | ns | ns | ns | ns | ns |
| 6 h | ||||||
| Bacterial Strain | E. faecalis | S. aureus | E. faecalis | S. aureus | E. faecalis | S. aureus |
| 107 | 108 | 109 | ||||
| A. baumanii | ns | ns | 0.007 | 0.007 | 0.01 | 0.04 |
| P. aeruginosa | ns | ns | 0.01 | 0.027 | 0.001 | 0.001 |
| E. coli | ns | ns | 0.004 | 0.004 | 0.002 | 0.003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Auletta, S.; Galli, F.; Varani, M.; Campagna, G.; Conserva, M.; Martinelli, D.; Santino, I.; Signore, A. In Vitro and In Vivo Evaluation of 99mTc-Polymyxin B for Specific Targeting of Gram-Bacteria. Biomolecules 2021, 11, 232. https://doi.org/10.3390/biom11020232
Auletta S, Galli F, Varani M, Campagna G, Conserva M, Martinelli D, Santino I, Signore A. In Vitro and In Vivo Evaluation of 99mTc-Polymyxin B for Specific Targeting of Gram-Bacteria. Biomolecules. 2021; 11(2):232. https://doi.org/10.3390/biom11020232
Chicago/Turabian StyleAuletta, Sveva, Filippo Galli, Michela Varani, Giuseppe Campagna, Martina Conserva, Daniela Martinelli, Iolanda Santino, and Alberto Signore. 2021. "In Vitro and In Vivo Evaluation of 99mTc-Polymyxin B for Specific Targeting of Gram-Bacteria" Biomolecules 11, no. 2: 232. https://doi.org/10.3390/biom11020232






