Cell Culture Media, Unlike the Presence of Insulin, Affect α-Synuclein Aggregation in Dopaminergic Neurons
Abstract
:1. Introduction
2. Materials and Methods
2.1. Primary Mouse Embryonic Neuronal Cultures
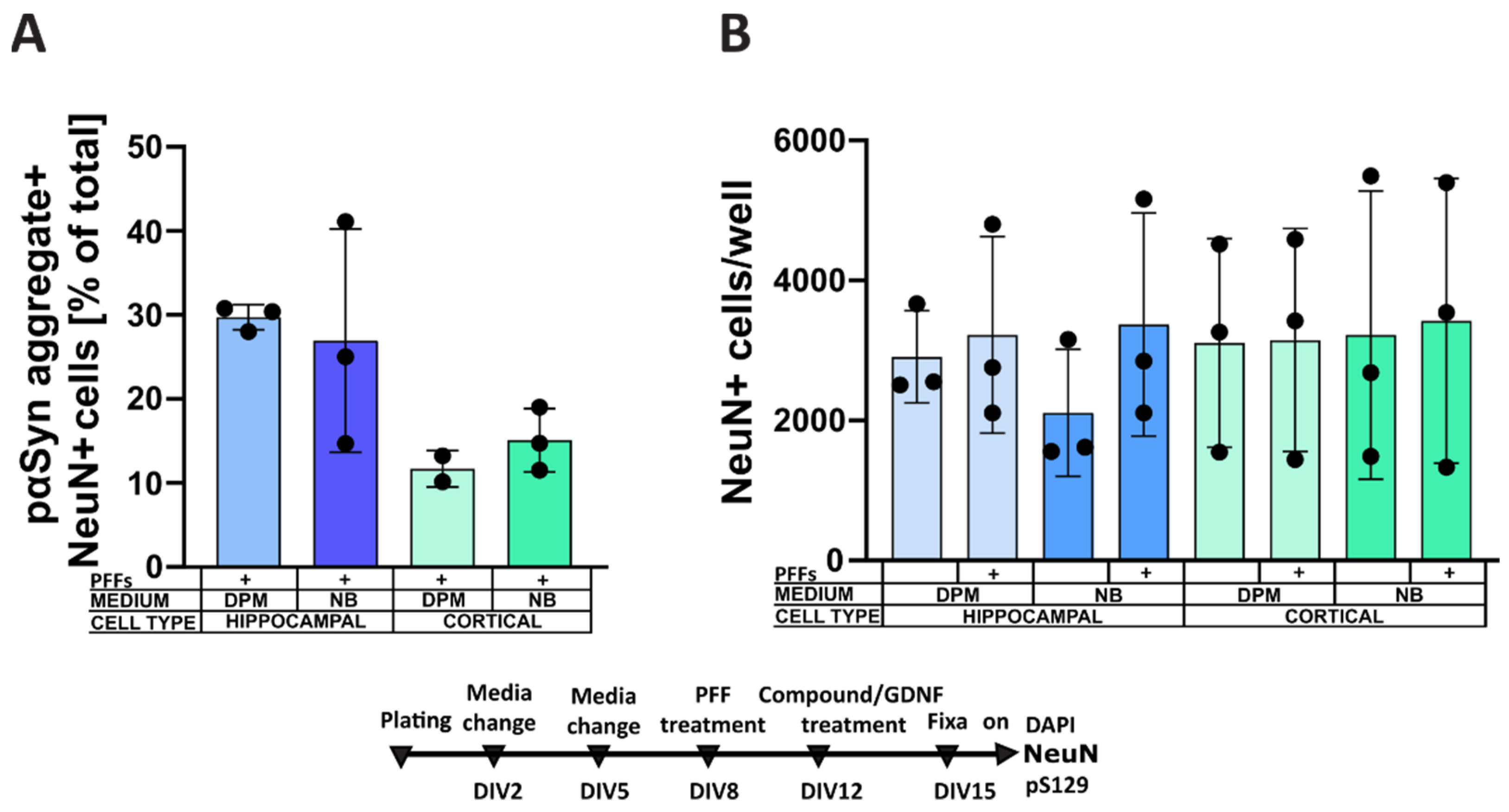
- Dopamine neuron medium (DPM) (Dulbecco’s Modified Eagle’s Medium DMEM/F12 (Thermo Scientific (Gibco) #21331–020,Waltham, MA, USA), 5 µM L-Glutamine (Thermo Scientific (Gibco), #25030–032, Waltham, MA, USA), 1× N-2 serum supplement (Thermo Scientific, #17502–048, Waltham, MA, USA), 150 µM D-glucose (Sigma-Aldrich, #G8769, St. Louis, MO, USA) and 200 ng/mL Primocin (Invivogen; ant-pm-1, ant-pm-2, San Diego, CA, USA));
- Neurobasal Medium cocktail (NB) (Neurobasal Medium (-) L-Glutamine (Thermo Scientific (Gibco), #21103-049, Waltham, MA, USA), 1× B-27 Supplement (Thermo Scientific (Gibco), #17504-044, Waltham, MA, USA), 1.25 µM L-Glutamine (Thermo Scientific (Gibco), #25030–032, Waltham, MA, USA) and 200 ng/mL Primocin (Invivogen; ant-pm-1, ant-pm-2, San Diego, CA, USA));
- Neurobasal Medium cocktail sans insulin (NB-ins), essentially similar to NB, but having 1× B-27 Supplement Minus Insulin (Thermo Scientific (Gibco), #A18956-02, Waltham, MA, USA).
2.2. Compounds and Treatments
2.3. Imaging and Analysis
2.4. Statistical Analysis
3. Results
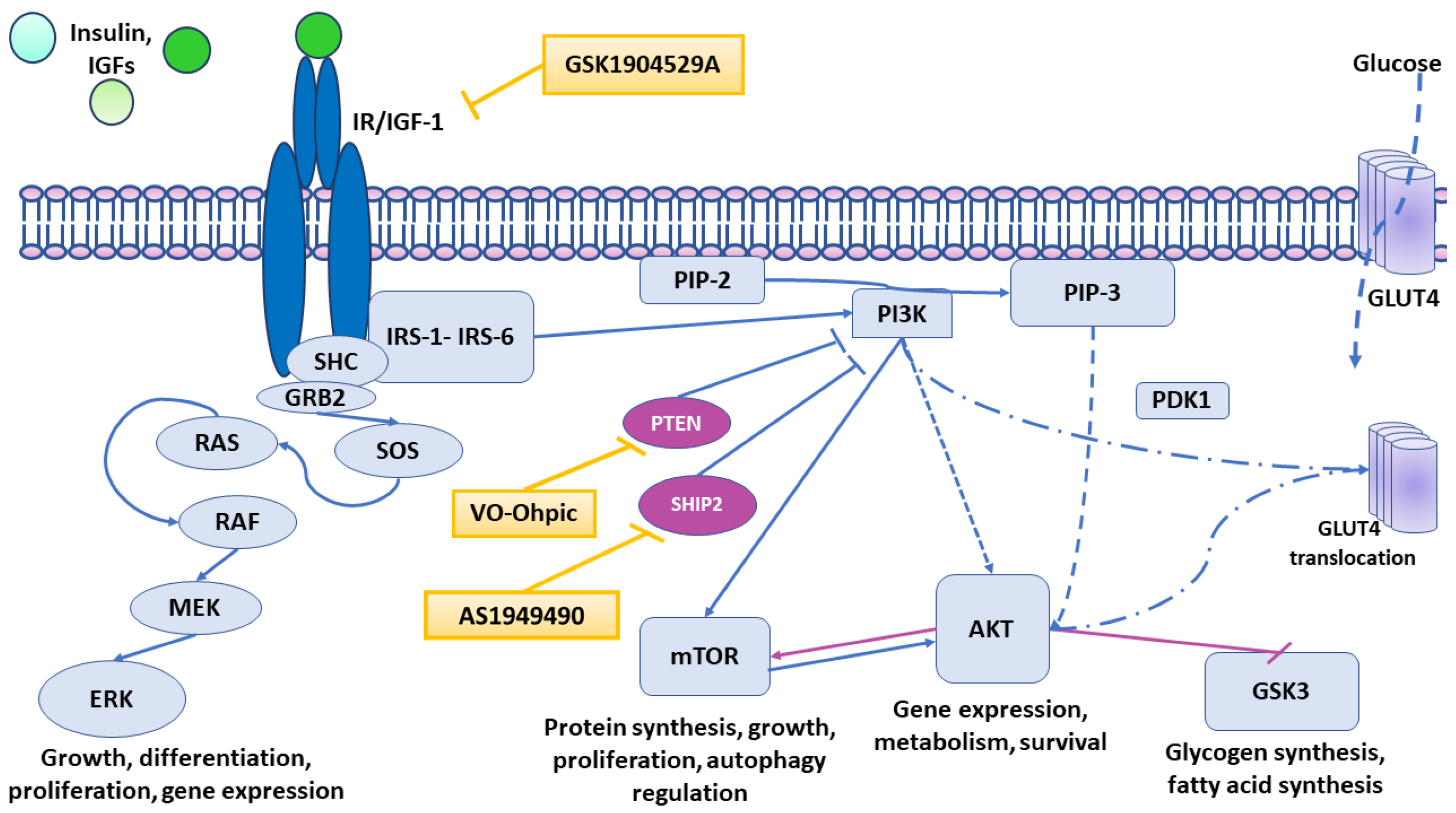
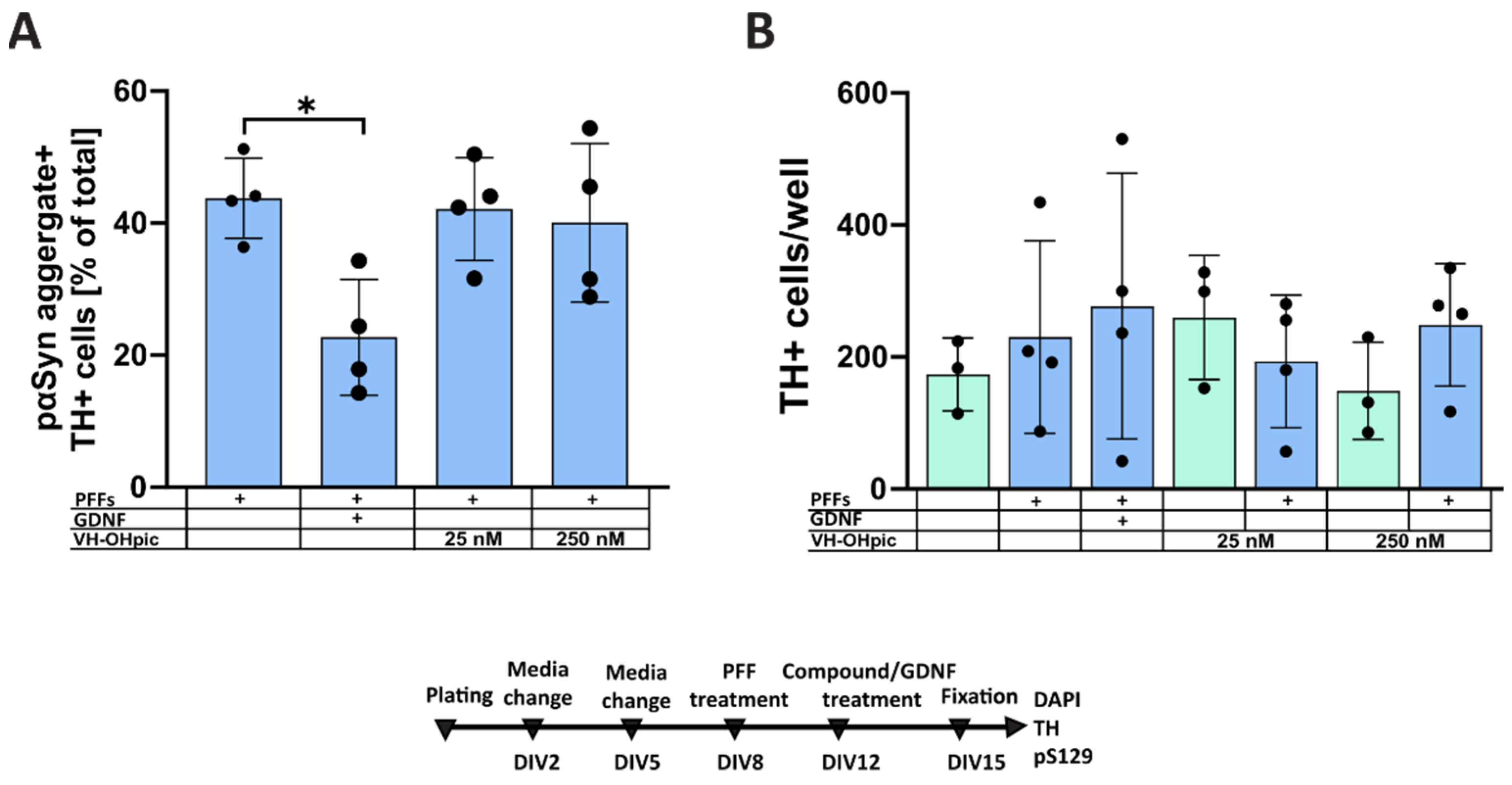
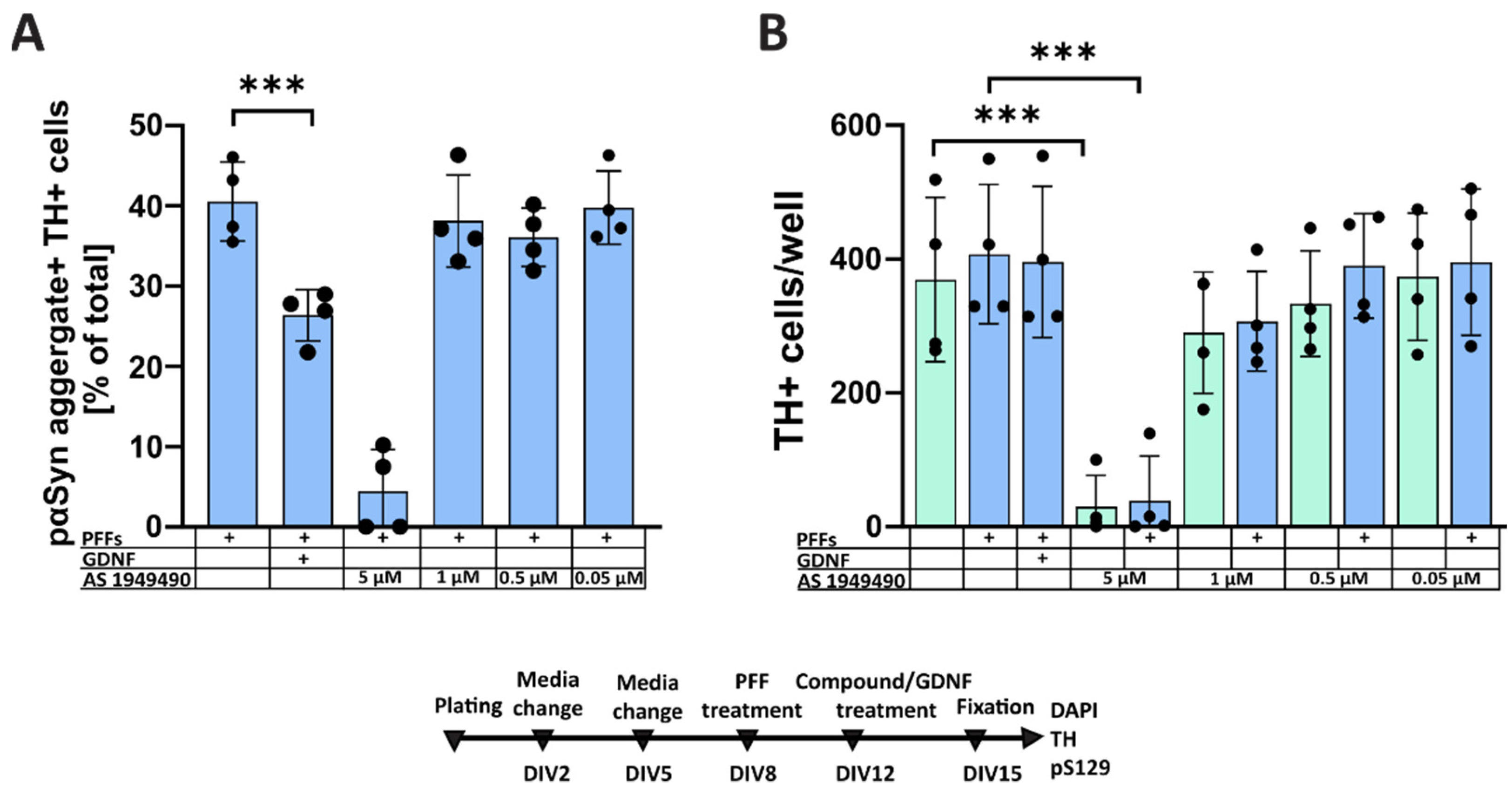
3.1. Pharmacological Modulation of Insulin Signaling Does Not Affect α-Syn Aggregation
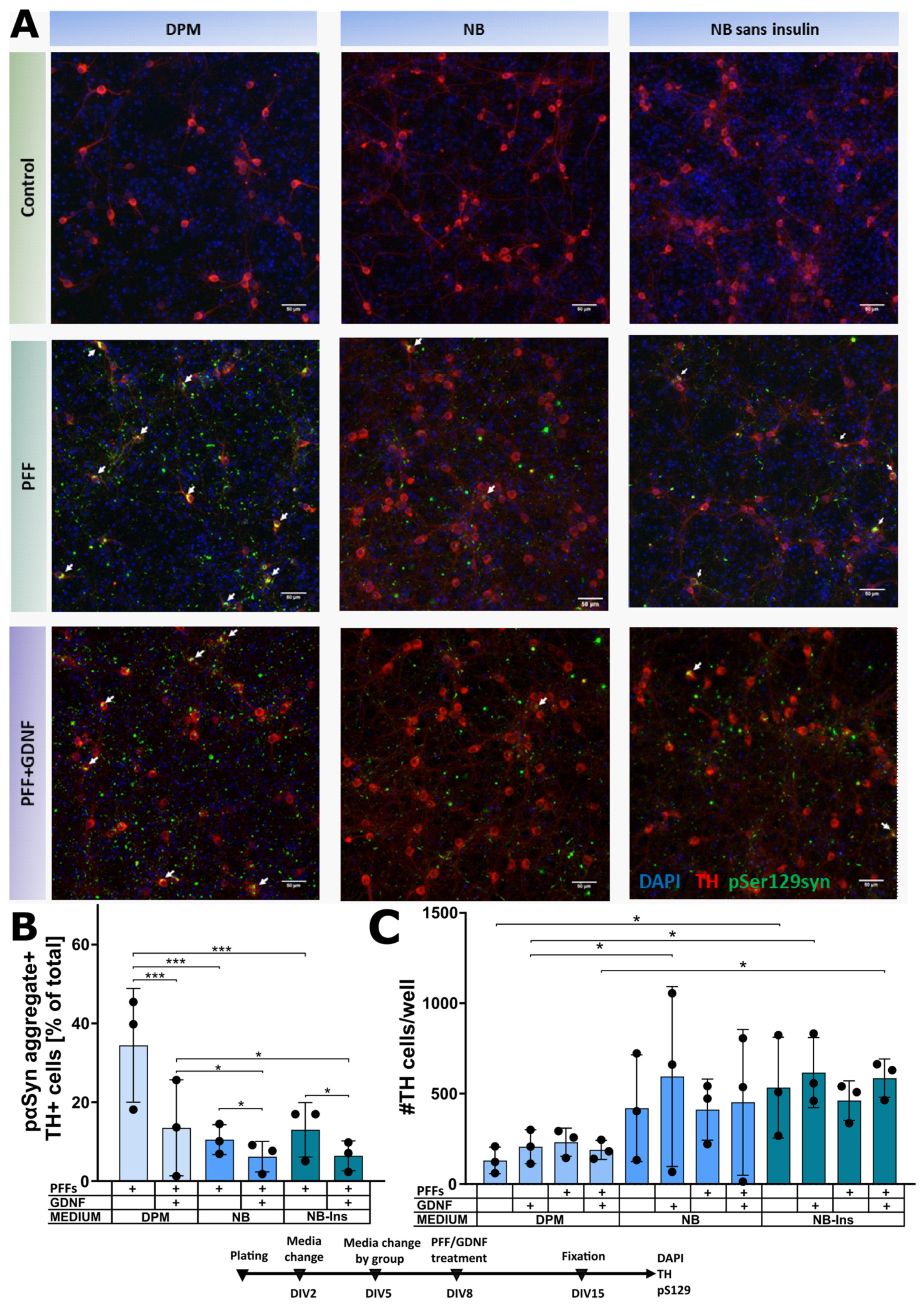
3.2. Culture Media Affect α-Syn Aggregation in Dopaminergic Neurons
4. Discussion
4.1. Formation of Intracellular α-Synuclein Accumulation in Dopaminergic Neurons Treated with a Selective Inhibitor of IGF-1R and IR
4.2. Formation of Intracellular α-Synuclein Accumulation in Dopaminergic Neurons Treated with Selective PTEN and SHIP2 Inhibitors
4.3. Formation of Intracellular α-Synuclein Aggregates in Dopaminergic Neurons Cultured in Different Media
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gustavsson, A.; Svensson, M.; Jacobi, F.; Allgulander, C.; Alonso, J.; Beghi, E.; Dodel, R.; Ekman, M.; Faravelli, C.; Fratiglioni, L.; et al. Cost of disorders of the brain in Europe 2010. Eur. Neuropsychopharmacol. 2011, 21, 718–779. [Google Scholar] [CrossRef] [Green Version]
- The Lewin Group, Inc. 2019 Parkinson’s Economic Burden Study (Final Report); The Lewin Group, Inc.: Falls Church, VA, USA, 2019. [Google Scholar]
- Spillantini, M.G.; Schmidt, M.L.; Lee, V.M.-Y.; Trojanowski, J.Q.; Jakes, R.; Goedert, M. α-Synuclein in Lewy Bodies. Nature 1997, 388, 839–840. [Google Scholar] [CrossRef]
- Braak, H.; Sandmann-Keil, D.; Gai, W.; Braak, E. Extensive axonal lewy neurites in parkinson’s disease: A novel pathological feature revealed by α-synuclein immunocytochemistry. Neurosci. Lett. 1999, 265, 67–69. [Google Scholar] [CrossRef]
- Shahmoradian, S.H.; Lewis, A.J.; Genoud, C.; Hench, J.; Moors, T.E.; Navarro, P.P.; Castaño-Díez, D.; Schweighauser, G.; Graff-Meyer, A.; Goldie, K.N.; et al. Lewy pathology in parkinson’s disease consists of crowded organelles and lipid membranes. Nat. Neurosci. 2019, 22, 1099–1109. [Google Scholar] [CrossRef] [Green Version]
- Lashuel, H.A. Do lewy bodies contain alpha-synuclein fibrils? And does it matter? A brief history and critical analysis of recent reports. Neurobiol. Dis. 2020, 141, 104876. [Google Scholar] [CrossRef]
- Van der Heide, L.P.; Ramakers, G.M.J.; Smidt, M.P. Insulin signaling in the central nervous system: Learning to survive. Prog. Neurobiol. 2006, 79, 205–221. [Google Scholar] [CrossRef]
- Feld, G.B.; Wilhem, I.; Benedict, C.; Rüdel, B.; Klameth, C.; Born, J.; Hallschmid, M. Central nervous insulin signaling in sleep-associated memory formation and neuroendocrine regulation. Neuropsychopharmacology 2016, 41, 1540–1550. [Google Scholar] [CrossRef] [Green Version]
- Pang, Y.; Lin, S.; Wright, C.; Shen, J.; Carter, K.; Bhatt, A.; Fan, L.-W. Intranasal insulin protects against substantia nigra dopaminergic neuronal loss and alleviates motor deficits induced by 6-OHDA in rats. Neuroscience 2016, 318, 157–165. [Google Scholar] [CrossRef] [Green Version]
- Chiu, S.-L.; Cline, H.T. Insulin receptor signaling in the development of neuronal structure and function. Neural Dev. 2010, 5, 7. [Google Scholar] [CrossRef] [Green Version]
- Bassil, F.; Fernagut, P.-O.; Bezard, E.; Meissner, W.G. Insulin, IGF-1 and GLP-1 signaling in neurodegenerative disorders: Targets for disease modification? Prog. Neurobiol. 2014, 118, 1–18. [Google Scholar] [CrossRef]
- Broughton, S.; Partridge, L. Insulin/IGF-like signalling, the central nervous system and aging. Biochem. J. 2009, 418, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duarte, A.I.; Moreira, P.I.; Oliveira, C.R. Insulin in central nervous system: More than just a peripheral hormone. J. Aging Res. 2012, 2012, 384017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, G.; Jousilahti, P.; Bidel, S.; Antikainen, R.; Tuomilehto, J. Type 2 diabetes and the risk of Parkinson’s disease. Diabetes Care 2007, 30, 842–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rotermund, C.; Truckenmüller, F.M.; Schell, H.; Kahle, P.J. Diet-induced obesity accelerates the onset of terminal phenotypes in α-synuclein transgenic mice. J. Neurochem. 2014, 131, 848–858. [Google Scholar] [CrossRef] [PubMed]
- Craft, S.; Baker, L.D.; Montine, T.J.; Minoshima, S.; Watson, G.S.; Claxton, A.; Arbuckle, M.; Callaghan, M.; Tsai, E.; Plymate, S.R.; et al. Intranasal insulin therapy for alzheimer disease and amnestic mild cognitive impairment: A pilot clinical trial. Arch. Neurol. 2012, 69, 29–38. [Google Scholar] [CrossRef] [Green Version]
- Koenig, A.M.; Mechanic-Hamilton, D.; Xie, S.X.; Combs, M.F.; Cappola, A.R.; Xie, L.; Detre, J.A.; Wolk, D.A.; Arnold, S.E. Effects of the insulin sensitizer metformin in alzheimer disease: Pilot data from a randomized placebo-controlled crossover study. Alzheimer Dis. Assoc. Disord. 2017, 31, 107–113. [Google Scholar] [CrossRef]
- Gray, S.M.; Meijer, R.I.; Barrett, E.J. Insulin regulates brain function, but how does it get there? Diabetes 2014, 63, 3992–3997. [Google Scholar] [CrossRef] [Green Version]
- Rhea, E.M.; Banks, W.A. Role of the blood-brain barrier in central nervous system insulin resistance. Front. Neurosci. 2019, 13, 521. [Google Scholar] [CrossRef] [Green Version]
- De Mello, N.P.; Orellana, A.M.; Mazucanti, C.H.; de Morais Lima, G.; Scavone, C.; Kawamoto, E.M. Insulin and autophagy in neurodegeneration. Front. Neurosci. 2019, 13, 491. [Google Scholar] [CrossRef] [Green Version]
- Batista, T.M.; Garcia-Martin, R.; Cai, W.; Konishi, M.; O’Neill, B.T.; Sakaguchi, M.; Kim, J.H.; Jung, D.Y.; Kim, J.K.; Kahn, C.R. Multi-dimensional transcriptional remodeling by physiological insulin in vivo. Cell Rep. 2019, 26, 3429–3443.e3. [Google Scholar] [CrossRef] [Green Version]
- Greenhill, C. Insulin and the insulin receptor regulate gene expression. Nat. Rev. Endocrinol. 2019, 15, 315. [Google Scholar] [CrossRef]
- Boucher, J.; Kleinridders, A.; Kahn, C.R. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb. Perspect. Biol. 2014, 6. [Google Scholar] [CrossRef] [Green Version]
- Slaaby, R. Specific insulin/IGF1 hybrid receptor activation assay reveals IGF1 as a more potent ligand than insulin. Sci. Rep. 2015, 5, 7911. [Google Scholar] [CrossRef] [Green Version]
- Belfiore, A.; Frasca, F.; Pandini, G.; Sciacca, L.; Vigneri, R. Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocr. Rev. 2009, 30, 586–623. [Google Scholar] [CrossRef] [Green Version]
- Slaaby, R.; Schäffer, L.; Lautrup-Larsen, I.; Andersen, A.S.; Shaw, A.C.; Mathiasen, I.S.; Brandt, J. Hybrid receptors formed by insulin receptor (IR) and insulin-like growth factor I receptor (IGF-IR) have low insulin and high IGF-1 affinity irrespective of the IR splice variant. J. Biol. Chem. 2006, 281, 25869–25874. [Google Scholar] [CrossRef] [Green Version]
- Belfiore, A.; Malaguarnera, R.; Vella, V.; Lawrence, M.C.; Sciacca, L.; Frasca, F.; Morrione, A.; Vigneri, R. Insulin receptor isoforms in physiology and disease: An updated view. Endocr. Rev. 2017, 38, 379–431. [Google Scholar] [CrossRef]
- Mielke, J.G.; Wang, Y.T. Insulin exerts neuroprotection by counteracting the decrease in cell-surface GABAA receptors following oxygen–glucose deprivation in cultured cortical neurons. J. Neurochem. 2005, 92, 103–113. [Google Scholar] [CrossRef]
- Ramalingam, M.; Kim, S.-J. The neuroprotective role of insulin against MPP(+) -induced Parkinson’s disease in differentiated SH-SY5Y cells. J. Cell Biochem. 2016, 117, 917–926. [Google Scholar] [CrossRef]
- Chmielarz, P.; Er, Ş.; Konovalova, J.; Bandres, L.; Hlushchuk, I.; Albert, K.; Panhelainen, A.; Luk, K.; Airavaara, M.; Domanskyi, A. GDNF/RET signaling pathway activation eliminates lewy body pathology in midbrain dopamine neurons. Mov. Disord. 2020, 35, 2279–2289. [Google Scholar] [CrossRef]
- Wie, J.; Liu, Z.; Song, H.; Tropea, T.F.; Yang, L.; Wang, H.; Liang, Y.; Cang, C.; Aranda, K.; Lohmann, J.; et al. A Growth-factor-activated lysosomal K+ channel regulates Parkinson’s pathology. Nature 2021, 591, 431–437. [Google Scholar] [CrossRef]
- Konnova, E.A.; Swanberg, M. Animal Models of Parkinson’s Disease. In Parkinson’s Disease: Pathogenesis and Clinical Aspects; Stoker, T.B., Greenland, J.C., Eds.; Codon Publications: Brisbane, Australia, 2018; ISBN 978-0-9944381-6-4. [Google Scholar]
- Dauer, W.; Przedborski, S. Parkinson’s disease: Mechanisms and models. Neuron 2003, 39, 889–909. [Google Scholar] [CrossRef] [Green Version]
- Barker, R.A.; Björklund, A. Animal models of Parkinson’s disease: Are they useful or not? J. Parkinsons Dis. 2020. [Google Scholar] [CrossRef] [PubMed]
- Smits, L.M.; Reinhardt, L.; Reinhardt, P.; Glatza, M.; Monzel, A.S.; Stanslowsky, N.; Rosato-Siri, M.D.; Zanon, A.; Antony, P.M.; Bellmann, J.; et al. Modeling Parkinson’s disease in midbrain-like organoids. NPJ Parkinsons Dis. 2019, 5, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falkenburger, B.H.; Saridaki, T.; Dinter, E. Cellular models for Parkinson’s disease. J. Neurochem. 2016, 139 (Suppl. S1), 121–130. [Google Scholar] [CrossRef] [PubMed]
- Airavaara, M.; Parkkinen, I.; Konovalova, J.; Albert, K.; Chmielarz, P.; Domanskyi, A. Back and to the future: From neurotoxin-induced to human Parkinson’s disease models. Curr. Protoc. Neurosci. 2020, 91, e88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Er, S.; Hlushchuk, I.; Airavaara, M.; Chmielarz, P.; Domanskyi, A. Studying pre-formed fibril induced α-synuclein accumulation in primary embryonic mouse midbrain dopamine neurons. JoVE 2020, e61118. [Google Scholar] [CrossRef] [PubMed]
- Volpicelli-Daley, L.A.; Luk, K.C.; Patel, T.P.; Tanik, S.A.; Riddle, D.M.; Stieber, A.; Meaney, D.F.; Trojanowski, J.Q.; Lee, V.M.-Y. Exogenous α-synuclein fibrils induce lewy body pathology leading to synaptic dysfunction and neuron death. Neuron 2011, 72, 57–71. [Google Scholar] [CrossRef] [Green Version]
- Volpicelli-Daley, L.A.; Luk, K.C.; Lee, V.M.-Y. Addition of exogenous α-synuclein pre-formed fibrils to primary neuronal cultures to seed recruitment of endogenous α-synuclein to lewy body and lewy neurite-like aggregates. Nat. Protoc. 2014, 9, 2135–2146. [Google Scholar] [CrossRef] [Green Version]
- Mahul-Mellier, A.-L.; Burtscher, J.; Maharjan, N.; Weerens, L.; Croisier, M.; Kuttler, F.; Leleu, M.; Knott, G.W.; Lashuel, H.A. The process of lewy body formation, rather than simply α-synuclein fibrillization, is one of the major drivers of neurodegeneration. Proc. Natl. Acad. Sci. USA 2020, 117, 4971–4982. [Google Scholar] [CrossRef] [Green Version]
- Luk, K.C.; Kehm, V.; Carroll, J.; Zhang, B.; O’Brien, P.; Trojanowski, J.Q.; Lee, V.M.-Y. Pathological α-synuclein transmission initiates parkinson-like neurodegeneration in non-transgenic mice. Science 2012, 338, 949–953. [Google Scholar] [CrossRef] [Green Version]
- Planken, A.; Porokuokka, L.L.; Hänninen, A.-L.; Tuominen, R.K.; Andressoo, J.-O. Medium-throughput computer aided micro-island method to assay embryonic dopaminergic neuron cultures in vitro. J. Neurosci. Methods 2010, 194, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Hlushchuk, I.; Ruskoaho, H.; Domanskyi, A.; Airavaara, M.; Välimäki, M.J. Domain-independent inhibition of CBP/P300 attenuates α-synuclein aggregation. ACS Chem. Neurosci. 2021, 12, 2273–2279. [Google Scholar] [CrossRef] [PubMed]
- Oueslati, A. Implication of alpha-synuclein phosphorylation at S129 in synucleinopathies: What have we learned in the last decade? JPD 2016, 6, 39–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carpenter, A.E.; Jones, T.R.; Lamprecht, M.R.; Clarke, C.; Kang, I.H.; Friman, O.; Guertin, D.A.; Chang, J.H.; Lindquist, R.A.; Moffat, J.; et al. CellProfiler: Image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 2006, 7, R100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lew, M. Good statistical practice in pharmacology. Problem 2. Br. J. Pharmacol 2007, 152, 299–303. [Google Scholar] [CrossRef]
- Soeda, Y.; Tsuneki, H.; Muranaka, H.; Mori, N.; Hosoh, S.; Ichihara, Y.; Kagawa, S.; Wang, X.; Toyooka, N.; Takamura, Y.; et al. The inositol phosphatase SHIP2 negatively regulates insulin/igf-i actions implicated in neuroprotection and memory function in mouse brain. Mol. Endocrinol. 2010, 24, 1965–1977. [Google Scholar] [CrossRef]
- Ramalingam, M.; Kim, S.-J. The role of insulin against hydrogen peroxide-induced oxidative damages in differentiated SH-SY5Y Cells. J. Recept. Signal. Transduct Res. 2014, 34, 212–220. [Google Scholar] [CrossRef]
- Duarte, A.I.; Santos, P.; Oliveira, C.R.; Santos, M.S.; Rego, A.C. Insulin neuroprotection against oxidative stress is mediated by Akt and GSK-3beta signaling pathways and changes in protein expression. Biochim. Biophys. Acta 2008, 1783, 994–1002. [Google Scholar] [CrossRef] [Green Version]
- Castilla-Cortázar, I.; Aguirre, G.A.; Femat-Roldán, G.; Martín-Estal, I.; Espinosa, L. Is insulin-like growth factor-1 involved in Parkinson’s disease development? J. Transl Med. 2020, 18, 70. [Google Scholar] [CrossRef] [Green Version]
- Sabbatini, P.; Rowand, J.L.; Groy, A.; Korenchuk, S.; Liu, Q.; Atkins, C.; Dumble, M.; Yang, J.; Anderson, K.; Wilson, B.J.; et al. Antitumor activity of GSK1904529A, a small-molecule inhibitor of the insulin-like growth factor-i receptor tyrosine kinase. Clin. Cancer Res. 2009, 15, 3058–3067. [Google Scholar] [CrossRef] [Green Version]
- Kao, S.-Y. Rescue of alpha-synuclein cytotoxicity by insulin-like growth factors. Biochem. Biophys. Res. Commun. 2009, 385, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.-Y.; Lee, S.-J.; Lee, S.-H.; Jung, Y.S.; Ha, N.-C.; Seol, W.; Park, B.-J. Direct interaction of α-synuclein and AKT regulates IGF-1 signaling: Implication of Parkinson disease. Neurosignals 2011, 19, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Xicoy, H.; Wieringa, B.; Martens, G.J.M. The SH-SY5Y Cell line in Parkinson’s disease research: A systematic review. Mol. Neurodegener. 2017, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Yen, C.; Liaw, D.; Podsypanina, K.; Bose, S.; Wang, S.I.; Puc, J.; Miliaresis, C.; Rodgers, L.; McCombie, R.; et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science 1997, 275, 1943–1947. [Google Scholar] [CrossRef] [PubMed]
- Haeusler, R.A.; McGraw, T.E.; Accili, D. Biochemical and cellular properties of insulin receptor signalling. Nat. Rev. Mol. Cell Biol. 2018, 19, 31–44. [Google Scholar] [CrossRef]
- Yang, L.; Wang, H.; Liu, L.; Xie, A. The role of insulin/IGF-1/PI3K/Akt/GSK3β signaling in Parkinson’s disease dementia. Front. Neurosci. 2018, 12, 73. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.-H.; Yan, W.-F.; Sun, J.-D.; Huang, J.-Y.; Mu, Z.; Chen, N.-H. The molecular mechanism of rotenone-induced α-synuclein aggregation: Emphasizing the role of the calcium/GSK3β pathway. Toxicol. Lett. 2015, 233, 163–171. [Google Scholar] [CrossRef]
- Golpich, M.; Amini, E.; Hemmati, F.; Ibrahim, N.M.; Rahmani, B.; Mohamed, Z.; Raymond, A.A.; Dargahi, L.; Ghasemi, R.; Ahmadiani, A. Glycogen synthase kinase-3 beta (GSK-3β) signaling: Implications for Parkinson’s disease. Pharmacol. Res. 2015, 97, 16–26. [Google Scholar] [CrossRef]
- Loria, F.; Vargas, J.Y.; Bousset, L.; Syan, S.; Salles, A.; Melki, R.; Zurzolo, C. α-synuclein transfer between neurons and astrocytes indicates that astrocytes play a role in degradation rather than in spreading. Acta Neuropathol. 2017, 134, 789–808. [Google Scholar] [CrossRef]
- Tsunemi, T.; Ishiguro, Y.; Yoroisaka, A.; Valdez, C.; Miyamoto, K.; Ishikawa, K.; Saiki, S.; Akamatsu, W.; Hattori, N.; Krainc, D. Astrocytes protect human dopaminergic neurons from α-synuclein accumulation and propagation. J. Neurosci. 2020, 40, 8618–8628. [Google Scholar] [CrossRef]
- Duarte Azevedo, M.; Sander, S.; Tenenbaum, L. GDNF, a neuron-derived factor upregulated in glial cells during disease. J. Clin. Med. 2020, 9, 456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-García, I.; Gruber, T.; García-Cáceres, C. Insulin action on astrocytes: From energy homeostasis to behaviour. J. Neuroendocrinol. 2021, 33, e12953. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hlushchuk, I.; Barut, J.; Airavaara, M.; Luk, K.; Domanskyi, A.; Chmielarz, P. Cell Culture Media, Unlike the Presence of Insulin, Affect α-Synuclein Aggregation in Dopaminergic Neurons. Biomolecules 2022, 12, 563. https://doi.org/10.3390/biom12040563
Hlushchuk I, Barut J, Airavaara M, Luk K, Domanskyi A, Chmielarz P. Cell Culture Media, Unlike the Presence of Insulin, Affect α-Synuclein Aggregation in Dopaminergic Neurons. Biomolecules. 2022; 12(4):563. https://doi.org/10.3390/biom12040563
Chicago/Turabian StyleHlushchuk, Irena, Justyna Barut, Mikko Airavaara, Kelvin Luk, Andrii Domanskyi, and Piotr Chmielarz. 2022. "Cell Culture Media, Unlike the Presence of Insulin, Affect α-Synuclein Aggregation in Dopaminergic Neurons" Biomolecules 12, no. 4: 563. https://doi.org/10.3390/biom12040563






