The Effectiveness of Cold Atmospheric Plasma (CAP) on Bacterial Reduction in Dental Implants: A Systematic Review
Abstract
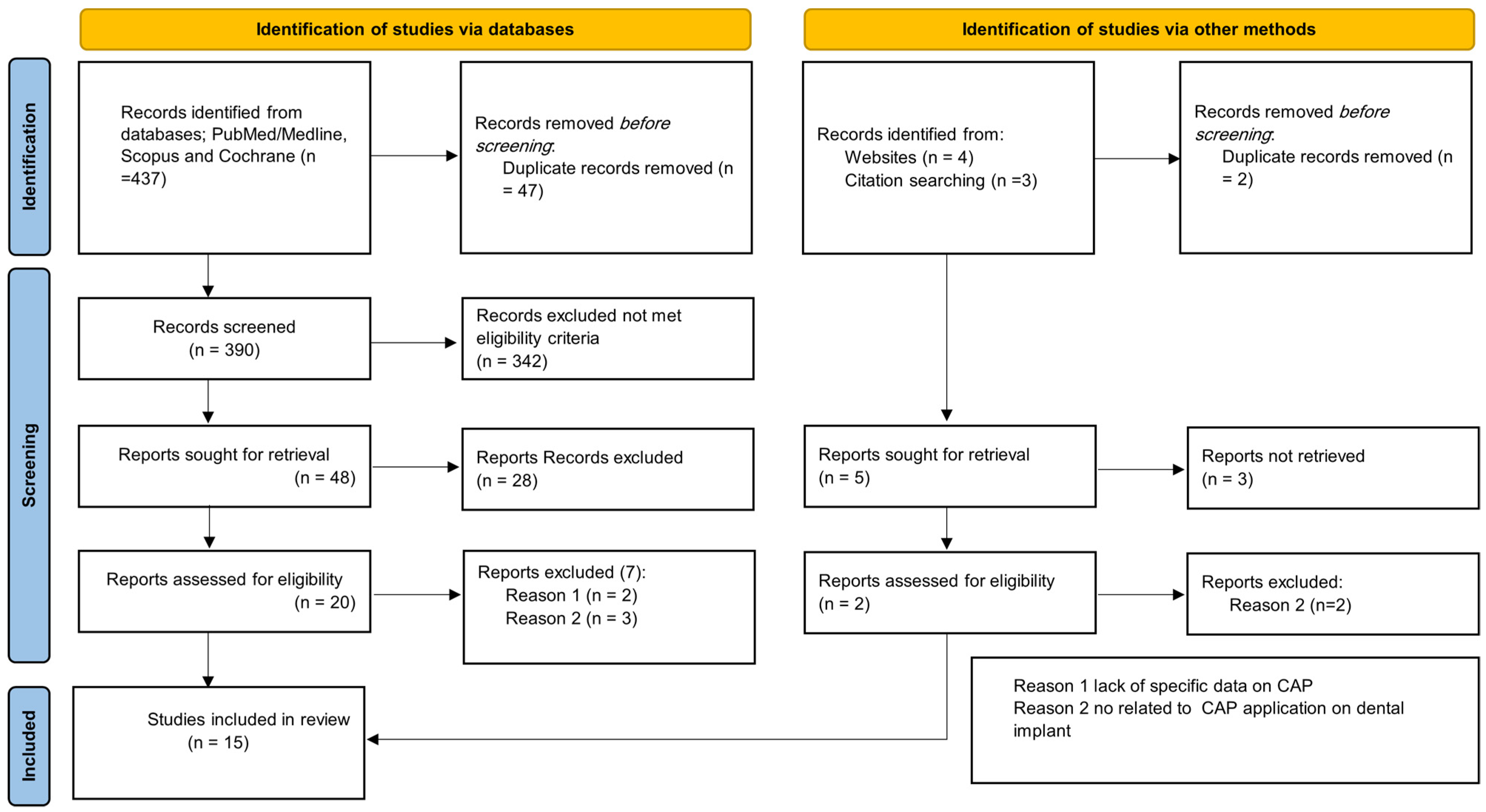
:1. Introduction
2. Review Method
3. Results
| Study ID | Year | Device | Plasma Mode | Gas | Device Parameters | Implant Material | Sample Size | Control | Incubation (hours) | Species | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Power (W) | Voltage (kV) | Frequency (kHz) | Gas Flow Rates (slm) | ||||||||||
| Canullo et al. | 2017 | argon atmospheric pressure dielectric barrier discharge | Plasma Jet | Argon | 8 | NR | NR | None | Titanium Grade 4 discs (Sweden and Martina) | 720 discs | Untreated titanium discs | 24 | Streptococcus mitis |
| Duske et al. | 2015 | (kINPen08, INP Greifswald, Germany) | Plasma Jet | Argon (99%) with 1% oxygen | 2–3 | 2–6 | 1.82 | 5 | Titanium Grade 4, diameter 15 mm, and thickness 1 mm Straumann, Freiburg, Germany) | 80 discs | Discs without biofilm, untreated biofilm and autoclaved biofilm | 24 & 120 | Sub-gingival plaque from deep pockets |
| Flörke et al. | 2022 | kINPen® MED (neoplas tools GmbH, Greifswald, Germany) | Plasma Jet | NR | 5 | NR | NR | NR | Titanium (TiPure Plus BEGO Semados® SC, BEGO GmbH & Co. KG, Bremen, Germany, 3.75 × 8.5 mm) | 45 implants | Negative: 2 implants neither infected nor decontaminated + 2 implants had been kept. free of contamination and treatment. Positive: before the decontamination procedure, one implant was removed. | 24 | Enterococcus faecalis |
| Ibis et al. | 2016 | Advanced plasma Solutions, Malvern, PA, USA | Atmospheric Pressure | NR | 0.29 | 31 | 1.5 | None | Steel, titanium, and polyethylene rods cut into disc | NR | Untreated disc as positive control | 24 | Escherichia coli and Staphylococcus aureus |
| Jungbauer et al. | 2022 | piezobrush® PZ3; Relyon Plasma, Regensburg, Germany | Plasma brush | NR | 8 | Non | 50 | NR | Polystyrene, dentin, titanium | 60 | NR | 84 | 12 bacterial strains |
| Kamionka et al. | 2022 | kINPen® | Plasma Jet | Argon | 3.5 | 2–6 | 1 | 5 | Titanium discs (Nobel Biocare AB, Göteborg, Sweden’s TiUnite) 5 mm in diameter and 1 mm in thickness | 280 discs | Untreated discs with biofilm and sterile discs | 168 | Subgingival plaque |
| Lai Hui et al. | 2021 | Atmospheric experimental plasma pen jet | Plasma Jet | NR | NR | 10 | 1.4 | NR | Grade 4 titanium discs diameter 10 mm, thickness 1.5/2 mm with two different surfaces | 112 discs | Negative control: treated titanium discs that have not been contaminated. Positive control: contaminated and untreated discs | 96 | 10% of a patient’s peri-implantitis human saliva |
| Hui et al. | 2021 | NR | Plasma Jet | NR | NR | 10 | 1.4 | NR | Dental implants made of grade 4 pure Ti | 35 implants | Negative (2 S non-contaminated, treated by AA and CAP) Positive control group: (3 S untreated, contaminated) | 96 | Saliva from peri-implantitis patient |
| Lee et al. | 2019 | Dawonsys, Ansan, Republic of Korea’s MF plasma power supply | Plasma Jet | Helium (He) | NR | 7 | 10 | 5 | Grade 4 titanium discs (Osstem Implant Co., Ltd., Busan, Republic of Korea) 10 mm diameter, 1 mm thickness | 12 discs | Untreated discs | 144 | Porphyromonas gingivalis |
| Matthes et al. | 2017 | neoplas GmbH, Greifswald, Germany, kINPen 09 | Plasma Jet | Argon | 3.5 | 2–6 | 1 | Grade 4 titanium discs (BIOMET 3i LLC, Palm Beach Garden, FL, USA) | 18 discs | Negative control: untreated biofilm Positive control: sterile pristine discs | 24 | MG63 cells, bacteria, or biofilm are present | |
| Ulu et al. | 2018 | Bad Ems, Germany, Plasma Medical Systems | NR | NR | 5 | 7 | 1.2 | None | Large-grit, acid-etched (SLA), sandblasted titanium discs | 76 discs | Er: YAG laser | 168 | Staphylococcus aureus |
| Idlibi et al. | 2013 | Leibniz Institute of Surface Modification, Leipzig, Germany | Plasma Jet | Helium, O2 | 3–5 | None | 2.45 | 2 | Titanium discs | 200 discs | untreated and treated controls (diode laser, air-abrasion, chlorhexidine) | 72 | Oral biofilms |
| Preissner et al. | 2016 | Tissue tolerable plasma (TTP120) | Plasma jet | Argon gas | 2 | 10–15 | 28: direct sonication 35: indirect sonication | 4.3 | Titanium (2.5 × 13 mm tiny Implant, Biotechnology Institute BTI, Miñano, Spain, REF: IRT2513) | 32 implants | Negative control: rinsed with 1 NaCl, Positive control: irradiated with diode or plasma | 84 | Strepto-coccus Mitis |
| Rupf et al. | 2011 | custom-built (Leipzig, Germany, Leibniz Institute of Surface Modification) | plasma jet | Helium | 3–5 | None | 2.45 | None | Titanium discs grade 2, friadent, 5 mm in diameter and 1 mm in thickness, Mannheim, Germany | Total 334 298 with biofilm 36 without | Biofilms with and without water/air treatment. | 72 and 24 | Oral cavities biofilm |
| Yang et al. | 2020 | Cold atomosphirc plasma | Plasma jet | Helium | 0.95 | 2.85 | 17 | 13.5 | Yttrium-stabilized zirconia discs (Wieland, Pforzheim, Germany) | 24 discs | The control group left untreated. | 72 | Streptococcus mutans, Porphyromonas gingivalis |
| Study ID | Item 1 | Item 2 | Item 3 | Item 4 | Item 5 | Item 6 | Item 7 | Item 8 | Item 9 | Item 10 | Item 11 | Total | Score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Canullo et al. | 1 | 2 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 11 | 84.6 |
| Duske et al. | 1 | 1 | 2 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 9 | 69.2 |
| Flörke et al. | 1 | 2 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 10 | 76.9 |
| Ibis et al. | 0 | 2 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 4 | 30.8 |
| Jungbauer et al. | 1 | 1 | 2 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 8 | 61.5 |
| Kamionka et al. | 1 | 2 | 2 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 12 | 92.3 |
| Lai Hui et al. | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 9 | 69.2 |
| Lai Hui et al. | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 10 | 76.9 |
| Lee. et al. | 1 | 2 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 7 | 53.8 |
| Matthes et al. | 1 | 2 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 9 | 69.2 |
| Ulu et al. | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 8 | 61.5 |
| Idlibi et al. | 1 | 2 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 9 | 69.2 |
| Preissner et al. | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 8 | 61.5 |
| Rupf et al. | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 5 | 38.5 |
| Yang et al. | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 8 | 61.5 |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nørgaard Petersen, F.; Jensen, S.S.; Dahl, M. Implant treatment after traumatic tooth loss: A systematic review. Dent. Traumatol. 2022, 38, 105–116. [Google Scholar] [CrossRef]
- Elani, H.; Starr, J.; Da Silva, J.; Gallucci, G. Trends in dental implant use in the US, 1999–2016, and projections to 2026. J. Dent. Res. 2018, 97, 1424–1430. [Google Scholar] [CrossRef]
- Pavel, K.; Seydlova, M.; Dostalova, T.; Zdenek, V.; Chleborad, K.; Jana, Z.; Feberova, J.; Radek, H. Dental implants and improvement of oral health-related quality of life. Community Dent. Oral Epidemiol. 2012, 40, 65–70. [Google Scholar] [CrossRef]
- AlOtaibi, N.M.; Dunne, M.; Ayoub, A.F.; Naudi, K.B. A novel surgical model for the preclinical assessment of the osseointegration of dental implants: A surgical protocol and pilot study results. J. Transl. Med. 2021, 19, 276. [Google Scholar] [CrossRef]
- Khurshid, Z.; Hafeji, S.; Tekin, S.; Habib, S.R.; Ullah, R.; Sefat, F.; Zafar, M.S. Titanium, zirconia, and polyetheretherketone (PEEK) as a dental implant material. In Dental Implants; Elsevier: Amsterdam, The Netherlands, 2020; pp. 5–35. [Google Scholar]
- Nunes, M.P.; Nunes, L.F.P.; Nunes Filho, D.P.; Pinho, R.M.; Cimões, R. Prosthetic Rehabilitation in Older Adult with Free Gingival Graft: Case Report. Int. Arch. Med. 2019, 12, 1–9. [Google Scholar] [CrossRef]
- Heitz-Mayfield, L.J.; Mombelli, A. The therapy of peri-implantitis: A systematic review. Int. J. Oral Maxillofac. Implant. 2014, 29, 325–345. [Google Scholar] [CrossRef]
- Alqutaibi, A.Y.; Aboalrejal, A.N. Microgap and micromotion at the implant abutment interface cause marginal bone loss around dental implant but more evidence is needed. J. Evid. Based Dent. Pract. 2018, 18, 171–172. [Google Scholar] [CrossRef]
- Idlibi, A.N.; Al-Marrawi, F.; Hannig, M.; Lehmann, A.; Rueppell, A.; Schindler, A.; Jentsch, H.; Rupf, S. Destruction of oral biofilms formed in situ on machined titanium (Ti) surfaces by cold atmospheric plasma. Biofouling 2013, 29, 369–379. [Google Scholar] [CrossRef]
- Armitage, G.C.; Xenoudi, P.J.P. Post-treatment supportive care for the natural dentition and dental implants. Periodontology 2000 2016, 71, 164–184. [Google Scholar] [CrossRef]
- Khoshkam, V.; Del Amo, F.S.-L.; Monje, A.; Lin, G.-h.; Chan, H.-L.; Wang, H.-L. Long-term Radiographic and Clinical Outcomes of Regenerative Approach for Treating Peri-implantitis: A Systematic Review and Meta-analysis. Int. J. Oral Maxillofac. Implant. 2016, 31, 1303–1310. [Google Scholar] [CrossRef]
- Faggion, C.M., Jr.; Listl, S.; Fruehauf, N.; Chang, H.J.; Tu, Y.K. A systematic review and Bayesian network meta-analysis of randomized clinical trials on non-surgical treatments for peri-implantitis. J. Clin. Periodontol. 2014, 41, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- Tomasi, C.; Regidor, E.; Ortiz-Vigón, A.; Derks, J. Efficacy of reconstructive surgical therapy at peri-implantitis-related bone defects. A systematic review and meta-analysis. J. Clin. Periodontol. 2019, 46, 340–356. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-Y.; Chen, Z.; Chiang, H.-L.; Pan, W.-L.; Wang, H.-L. The Impact of Implantoplasty in Regenerated and Nonregenerated Treatment Modalities in Peri-implantitis: A Systematic Review and Meta-analysis. Int. J. Oral Maxillofac. Implant. 2022, 37, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.; Grusovin, M.G.; Worthington, H.V. Interventions for replacing missing teeth: Treatment of peri-implantitis. Cochrane Database Syst. Rev. 2012, 1, CD004970. [Google Scholar] [CrossRef] [PubMed]
- Monje, A.; Aranda, L.; Diaz, K.; Alarcón, M.; Bagramian, R.; Wang, H.; Catena, A. Impact of maintenance therapy for the prevention of peri-implant diseases: A systematic review and meta-analysis. J. Dent. Res. 2016, 95, 372–379. [Google Scholar] [CrossRef]
- Salvi, G.E.; Ramseier, C.A. Efficacy of patient-administered mechanical and/or chemical plaque control protocols in the management of peri-implant mucositis. A systematic review. J. Clin. Periodontol. 2015, 42, S187–S201. [Google Scholar] [CrossRef]
- Salvi, G.E.; Zitzmann, N. The effects of anti-infective preventive measures on the occurrence of biologic implant complications and implant loss: A systematic review. Int. J. Oral Maxillofac. Implant. 2014, 29, 292–307. [Google Scholar] [CrossRef]
- Schwarz, F.; Becker, K.; Renvert, S. Efficacy of air polishing for the non-surgical treatment of peri-implant diseases: A systematic review. J. Clin. Periodontol. 2015, 42, 951–959. [Google Scholar] [CrossRef]
- Natto, Z.S.; Aladmawy, M.; Levi, P.A., Jr.; Wang, H.-L. Comparison of the efficacy of different types of lasers for the treatment of peri-implantitis: A systematic review. Int. J. Oral Maxillofac. Implant. 2015, 30, 338–345. [Google Scholar] [CrossRef]
- Caton, J.G.; Armitage, G.; Berglundh, T.; Chapple, I.L.; Jepsen, S.; Kornman, K.S.; Mealey, B.L.; Papapanou, P.N.; Sanz, M.; Tonetti, M.S. A new classification scheme for periodontal and peri-implant diseases and conditions–Introduction and key changes from the 1999 classification. J. Periodontol. 2018, 89, S1–S8. [Google Scholar] [CrossRef]
- Pei, X.; Lu, X.; Liu, J.; Liu, D.; Yang, Y.; Ostrikov, K.; Chu, P.K.; Pan, Y. Inactivation of a 25.5 µm Enterococcus faecalis biofilm by a room-temperature, battery-operated, handheld air plasma jet. J. Phys. D Appl. Phys. 2012, 45, 165205. [Google Scholar] [CrossRef]
- Van Oost, G. Basic Features of Plasmas. In Plasma Gasification and Pyrolysis; CRC Press: Boca Raton, FL, USA, 2022; pp. 1–14. [Google Scholar]
- Sakudo, A.; Yagyu, Y.; Onodera, T. Disinfection and sterilization using plasma technology: Fundamentals and future perspectives for biological applications. Int. J. Mol. Sci. 2019, 20, 5216. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, C.; Berganza, C.; Zhang, J. Cold Atmospheric Plasma: Methods of production and application in dentistry and oncology. Med. Gas Res. 2013, 3, 21. [Google Scholar] [CrossRef] [PubMed]
- Chaerony Siffa, I.; Gerling, T.; Masur, K.; Eschenburg, C.; Starkowski, F.; Emmert, S. Development of a Mobile Sensory Device to Trace Treatment Conditions for Various Medical Plasma Source Devices. Sensors 2022, 22, 7242. [Google Scholar] [CrossRef]
- Bernhardt, T.; Semmler, M.L.; Schäfer, M.; Bekeschus, S.; Emmert, S.; Boeckmann, L. Plasma medicine: Applications of cold atmospheric pressure plasma in dermatology. Oxid. Med. Cell. Longev. 2019, 2019, 3873928. [Google Scholar] [CrossRef]
- Cui, H.; Jiang, M.; Zhou, W.; Gao, M.; He, R.; Huang, Y.; Chu, P.K.; Yu, X.-F. Carrier-free cellular transport of CRISPR/Cas9 ribonucleoprotein for genome editing by cold atmospheric plasma. Biology 2021, 10, 1038. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Kim, K.-H.; Park, S.-Y.; Yoon, S.-Y.; Kim, G.-H.; Lee, Y.-M.; Rhyu, I.-C.; Seol, Y.-J. The bactericidal effect of an atmospheric-pressure plasma jet on Porphyromonas gingivalis biofilms on sandblasted and acid-etched titanium discs. J. Periodontal Implant Sci. 2019, 49, 319–329. [Google Scholar] [CrossRef]
- Fridman, G.; Brooks, A.D.; Balasubramanian, M.; Fridman, A.; Gutsol, A.; Vasilets, V.N.; Ayan, H.; Friedman, G. Comparison of direct and indirect effects of non-thermal atmospheric-pressure plasma on bacteria. Plasma Process. Polym. 2007, 4, 370–375. [Google Scholar] [CrossRef]
- Umair, M.; Jabbar, S.; Ayub, Z.; Muhammad Aadil, R.; Abid, M.; Zhang, J.; Liqing, Z. Recent advances in plasma technology: Influence of atmospheric cold plasma on spore inactivation. Food Rev. Int. 2022, 38, 789–811. [Google Scholar] [CrossRef]
- Lata, S.; Chakravorty, S.; Mitra, T.; Pradhan, P.K.; Mohanty, S.; Patel, P.; Jha, E.; Panda, P.K.; Verma, S.K.; Suar, M. Aurora Borealis in dentistry: The applications of cold plasma in biomedicine. Mater. Today Bio 2022, 13, 100200. [Google Scholar] [CrossRef]
- Borges, A.C.; Kostov, K.G.; Pessoa, R.S.; de Abreu, G.M.; Lima, G.d.M.; Figueira, L.W.; Koga-Ito, C.Y. Applications of cold atmospheric pressure plasma in dentistry. Appl. Sci. 2021, 11, 1975. [Google Scholar] [CrossRef]
- Dhaliwal, J.S.; Rahman, N.A.; Knights, J.; Ghani, H.; de Albuquerque Junior, R.F. The effect of different surface topographies of titanium implants on bacterial biofilm: A systematic review. SN Appl. Sci. 2019, 1, 615. [Google Scholar] [CrossRef]
- Dewhirst, F.E.; Chen, T.; Izard, J.; Paster, B.J.; Tanner, A.C.; Yu, W.-H.; Lakshmanan, A.; Wade, W.G. The human oral microbiome. J. Bacteriol. 2010, 192, 5002–5017. [Google Scholar] [CrossRef]
- Hui, W.L.; Ipe, D.; Perrotti, V.; Piattelli, A.; Fang, Z.; Ostrikov, K.; Quaranta, A. Novel technique using cold atmospheric plasma coupled with air-polishing for the treatment of titanium discs grown with biofilm: An in-vitro study. Dent. Mater. 2021, 37, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef] [PubMed]
- Jungbauer, G.; Moser, D.; Müller, S.; Pfister, W.; Sculean, A.; Eick, S. The antimicrobial effect of cold atmospheric plasma against dental pathogens—A systematic review of in-vitro studies. Antibiotics 2021, 10, 211. [Google Scholar] [CrossRef] [PubMed]
- EN 14561; Chemical Disinfectants and Antiseptics. Quantitative Carrier Test for the Evaluation of Bactericidal Activity for Instruments Used in the Medical Area. Test Method and Requirements (Phase 2, Step 2). British Standards Institute: London, UK, 2006.
- ANSI/AAMI/ISO 11737-1: 2006; Sterilization of Medical Devices—Microbiological Methods—Part 1: Determination of a Population of Microorganisms on Products. AAMI: Arlington, VA, USA, 2006.
- Chyderiotis, S.; Legeay, C.; Verjat-Trannoy, D.; Le Gallou, F.; Astagneau, P.; Lepelletier, D. New insights on antimicrobial efficacy of copper surfaces in the healthcare environment: A systematic review. Clin. Microbiol. Infect. 2018, 24, 1130–1138. [Google Scholar] [CrossRef] [PubMed]
- Canullo, L.; Genova, T.; Wang, H.-L.; Carossa, S.; Mussano, F. Plasma of argon increases cell attachment and bacterial decontamination on different implant surfaces. Int. J. Oral Maxillofac. Implant. 2017, 32, 1315–1323. [Google Scholar] [CrossRef]
- Preissner, S.; Wirtz, H.C.; Tietz, A.K.; Abu-Sirhan, S.; Herbst, S.R.; Hartwig, S.; Pierdzioch, P.; Schmidt-Westhausen, A.M.; Dommisch, H.; Hertel, M. Bactericidal efficacy of tissue tolerable plasma on microrough titanium dental implants: An in-vitro-study. J. Biophotonics 2016, 9, 637–644. [Google Scholar] [CrossRef]
- Ibis, F.; Oflaz, H.; Ercan, U.K. Biofilm inactivation and prevention on common implant material surfaces by nonthermal DBD plasma treatment. Plasma Med. 2016, 6, 33–45. [Google Scholar] [CrossRef]
- Ulu, M.; Pekbagriyanik, T.; Ibis, F.; Enhos, S.; Ercan, U.K. Antibiofilm efficacies of cold plasma and er: YAG laser on staphylococcus aureus biofilm on titanium for nonsurgical treatment of peri-implantitis. Niger. J. Clin. Pract. 2018, 21, 758–765. [Google Scholar] [PubMed]
- Kamionka, J.; Matthes, R.; Holtfreter, B.; Pink, C.; Schlüter, R.; von Woedtke, T.; Kocher, T.; Jablonowski, L. Efficiency of cold atmospheric plasma, cleaning powders and their combination for biofilm removal on two different titanium implant surfaces. Clin. Oral Investig. 2022, 26, 3179–3187. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zheng, M.; Yang, Y.; Li, J.; Su, Y.-F.; Li, H.-P.; Tan, J.-G. Inhibition of bacterial growth on zirconia abutment with a helium cold atmospheric plasma jet treatment. Clin. Oral Investig. 2020, 24, 1465–1477. [Google Scholar] [CrossRef] [PubMed]
- Flörke, C.; Janning, J.; Hinrichs, C.; Behrens, E.; Liedtke, K.R.; Sen, S.; Christofzik, D.; Wiltfang, J.; Gülses, A. In-vitro assessment of the efficiency of cold atmospheric plasma on decontamination of titanium dental implants. Int. J. Implant Dent. 2022, 8, 12. [Google Scholar] [CrossRef]
- Rupf, S.; Idlibi, A.N.; Marrawi, F.A.; Hannig, M.; Schubert, A.; von Mueller, L.; Spitzer, W.; Holtmann, H.; Lehmann, A.; Rueppell, A. Removing biofilms from microstructured titanium ex vivo: A novel approach using atmospheric plasma technology. PLoS ONE 2011, 6, e25893. [Google Scholar] [CrossRef]
- Hui, W.L.; Perrotti, V.; Piattelli, A.; Ostrikov, K.; Fang, Z.; Quaranta, A. Cold atmospheric plasma coupled with air abrasion in liquid medium for the treatment of peri-implantitis model grown with a complex human biofilm: An in vitro study. Clin. Oral Investig. 2021, 25, 6633–6642. [Google Scholar] [CrossRef]
- Matthes, R.; Duske, K.; Kebede, T.G.; Pink, C.; Schlüter, R.; von Woedtke, T.; Weltmann, K.D.; Kocher, T.; Jablonowski, L. Osteoblast growth, after cleaning of biofilm-covered titanium discs with air-polishing and cold plasma. J. Clin. Periodontol. 2017, 44, 672–680. [Google Scholar] [CrossRef]
- Duske, K.; Jablonowski, L.; Koban, I.; Matthes, R.; Holtfreter, B.; Sckell, A.; Nebe, J.B.; von Woedtke, T.; Weltmann, K.D.; Kocher, T. Cold atmospheric plasma in combination with mechanical treatment improves osteoblast growth on biofilm covered titanium discs. Biomaterials 2015, 52, 327–334. [Google Scholar] [CrossRef]
- Jungbauer, G.; Favaro, L.; Müller, S.; Sculean, A.; Eick, S. The in-vitro activity of a cold atmospheric plasma device utilizing ambient air against bacteria and biofilms associated with periodontal or peri-implant Diseases. Antibiotics 2022, 11, 752. [Google Scholar] [CrossRef]
- Alqutaibi, A.Y.; saleh Algabri, R. Limited evidence suggests high risk of implant failure rates among people with generalized aggressive periodontitis. J. Evid. Based Dent. Pract. 2015, 15, 187–189. [Google Scholar] [CrossRef]
- Gaur, N.; Szili, E.J.; Oh, J.-S.; Hong, S.-H.; Michelmore, A.; Graves, D.B.; Hatta, A.; Short, R.D. Combined effect of protein and oxygen on reactive oxygen and nitrogen species in the plasma treatment of tissue. Appl. Phys. Lett. 2015, 107, 103703. [Google Scholar] [CrossRef]
- Shi, Q.; Song, K.; Zhou, X.; Xiong, Z.; Du, T.; Lu, X.; Cao, Y. Effects of non-equilibrium plasma in the treatment of ligature-induced peri-implantitis. J. Clin. Periodontol. 2015, 42, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Küçük, D.; Savran, L.; Ercan, U.K.; Yarali, Z.B.; Karaman, O.; Kantarci, A.; Sağlam, M.; Köseoğlu, S. Evaluation of efficacy of non-thermal atmospheric pressure plasma in treatment of periodontitis: A randomized controlled clinical trial. Clin. Oral Investig. 2020, 24, 3133–3145. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Estevan, L.; Millan-Martínez, D.; Fons-Font, A.; Agustín-Panadero, R.; Román-Rodríguez, J.-L. Methodology in specimen fabrication for in vitro dental studies: Standardization of extracted tooth preparation. J. Clin. Exp. Dent. 2017, 9, e897. [Google Scholar] [CrossRef]
| Items | Points Attributed According to the Response for Each Critical Step |
|---|---|
| 1. Preparation of microorganisms | 1 if described 0 if not described |
| 2. Technical data of plasma generator | 2 if at least 3 parameters described or commercially device 1 if at least 1 parameter described 0 if not described |
| 3. Experimental size presented | 2 for theoretical + true inoculum sizes 1 for theoretical inoculum size 0 if not described |
| 4. Experimental temperature | 1 if described 0 if not described or over 47 °C |
| 5. Protection of samples | 1 if described 0 if not described |
| 6. Micro-organisms recovery | 1 if other method with mechanic action and validated with a test 0 if not clearly described or technic not validated |
| 7. Time, temperature and method indicated | 1 if described 0 if not or poorly described |
| 8. Culture media | 1 if described 0 if not described |
| 9. Number of experiments | 1 if described with more than one experiment 0 if not described or described with onlyone experiment |
| 10. Statistical method (to compare differences) | 1 if described 0 if not described |
| 11. Declaration | 1 if declared 0 if not declared |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alqutaibi, A.Y.; Aljohani, A.; Alduri, A.; Masoudi, A.; Alsaedi, A.M.; Al-Sharani, H.M.; Farghal, A.E.; Alnazzawi, A.A.; Aboalrejal, A.N.; Mohamed, A.-A.H.; et al. The Effectiveness of Cold Atmospheric Plasma (CAP) on Bacterial Reduction in Dental Implants: A Systematic Review. Biomolecules 2023, 13, 1528. https://doi.org/10.3390/biom13101528
Alqutaibi AY, Aljohani A, Alduri A, Masoudi A, Alsaedi AM, Al-Sharani HM, Farghal AE, Alnazzawi AA, Aboalrejal AN, Mohamed A-AH, et al. The Effectiveness of Cold Atmospheric Plasma (CAP) on Bacterial Reduction in Dental Implants: A Systematic Review. Biomolecules. 2023; 13(10):1528. https://doi.org/10.3390/biom13101528
Chicago/Turabian StyleAlqutaibi, Ahmed Yaseen, Abdulbari Aljohani, Abdullah Alduri, Abdulmajid Masoudi, Anas M. Alsaedi, Hesham Mohammed Al-Sharani, Ahmed E. Farghal, Ahmad Abdulkareem Alnazzawi, Afaf Noman Aboalrejal, Abdel-Aleam H. Mohamed, and et al. 2023. "The Effectiveness of Cold Atmospheric Plasma (CAP) on Bacterial Reduction in Dental Implants: A Systematic Review" Biomolecules 13, no. 10: 1528. https://doi.org/10.3390/biom13101528









