Mechanism by Which PF-3758309, a Pan Isoform Inhibitor of p21-Activated Kinases, Blocks Reactivation of HIV-1 Latency
Abstract
:1. Introduction
2. Materials and Methods
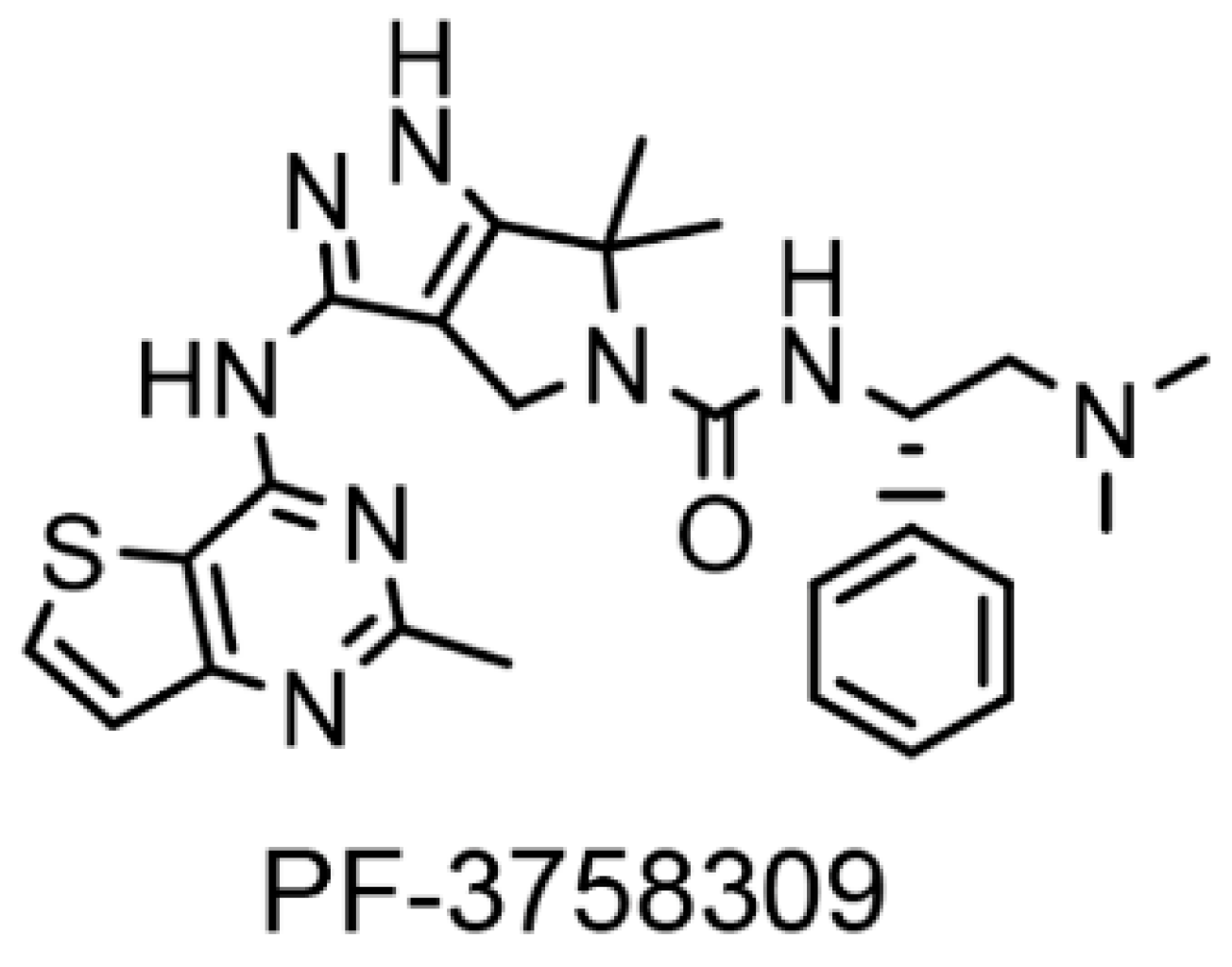
2.1. Reagents
2.2. Quantification of PAK Expression
2.3. siRNA Transfection of 24ST1NLESG Cells
2.4. PAK1 and PAK2 Overexpression
2.5. Phospho Explorer Antibody Array
2.6. Cellular Thermal Shift Assays
3. Results
3.1. PAK Isoform Expression in 24ST1NLESG Cells and in Purified CD4+ Naïve (TN) and Central Memory (TCM) T Cells
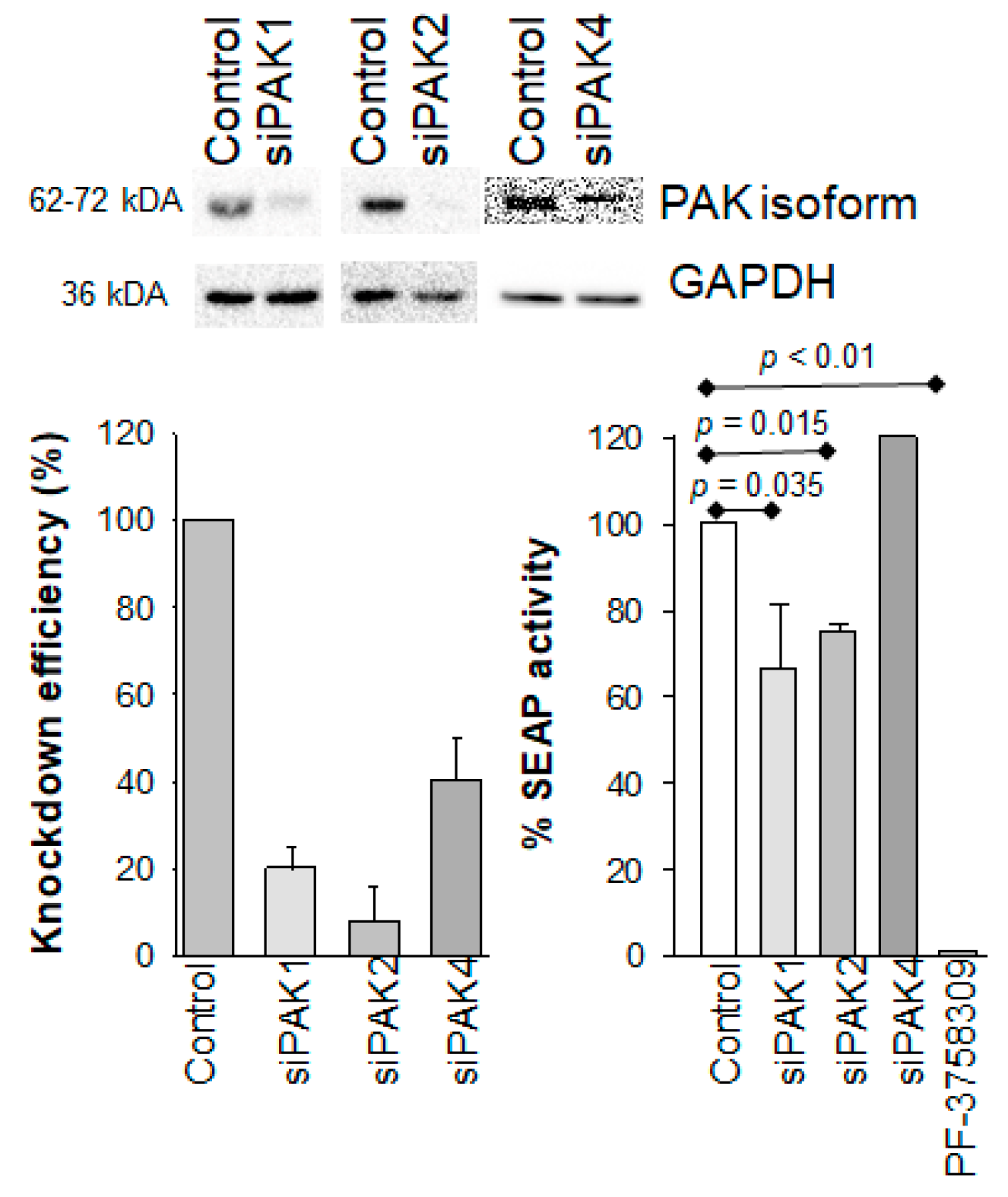
3.2. Knockdown of PAK1, PAK2, and PAK4 in 24ST1NLESG Cells
3.3. Overexpression of PAK1 and PAK2 in 24ST1NLESG Cells
3.4. PAK Signaling and HIV-1 Latency Reversal
3.5. Potential Off-Target Effects of PF-3758309
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siliciano, J.D.; Siliciano, R.F. In Vivo Dynamics of the Latent Reservoir for HIV-1: New Insights and Implications for Cure. Annu. Rev. Pathol. 2022, 17, 271–294. [Google Scholar] [CrossRef]
- Siliciano, J.D.; Siliciano, R.F. Low Inducibility of Latent Human Immunodeficiency Virus Type 1 Proviruses as a Major Barrier to Cure. J. Infect. Dis. 2021, 223 (Suppl. S2), 13–21. [Google Scholar] [CrossRef]
- Chitrakar, A.; Sanz, M.; Maggirwar, S.B.; Soriano-Sarabia, N. HIV Latency in Myeloid Cells: Challenges for a Cure. Pathogens 2022, 11, 611. [Google Scholar] [CrossRef]
- Moranguinho, I.; Valente, S.T. Block-And-Lock: New Horizons for a Cure for HIV-1. Viruses 2020, 12, 1443. [Google Scholar] [CrossRef]
- Vansant, G.; Bruggemans, A.; Janssens, J.; Debyser, Z. Block-And-Lock Strategies to Cure HIV Infection. Viruses 2020, 12, 84. [Google Scholar] [CrossRef] [Green Version]
- Mbonye, U.; Karn, J. Transcriptional control of HIV latency: Cellular signaling pathways, epigenetics, happenstance and the hope for a cure. Virology 2014, 454–455, 328–339. [Google Scholar] [CrossRef] [Green Version]
- Vargas, B.; Giacobbi, N.S.; Sanyal, A.; Venkatachari, N.J.; Han, F.; Gupta, P.; Sluis-Cremer, N. Inhibitors of Signaling Pathways That Block Reversal of HIV-1 Latency. Antimicrob. Agents Chemother. 2019, 63, e01744-18. [Google Scholar] [CrossRef] [Green Version]
- Murray, B.W.; Guo, C.; Piraino, J.; Westwick, J.K.; Zhang, C.; Lamerdin, J.; Dagostino, E.; Knighton, D.; Loi, C.M.; Zager, M.; et al. Small-molecule p21-activated kinase inhibitor PF-3758309 is a potent inhibitor of oncogenic signaling and tumor growth. Proc. Natl. Acad. Sci. USA 2010, 107, 9446–9451. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; Sanawar, R.; Li, X.; Li, F. Structure, biochemistry, and biology of PAK kinases. Gene 2017, 605, 20–31. [Google Scholar] [CrossRef] [Green Version]
- Semblat, J.P.; Doerig, C. PAK in pathogen-host interactions. Cell. Logist. 2012, 2, 126–131. [Google Scholar] [CrossRef]
- Nguyen, D.G.; Wolff, K.C.; Yin, H.; Caldwell, J.S.; Kuhen, K.L. “UnPAKing” human immunodeficiency virus (HIV) replication: Using small interfering RNA screening to identify novel cofactors and elucidate the role of group I PAKs in HIV infection. J. Virol. 2006, 80, 130–137. [Google Scholar] [CrossRef] [Green Version]
- Chan, C.P.; Siu, Y.T.; Kok, K.H.; Ching, Y.P.; Tang, H.M.; Jin, D.Y. Group I p21-activated kinases facilitate Tax-mediated transcriptional activation of the human T-cell leukemia virus type 1 long terminal repeats. Retrovirology 2013, 10, 47. [Google Scholar] [CrossRef] [Green Version]
- Kouwenhoven, A.; Minassian, V.D.; Marsh, J.W. HIV-1 Nef mediates Pak phosphorylation of Mek1 serine298 and elicits an active phospho-state of Pak2. Curr. HIV Res. 2013, 11, 198–209. [Google Scholar] [CrossRef]
- Micheva-Viteva, S.; Pacchia, A.L.; Ron, Y.; Peltz, S.W.; Dougherty, J.P. Human immunodeficiency virus type 1 latency model for high-throughput screening. Antimicrob. Agents Chemother. 2005, 49, 5185–5188. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Garavaglia, S.; Ye, Z.; Moretti, A.; Belyaeva, O.V.; Beiser, A.; Ibrahim, M.; Wilk, A.; McClellan, S.; Klyuyeva, A.V.; et al. A specific inhibitor of ALDH1A3 regulates retinoic acid biosynthesis in glioma stem cells. Commun. Biol. 2021, 4, 1420. [Google Scholar] [CrossRef]
- Jarzab, A.; Kurzawa, N.; Hopf, T.; Moerch, M.; Zecha, J.; Leijten, N.; Bian, Y.; Musiol, E.; Maschberger, M.; Stoehr, G.; et al. Meltome atlas-thermal proteome stability across the tree of life. Nat. Methods 2020, 17, 495–503. [Google Scholar] [CrossRef]
- Wiśniewski, J.R.; Zougman, A.; Nagaraj, N.; Mann, M. Universal sample preparation method for proteome analysis. Nat. Methods 2009, 6, 359–362. [Google Scholar] [CrossRef]
- Chu, P.C.; Wu, J.; Liao, X.C.; Pardo, J.; Zhao, H.; Li, C.; Mendenhall, M.K.; Pali, E.; Shen, M.; Yu, S.; et al. A novel role for p21-activated protein kinase 2 in T cell activation. J. Immunol. 2004, 172, 7324–7334. [Google Scholar] [CrossRef] [Green Version]
- Ng, Y.W.; Raghunathan, D.; Chan, P.M.; Baskaran, Y.; Smith, D.J.; Lee, C.H.; Verma, C.; Manser, E. Why an A-loop phospho-mimetic fails to activate PAK1: Understanding an inaccessible kinase state by molecular dynamics simulations. Structure 2010, 18, 879–890. [Google Scholar] [CrossRef] [Green Version]
- Ryu, B.J.; Lee, H.; Kim, S.H.; Heo, J.N.; Choi, S.W.; Yeon, J.T.; Lee, J.; Lee, J.; Cho, J.Y.; Kim, S.H.; et al. PF-3758309, p21-activated kinase 4 inhibitor, suppresses migration and invasion of A549 human lung cancer cells via regulation of CREB, NF-κB, and β-catenin signalings. Mol. Cell. Biochem. 2014, 389, 69–77. [Google Scholar] [CrossRef]
- Wong, L.M.; Jiang, G. NF-κB sub-pathways and HIV cure: A revisit. EBioMedicine 2021, 63, 103159. [Google Scholar] [CrossRef]
- Martinez Molina, D.; Nordlund, P. The Cellular Thermal Shift Assay: A Novel Biophysical Assay for In Situ Drug Target Engagement and Mechanistic Biomarker Studies. Annu. Rev. Pharmacol. Toxicol. 2016, 56, 141–161. [Google Scholar] [CrossRef]
- Vargas, B.; Sluis-Cremer, N. Toward a Functional Cure for HIV-1 Infection: The Block and Lock therapeutic Approach. Front. Virol. 2022, 2, 917941. [Google Scholar] [CrossRef]
- Chan, J.K.; Greene, W.C. NF-κB/Rel: Agonist and antagonist roles in HIV-1 latency. Curr. Opin. HIV AIDS 2011, 6, 12–18. [Google Scholar] [CrossRef]
- Shi, H.; Berger, E.A. Characterization of Site-Specific Phosphorylation of NF-κB p65 in Retinal Cells in Response to High Glucose and Cytokine Polarization. Mediat. Inflamm. 2018, 2018, 3020675. [Google Scholar] [CrossRef] [Green Version]
- Dai, Y.; Chen, S.; Wang, L.; Pei, X.Y.; Funk, V.L.; Kramer, L.B.; Dent, P.; Grant, S. Disruption of IkappaB kinase (IKK)-mediated RelA serine 536 phosphorylation sensitizes human multiple myeloma cells to histone deacetylase (HDAC) inhibitors. J. Biol. Chem. 2011, 286, 34036–34050. [Google Scholar] [CrossRef] [Green Version]
- Melikian, M.; Eluard, B.; Bertho, G.; Baud, V.; Evrard-Todeschi, N. Model of the Interaction between the NF-κB Inhibitory Protein p100 and the E3 Ubiquitin Ligase β-TrCP based on NMR and Docking Experiments. J. Chem. Inf. Model. 2017, 57, 223–233. [Google Scholar] [CrossRef]





| Protein (Phosphorylation Site) | Phospho Ratio | Protein (Phosphorylation Site) | Phospho Ratio |
|---|---|---|---|
| PKC zeta (Phospho-Thr560) | 1.4 | c-PLA2 (Phospho-Ser505) | 1.62 |
| Chk1 (Phospho-Ser345) | 1.4 | HSL (Phospho-Ser554) | 1.64 |
| EGFR (Phospho-Thr693) | 1.41 | FGFR1 (Phospho-Tyr766) | 1.64 |
| Paxillin (Phospho-Tyr31) | 1.42 | Raf1 (Phospho-Ser259) | 1.66 |
| Ephrin B1 (Phospho-Tyr317) | 1.42 | Cyclin D3 (Phospho-Thr283) | 1.67 |
| p53 (Phospho-Ser37) | 1.42 | IL3RB (Phospho-Tyr593) | 1.68 |
| PPAR-BP (Phospho-Thr1457) | 1.43 | Cyclin E1 (Phospho-Thr77) | 1.68 |
| BIM (Phospho-Ser69/65) | 1.43 | Stathmin 1 (Phospho-Ser15) | 1.7 |
| MEK1 (Phospho-Ser221) | 1.44 | Smad2/3 (Phospho-Thr8) | 1.71 |
| Chk2 (Phospho-Thr383) | 1.45 | Keratin 18 (Phospho-Ser52) | 1.88 |
| BAD (Phospho-Ser134) | 1.45 | B-RAF (Phospho-Thr598) | 1.96 |
| PKC theta (Phospho-Ser676) | 1.46 | Ephrin B2 (Phospho-Tyr330) | 1.98 |
| MEK1 (Phospho-Ser298) | 1.47 | c-Jun (Phospho-Thr93) | 2.18 |
| Calsenilin/KCNIP3 (Phospho-Ser63) | 1.48 | CD4 (Phospho-Ser433) | 2.25 |
| PKC alpha (Phospho-Tyr657) | 1.48 | HDAC1 (Phospho-Ser421) | 2.38 |
| HDAC2 (Phospho-Ser394) | 1.48 | p53 (Phospho-Ser315) | 2.72 |
| Synuclein alpha (Phospho-Tyr125) | 1.49 | Ras-GRF1 (Phospho-Ser916) | 0.35 |
| Estrogen Receptor-alpha (Phospho-Ser104) | 1.49 | Claudin 3 (Phospho-Tyr219) | 0.43 |
| IRS-1 (Phospho-Ser636) | 1.49 | BCR (Phospho-Tyr177) | 0.44 |
| Lamin A (Phospho-Ser22) | 1.5 | NFkB-p65 (Phospho-Thr254) | 0.48 |
| Cyclin D1 (Phospho-Thr286) | 1.5 | Caspase 9 (Phospho-Tyr153) | 0.5 |
| ALK (Phospho-Tyr1604) | 1.52 | Filamin A (Phospho-Ser2152) | 0.51 |
| Smad2 (Phospho-Ser467) | 1.53 | BID (Phospho-Ser78) | 0.52 |
| GSK3 alpha/beta (Phospho-Tyr216/279) | 1.53 | 4E-BP1 (Phospho-Ser65) | 0.52 |
| 14-3-3 zeta/delta (Phospho-Thr232) | 1.53 | PLCG1 (Phospho-Tyr771) | 0.56 |
| ERK3 (Phospho-Ser189) | 1.53 | ALK (Phospho-Tyr1507) | 0.56 |
| JunB (Phospho-Ser259) | 1.54 | HDAC3 (Phospho-Ser424) | 0.56 |
| c-Jun (Phospho-Tyr170) | 1.58 | Kv1.3/KCNA3 (Phospho-Tyr135) | 0.57 |
| LKB1 (Phospho-Ser428) | 1.6 | IKK-alpha/beta (Phospho-Ser180/181) | 0.58 |
| PP1 alpha (Phospho-Thr320) | 1.61 | NFkB-p100/p52 (Phospho-Ser865) | 0.59 |
| EGFR (Phospho-Tyr1069) | 1.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vargas, B.; Boslett, J.; Yates, N.; Sluis-Cremer, N. Mechanism by Which PF-3758309, a Pan Isoform Inhibitor of p21-Activated Kinases, Blocks Reactivation of HIV-1 Latency. Biomolecules 2023, 13, 100. https://doi.org/10.3390/biom13010100
Vargas B, Boslett J, Yates N, Sluis-Cremer N. Mechanism by Which PF-3758309, a Pan Isoform Inhibitor of p21-Activated Kinases, Blocks Reactivation of HIV-1 Latency. Biomolecules. 2023; 13(1):100. https://doi.org/10.3390/biom13010100
Chicago/Turabian StyleVargas, Benni, James Boslett, Nathan Yates, and Nicolas Sluis-Cremer. 2023. "Mechanism by Which PF-3758309, a Pan Isoform Inhibitor of p21-Activated Kinases, Blocks Reactivation of HIV-1 Latency" Biomolecules 13, no. 1: 100. https://doi.org/10.3390/biom13010100




