The Radial Bulging and Axial Strains of Intervertebral Discs during Creep Obtained with the 3D-DIC System
Abstract
:1. Introduction
2. Methods
2.1. System Setup and Calibration
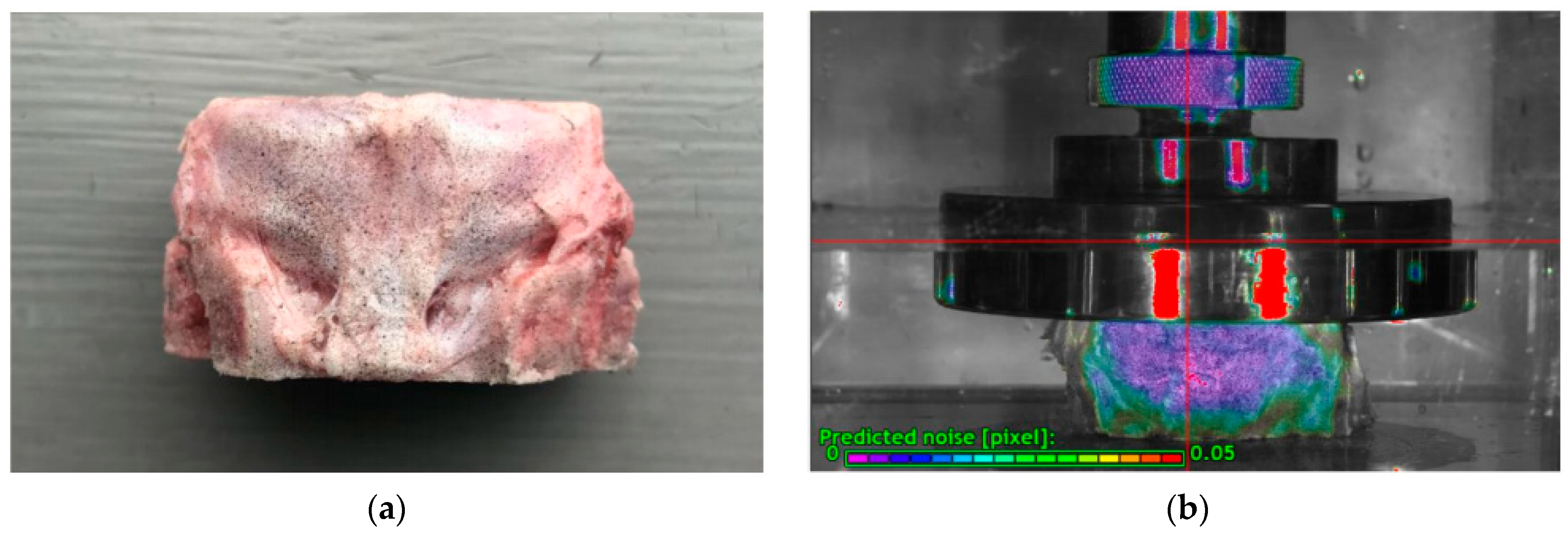
2.2. Specimen Preparation
2.3. Disc Bulging and Strains Computation
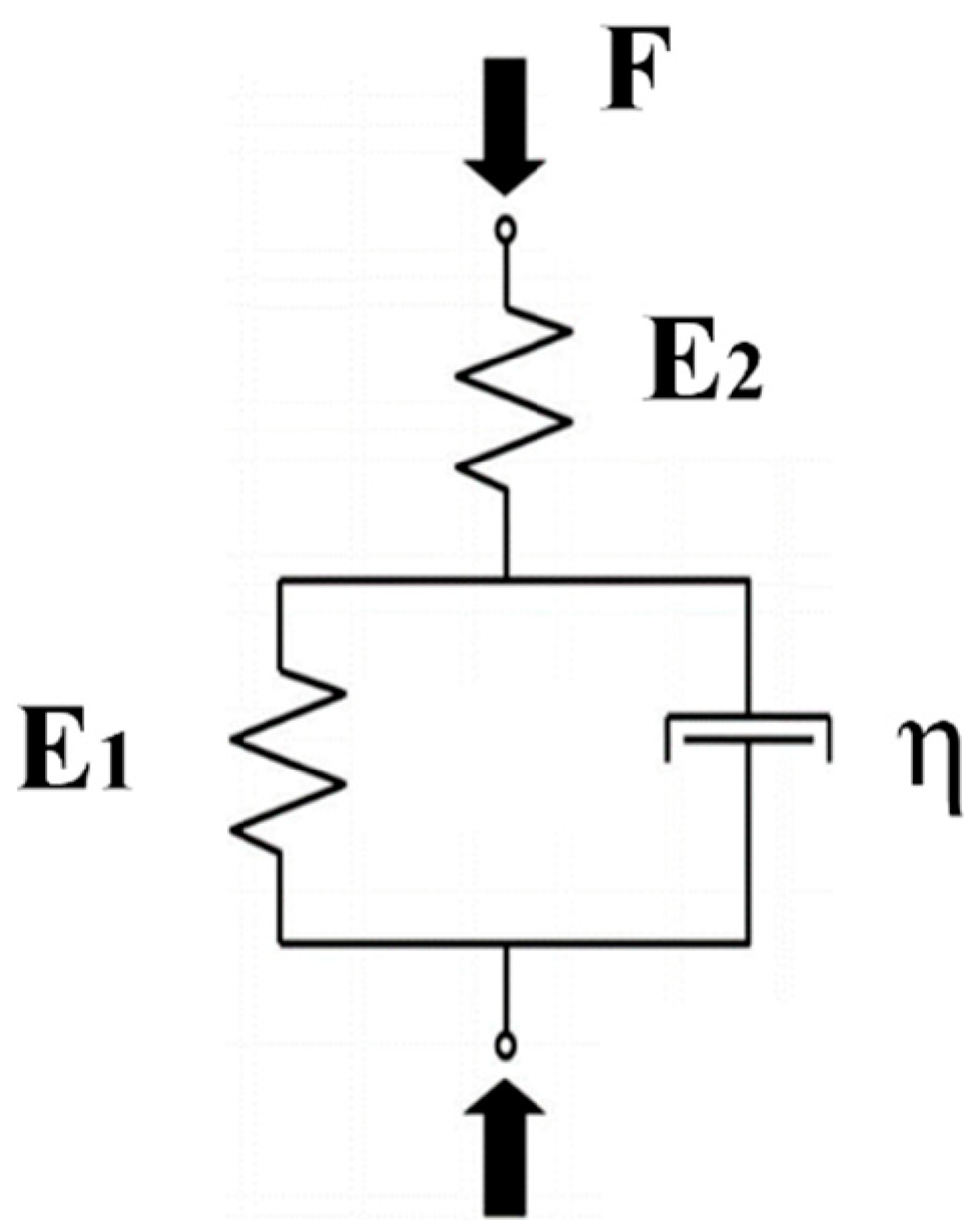
2.4. Fitting Models of Axial Strains and Lateral Bulging
2.5. Statistical Analysis
3. Results
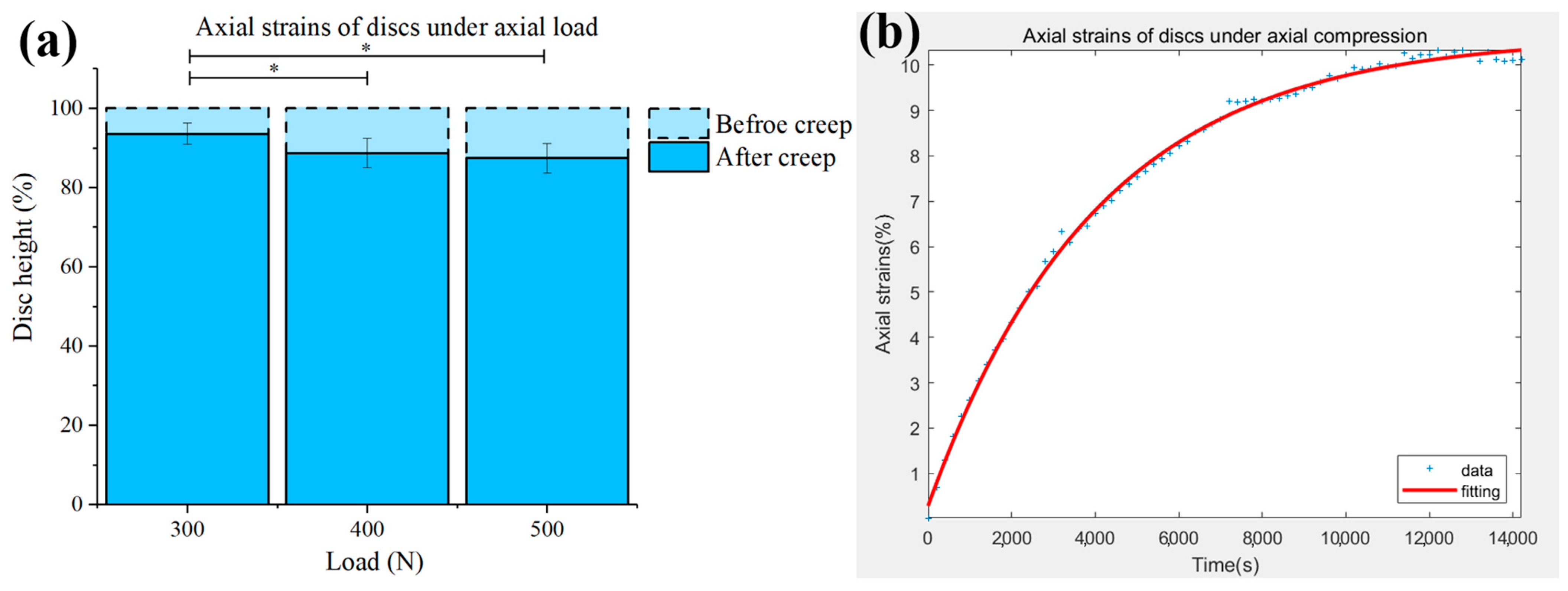
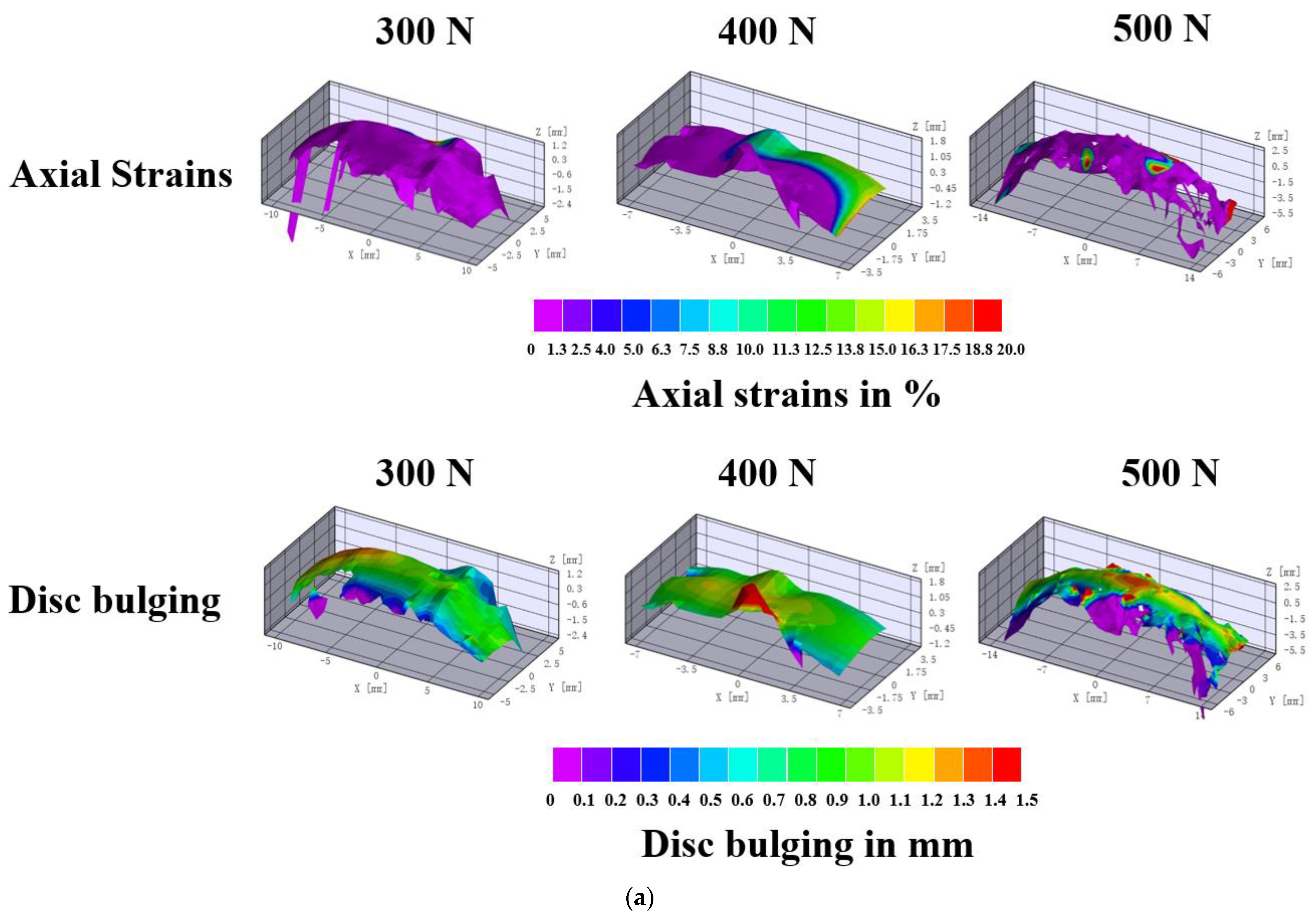
3.1. Effects of Loads on Disc Strains
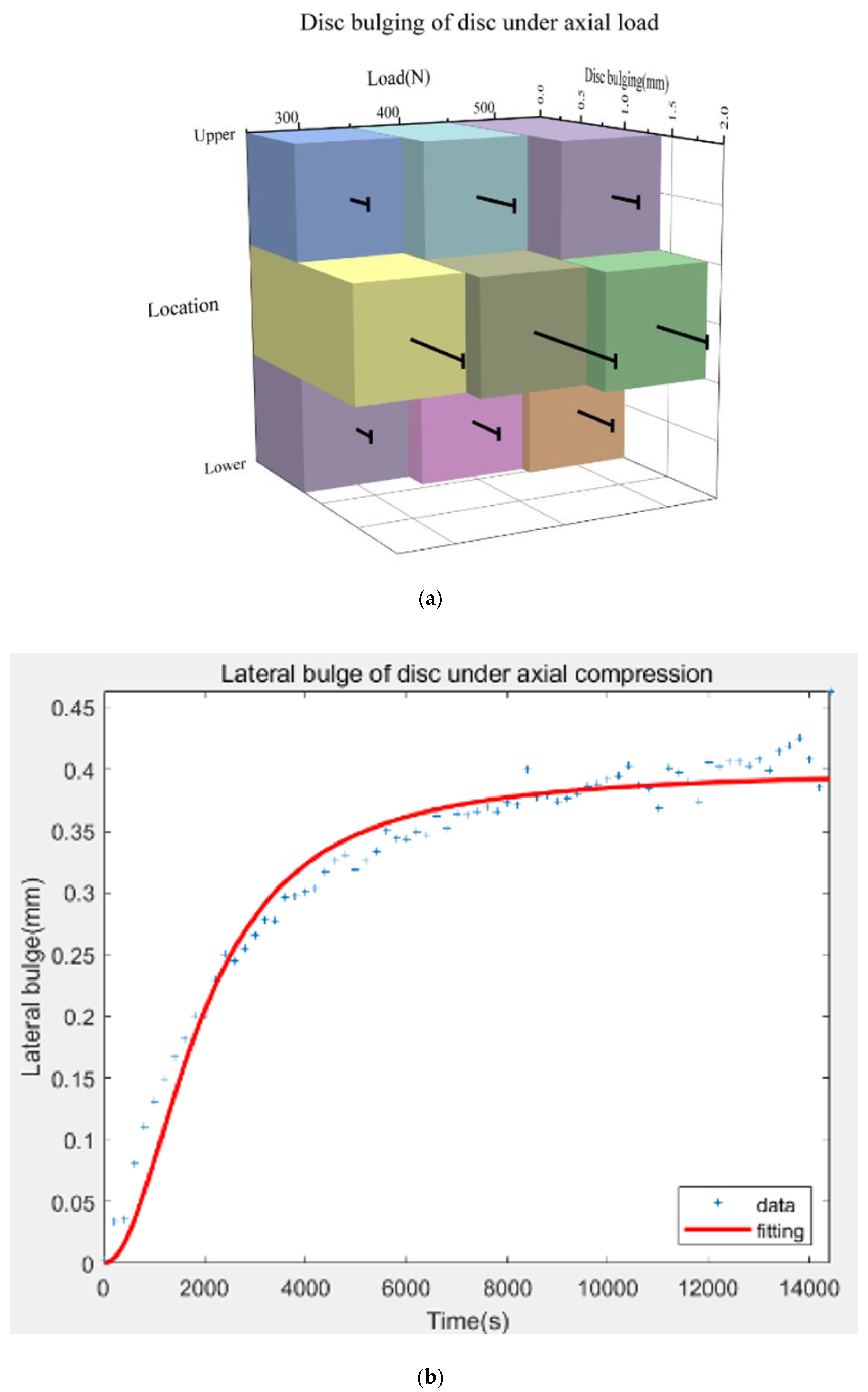
3.2. Effects of Loads on Disc Bulging
3.3. Relations between Disc Strains and Bulging
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Twomey, L.; Taylor, J. Flexion creep deformation and hysteresis in the lumbar vertebral column. Spine 1982, 7, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Leatt, P.; Reilly, T.; Troup, J.G. Spinal loading during circuit weight-training and running. Br. J. Sports Med. 1986, 20, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Tyrrell, A.R.; Reilly, T.; Troup, J.D.G. Circadian variation in stature and the effects of spinal loading. Spine 1985, 10, 161–164. [Google Scholar] [CrossRef] [PubMed]
- McMillan, D.W.; Garbutt, G.; Adams, M.A. Effect of sustained loading on the water content of intervertebral discs: Implications for disc metabolism. Ann. Rheum. Dis. 1996, 55, 880–887. [Google Scholar] [CrossRef] [PubMed]
- Reilly, T.; Tyrrell, A.; Troup, J.D.G. Circadian variation in human stature. Chronobiol. Int. 1984, 1, 121–126. [Google Scholar] [CrossRef]
- Adams, M.A.; Hutton, W.C. Gradual disc prolapse. Spine 1985, 10, 524–531. [Google Scholar] [CrossRef]
- Heuer, F.; Schmidt, H.; Schmidt, H.; Claes, L.; Wilke, H.J. Creep associated changes in intervertebral disc bulging obtained with a laser scanning device. Clin. Biomech. 2007, 22, 737–744. [Google Scholar] [CrossRef]
- Heuer, F.; Schmidt, H. The relation between intervertebral disc bulging and annular fiber associated strains for simple and complex loading. J. Biomech. 2008, 41, 1086–1094. [Google Scholar] [CrossRef]
- Heuer, F.; Schmidt, H.; Kafer, W.; Graf, N.; Wilke, H.J. Posterior motion preserving implants evaluated by means of intervertebral disc bulging and annular fiber strains. Clin. Biomech. 2012, 27, 218–225. [Google Scholar] [CrossRef]
- Fewster, K.M.; Noguchi, M.; Gooyers, C.E.; Wong, A.; Callaghan, J.P. Exploring the regional disc bulge response of the cervical porcine intervertebral disc under varying loads and posture. J. Biomech. 2020, 104, 109713. [Google Scholar] [CrossRef]
- Fewster, K.M.; Haider, S.; Gooyers, C.E.; Callaghan, J.; Wong, A. A computerised system for measurement of the radial displacement of the intervertebral disc using a laser scanning device. Comput. Methods Biomech. Biomed. Eng. Imaging Vis. 2020, 8, 287–293. [Google Scholar] [CrossRef]
- Cuchanski, M.; Cook, D.; Whiting, D.M.; Cheng, B.C. Measurement of occlusion of the spinal canal and intervertebral foramen by intervertebral disc bulge. SAS J. 2011, 5, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Dupré, D.A.; Cook, D.J.; Bellotte, J.B.; Oh, M.Y.; Whiting, D.; Cheng, B.C. Disc nucleus fortification for lumbar degenerative disc disease: A biomechanical study. J. Neurosurg. Spine 2016, 24, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Pei, B.Q.; Hui, L.; Li, D.Y.; Fan, Y.B.; Wang, C.; Wu, S.Q. Creep bulging deformation of intervertebral disc under axial compression. Bio-Med. Mater. Eng. 2013, 23, S191–S198. [Google Scholar] [CrossRef]
- Mengoni, M.; Zapata-Cornelio, F.Y.; Wijayathunga, V.N.; Wilcox, R.K. Experimental and computational comparison of intervertebral disc bulge for specimen-specific model evaluation based on imaging. Front. Bioeng. Biotechnol. 2021, 9, 661469. [Google Scholar] [CrossRef]
- Mayerhoefer, M.E.; Stelzeneder, D.; Bachbauer, W.; Welsch, G.H.; Mamisch, T.C.; Szczypinski, P.; Weber, M.; Peters, N.H.G.M.; Fruehwald-Pallamar, J.; Puchner, S.; et al. Quantitative analysis of lumbar intervertebral disc abnormalities at 3.0 Tesla: Value of T2 texture features and geometric parameters. NMR Biomed. 2012, 25, 866–872. [Google Scholar] [CrossRef]
- Hung, I.Y.-J.; Shih, T.T.F.; Chen, B.-B.; Guo, Y.L. Prediction of Lumbar Disc Bulging and Protrusion by Anthropometric Factors and Disc Morphology. Int. J. Environ. Res. Public Health 2021, 18, 2521. [Google Scholar] [CrossRef]
- Sundarsingh, S.; Kesavan, R. Diagnosis of disc bulge and disc desiccation in lumbar MRI using concatenated shape and texture features with random forest classifier. Int. J. Imaging Syst. Technol. 2020, 30, 340–347. [Google Scholar] [CrossRef]
- Beulah, A.; Sharmila, T.S.; Pramod, V.K. Disc bulge diagnostic model in axial lumbar MR images using Intervertebral disc Descriptor (IdD). Multimed. Tools Appl. 2018, 77, 27215–27230. [Google Scholar] [CrossRef]
- Lao, L.; Daubs, M.D.; Scott, T.P.; Phan, K.H.; Wang, J.C. Missed cervical disc bulges diagnosed with kinematic magnetic resonance imaging. Eur. Spine J. 2014, 23, 1725–1729. [Google Scholar] [CrossRef]
- Sutton, M.A.; Yan, J.H.; Tiwari, V.; Schreier, H.W.; Orteu, J.J. The effect of out-of-plane motion on 2D and 3D digital image correlation measurements. Opt. Lasers Eng. 2008, 46, 746–757. [Google Scholar] [CrossRef]
- Helm, J.D.; Sutton, M.A.; McNeill, S.R. Deformations in wide, center-notched, thin panels: Part I: Three dimensional shape and deformation measurements by computer vision. Opt. Eng. 2003, 42, 1293–1305. [Google Scholar]
- Helm, J.D.; Sutton, M.A.; McNeill, S.R. Deformations in wide, center-notched, thin panels: Part II: Finite element analysis and comparison to experimental measurements. Opt. Eng. 2003, 42, 1306–1320. [Google Scholar]
- Schreier, H.W.; Garcia, D.; Sutton, M.A. Advances in light microscope stereo vision. Exp. Mech. 2004, 44, 278–288. [Google Scholar] [CrossRef]
- Sutton, M.A.; Ke, X.; Lessner, S.M.; Goldbach, M.; Yost, M.; Zhao, F.; Schreier, H.W. Strain field measurements on mouse carotid arteries using microscopic three-dimensional digital image correlation. J. Biomed. Mater. Res. A 2008, 84, 178–190. [Google Scholar] [CrossRef]
- Sutton, M.A.; Helm, J.D.; Boone, M.L. Experimental study of crack growth in thin sheet 2024-T3 aluminum under tension–torsion loading. Int. J. Fract. 2001, 109, 285–301. [Google Scholar] [CrossRef]
- Yan, J.H.; Sutton, M.A.; Deng, X.; Cheng, C.S. Mixed mode fracture of ductile thin sheet materials under combined in-plane and out-of-plane loading. Int. J. Fract. 2007, 144, 297–321. [Google Scholar] [CrossRef]
- Sutton, M.A.; Yan, J.H.; Deng, X.M.; Cheng, C.S.; Zavattieri, P. 3D digital image correlation to quantify deformation and COD in ductile aluminum under mixed-mode I/III loading. Opt. Eng. 2007, 46, 051003. [Google Scholar] [CrossRef]
- Paul, C.P.L.; Schoorl, T.; Zuiderbaan, H.A.; van der Veen, A.J.; ban de Ven, P.M.; Mullender, M.G. Dynamic and Static Overloading Induce Early Degenerative Processes in Caprine Lumbar Intervertebral Discs. PLoS ONE 2013, 8, e62411. [Google Scholar] [CrossRef]
- O’Connell, G.D.; Jacobs, N.T.; Sen, S.; Vresilovic, E.J.; Elliott, D.M. Axial Creep Loading and Unloaded Recovery of the Human Intervertebral Disc and the Effect of Degeneration. J. Mech. Behav. Biomed. Mater. 2011, 4, 933–942. [Google Scholar] [CrossRef]
- Yang, M.Y.; Cui, Y.Y.; Zhang, Y.; Wu, H.K.; Hu, B.B.; Wang, S.; Liu, W.Q. Quantitative Characterization of the Elasticity, Net Creep, and Swelling of the Intervertebral Disc: An In Vitro Experiment. J. Bionic Eng. 2022, 19, 1077–1086. [Google Scholar] [CrossRef]
- Yang, M.Y.; Xiang, D.D.; Wang, S.; Liu, W.Q. In Vitro Studies for Investigating Creep of Intervertebral Discs under Axial Compression: A Review of Testing Environment and Results. Materials 2022, 15, 2500. [Google Scholar] [CrossRef] [PubMed]
- Keller, T.S.; Spengler, D.M.; Hansson, T.H. Mechanical behavior of the human lumbar spine. I. Creep analysis during static compressive loading. J. Orthop. Res. 1987, 5, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Burns, M.L.; Kaleps, I.; Kazarian, L.E. Analysis of compressive creep behavior of the vertebral unit subjected to a uniform axial loading using exact parametric solution equations of Kelvin-solid models–Part, I. Human intervertebral joints. J. Biomech. 1984, 17, 113–130. [Google Scholar] [CrossRef]
- Kaleps, I.; Kazarian, L.E.; Burns, M.L. Analysis of compressive creep behavior of the vertebral unit subjected to a uniform axial loading using exact parametric solution equations of Kelvin-solid models–Part II. Rhesus monkey intervertebral joints. J. Biomech. 1984, 17, 131–136. [Google Scholar] [CrossRef]
- Showalter, B.L.; Beckstein, J.C.; Martin, J.T.; Beattie, E.E.; Elliott, D.M. Comparison of Animal Discs Used in Disc Research to Human Lumbar Disc. Spine 2012, 37, E900–E907. [Google Scholar] [CrossRef]
- Reuber, M.; Schultz, A.; Denis, F.; Spencer, D. Bulging of lumbar intervertebral disks. J. Biomech. Eng. 1982, 104, 187–192. [Google Scholar] [CrossRef]
- Wenger, K.H.; Schlegel, J.D. Annular bulge contours from an axial photogrammetric method. Clin. Biomech. 1997, 12, 438–444. [Google Scholar] [CrossRef]
- Meakin, J.R.; Hukins, D.W.L. Effect of removing the nucleus pulposus on the deformation of the annulus fibrosus during compression of the intervertebral disc. J. Biomech. 2000, 33, 575–580. [Google Scholar] [CrossRef]
- O’Connell, G.D.; Malhotra, N.R.; Vresilovic, E.J.; Elliott, D.M. The Effect of Nucleotomy and the Dependence of Degeneration of Human Intervertebral Disc Strain in Axial Compression. Spine 2011, 36, 1765–1771. [Google Scholar] [CrossRef]
- O’Connell, G.D.; Johannessen, W. Human internal disc strains in axial compression measured noninvasively using magnetic resonance imaging. Spine 2007, 32, 2860–2868. [Google Scholar] [CrossRef] [PubMed]
- Ekstrm, L.; Holm, S.; Holm, A.K.; Hansson, T. In Vivo Porcine Intradiscal Pressure as a Function of External Loading. Clin. Spine Surg. 2004, 17, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Pollintine, P.; Tunen, M.V.; Luo, J.; Brown, M.D.; Dolan, P.; Adams, M.A. Time-dependent Compressive Deformation of the Ageing Spine: Relevance to Spinal Stenosis. Spine 2010, 35, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Gullbrand, S.E.; Ashinsky, B.G.; Martin, J.T.; Pickup, S.; Smith, L.J.; Mauck, R.L.; Smith, H.E. Correlations between quantitative T2 and T1ρ MRI, mechanical properties and biochemical composition in a rabbit lumbar intervertebral disc degeneration model. J. Orthop. Res. 2016, 34, 1382–1388. [Google Scholar] [CrossRef] [PubMed]
- Berberan-Santos, M.N.; Bodunov, E.N.; Valeur, B. Mathematical functions for the analysis of luminescence decays with un-derlying distributions. Kohlrausch decay function (stretched exponential). Chem. Phys. 2005, 315, 171–182. [Google Scholar] [CrossRef]
- Van der Veen, A.J.; Bisschop, A.; Mullender, M.G.; van Dieën, J.H. Modelling creep behaviour of the human intervertebral disc. J. Biomech. 2013, 46, 2101–2103. [Google Scholar] [CrossRef]
- Yang, M.Y.; Xiang, D.D.; Chen, Y.R.; Cui, Y.Y.; Wang, S.; Liu, W.Q. An Artificial PVA-BC Composite That Mimics the Biomechanical Properties and Structure of a Natural Intervertebral Disc. Materials 2022, 15, 1481. [Google Scholar] [CrossRef]
- Beckstein, J.C.; Sen, S.; Schaer, T.P.; Vresilovic, E.J.; Elliott, D.M. Comparison of animal discs used in disc research to human lumbar disc: Axial compression mechanics and glycosaminoglycan content. Spine 2008, 33, E166–E173. [Google Scholar] [CrossRef]
- Oxland, T.R.; Panjabi, M.M.; Southern, E.P.; Duranceau, J.S. An anatomic basis for spinal instability: A porcine trauma model. J. Orthop. Res. 1991, 9, 452–462. [Google Scholar] [CrossRef]
- Yingling, V.R.; Callaghan, J.P.; McGill, S.M. The porcine cervical spine as a model of the human lumbar spine: An anatomical, geometric, and functional comparison. J. Spinal Disord. 1999, 12, 415–423. [Google Scholar] [CrossRef]
- Chan, S.C.W.; Gantenbein-Ritter, B.; Leung, V.Y.L.; Chan, D.; Cheung, K.M.C.; Ito, K. Cryopreserved intervertebral disc with injected bone marrow-derived stromal cells: A feasibility study using organ culture. Spine J. 2010, 10, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Miles, D.E.; Mitchell, E.A.; Kapur, N.; Beales, P.A.; Wilcox, R.K. Peptide: Glycosaminoglycan hybrid hydrogels as an injectable intervention for spinal disc degeneration. J. Mater. Chem. B 2016, 4, 3225–3231. [Google Scholar] [CrossRef] [PubMed]
- Hom, W.W.; Tschopp, M.; Lin, H.A.; Nasser, P.; Laudier, D.M.; Hecht, A.C. Composite biomaterial repair strategy to restore biomechanical function and reduce herniation risk in an ex vivo large animal model of intervertebral disc herniation with varying injury severity. PLoS ONE 2019, 14, e0217357. [Google Scholar] [CrossRef]
- Sapiee, N.H.; Thambyah, A.; Robertson, P.A.; Broom, N.D. Sagittal alignment with downward slope of the lower lumbar motion segment influences its modes of failure in direct compression: A mechanical and microstructural investigation. Spine 2019, 44, 1118–1128. [Google Scholar] [CrossRef]
- Feki, F.; Taktak, R.; Kandil, K.; Derrouiche, A.; Moulart, M.; Haddar, N.; Zaïri, F.; Zaïri, F. How osmoviscoelastic coupling affects recovery of cyclically compressed intervertebral disc. Spine 2020, 45, E1376–E13851. [Google Scholar] [CrossRef] [PubMed]
- Derrouiche, A.; Feki, F.; Zaïri, F.; Taktak, R.; Moulart, M.; Qu, Z.; Ismail, J.; Charfi, S.; Haddar, N.; Zaïri, F. How pre-strain affects the chemo-torsional response of the intervertebral disc. Clin. Biomech. 2020, 76, 105020. [Google Scholar] [CrossRef]
- Werbner, B.; Spack, K.; O’Connell, G.D. Bovine annulus fibrosus hydration affects rate-dependent failure mechanics in tension. J. Biomech. 2019, 89, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, J.; Diwan, A.D.; Tipper, J.L. Elastic fibers: The missing key to improve engineering concepts for reconstruction of the nucleus pulposus in the intervertebral disc. Acta Biomater. 2020, 113, 407–416. [Google Scholar] [CrossRef]
- Du, Y.; Tavana, S.; Rahman, T.; Baxan, N.; Hansen, U.N.; Newell, N. Sensitivity of intervertebral disc finite element models to internal geometric and non-geometric parameters. Front. Bioeng. Biotechnol. 2021, 9, 660013. [Google Scholar] [CrossRef]
- Tamoud, A.; Zaïri, F.; Mesbah, A.; Zaïri, F. A fully three-dimensional model of interpenetrating collagen fibrillar networks for intervertebral disc mechanics. Int. J. Mech. Sci. 2022, 223, 107310. [Google Scholar] [CrossRef]
- Yoon, D.H.E.; Weber, C.I.; Easson, G.W.D.; Broz, K.S.; Tang, S.Y. Rapid determination of internal strains in soft tissues using an experimentally calibrated finite element model derived from magnetic resonance imaging. Quant. Imaging Med. Surg. 2020, 10, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Komeili, A.; Rasoulian, A.; Moghaddam, F.; El-Rich, M.; Li, L.P. The importance of intervertebral disc material model on the prediction of mechanical function of the cervical spine. BMC Musculoskelet. Disord. 2021, 22, 324. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.P.G.; Alves, J.L. Numerical implementation of an osmo-poro-visco-hyperelastic finite element solver: Application to the intervertebral disc. Comput. Methods Biomech. Biomed. Eng. 2020, 24, 538–550. [Google Scholar] [CrossRef]
- Subramani, A.V.; Whitley, P.E.; Garimella, H.T.; Kraft, R.H. Fatigue damage prediction in the annulus of cervical spine intervertebral discs using finite element analysis. Comput. Methods Biomech. Biomed. Eng. 2020, 23, 773–784. [Google Scholar] [CrossRef]
- Kandil, K.; Zaïri, F.; Messager, T.; Zaïri, F. A microstructure-based model for a full lamellar-interlamellar displacement and shear strain mapping inside human intervertebral disc core. Comput. Biol. Med. 2021, 125, 104629. [Google Scholar] [CrossRef]


| Load | η (MPa·s) | R2 | ||||
|---|---|---|---|---|---|---|
| 300 N | 0.375 | 8.361 | 0.196 | 7008.480 | 1373.662 | 0.9993 |
| 400 N | 0.500 | 2.171 | 0.060 | 4056.267 | 243.376 | 0.9979 |
| 500 N | 0.625 | 0.706 | 0.049 | 3379.265 | 165.584 | 0.9979 |
| Equation (2) | Location | a | b | c | R2 |
| 300 N | Upper | 3.891 | 3.891 × 107 | 16.62 | 0.9194 |
| Middle | 7.859 | 9.956 × 107 | 36.689 | 0.9056 | |
| Lower | 4.186 | 1.559 × 107 | 24.033 | 0.9321 | |
| 400 N | Upper | 2.457 | 1.648 × 107 | 5.823 | 0.9687 |
| Middle | 2.897 | 4.757 × 107 | 5.438 | 0.9220 | |
| Lower | 1.147 | 1.889 × 107 | 2.624 | 0.9109 | |
| 500 N | Upper | 3.545 | 7.101 × 108 | 12.905 | 0.9122 |
| Middle | 8.968 | 2.897 × 107 | 15.507 | 0.9543 | |
| Lower | 1.288 | 1.504 × 106 | 5.138 | 0.9238 |
| Reference | Specimen | Number | Load (N) | Time (min) | Bulging (mm) |
|---|---|---|---|---|---|
| Reuber et al., 1982 [37] | human lumbar | 14 | 400 | - | 0.55 |
| Wenger et al., 1997 [38] | human lumbar | 16 | 2500 | 2.5 | 0.65 ± 0.42 |
| Meakin et al., 2000 [39] | sheep lumbar | 18 | 1000 | - | 0.277 ± 0.218 |
| Heuer et al., 2007 [7] | human lumbar | 7 | 500 | 15 | 1.1 |
| Heuer et al., 2008 [8] | human lumbar | 6 | 500 | 15 | 0.86 |
| Heuer et al., 2012 [9] | human lumbar | 6 | 500 | 15 | 0.8 |
| Pei et al., 2013 [14] | ovine lumbar | 15 | 1000 | 5 | 0.343 ± 0.141 |
| Lao et al., 2014 [20] | human cervical | 3000 | in vivo | in vivo | C23-0.6 |
| C34-1.5 | |||||
| C45-1.7 | |||||
| C56-1.8 | |||||
| C67-1.4 | |||||
| C7T1-0.3 | |||||
| Dupré et al., 2016 [13] | human lumbar | 25 | 250 | - | 0.4 |
| Fewster et al., 2020 [10] | porcine cervical | 12 | 10 | 15 | 1.27 |
| 300 | 1.46 | ||||
| 600 | 1.43 | ||||
| 1200 | 1.45 | ||||
| Mengoni et al., 2021 [15] | bovine caudal | 6 | 110 | 90 | 1.2 |
| Our results | porcine cervical | 10 | 300 | 240 | 1.50 ± 0.61 |
| 400 | 240 | 1.67 ± 0.83 | |||
| 500 | 240 | 1.87 ± 0.83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, M.; Xiang, D.; Wang, S.; Liu, W. The Radial Bulging and Axial Strains of Intervertebral Discs during Creep Obtained with the 3D-DIC System. Biomolecules 2022, 12, 1097. https://doi.org/10.3390/biom12081097
Yang M, Xiang D, Wang S, Liu W. The Radial Bulging and Axial Strains of Intervertebral Discs during Creep Obtained with the 3D-DIC System. Biomolecules. 2022; 12(8):1097. https://doi.org/10.3390/biom12081097
Chicago/Turabian StyleYang, Mengying, Dingding Xiang, Song Wang, and Weiqiang Liu. 2022. "The Radial Bulging and Axial Strains of Intervertebral Discs during Creep Obtained with the 3D-DIC System" Biomolecules 12, no. 8: 1097. https://doi.org/10.3390/biom12081097






