Recombinant α1-Microglobulin (rA1M) Protects against Hematopoietic and Renal Toxicity, Alone and in Combination with Amino Acids, in a 177Lu-DOTATATE Mouse Radiation Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Recombinant Human A1M
2.2. Radiopharmaceuticals
2.3. Animal Studies
2.4. Pharmacokinetics
2.5. I.v. and S.c. Comparison
2.6. Dose Escalation
2.7. Biodistribution
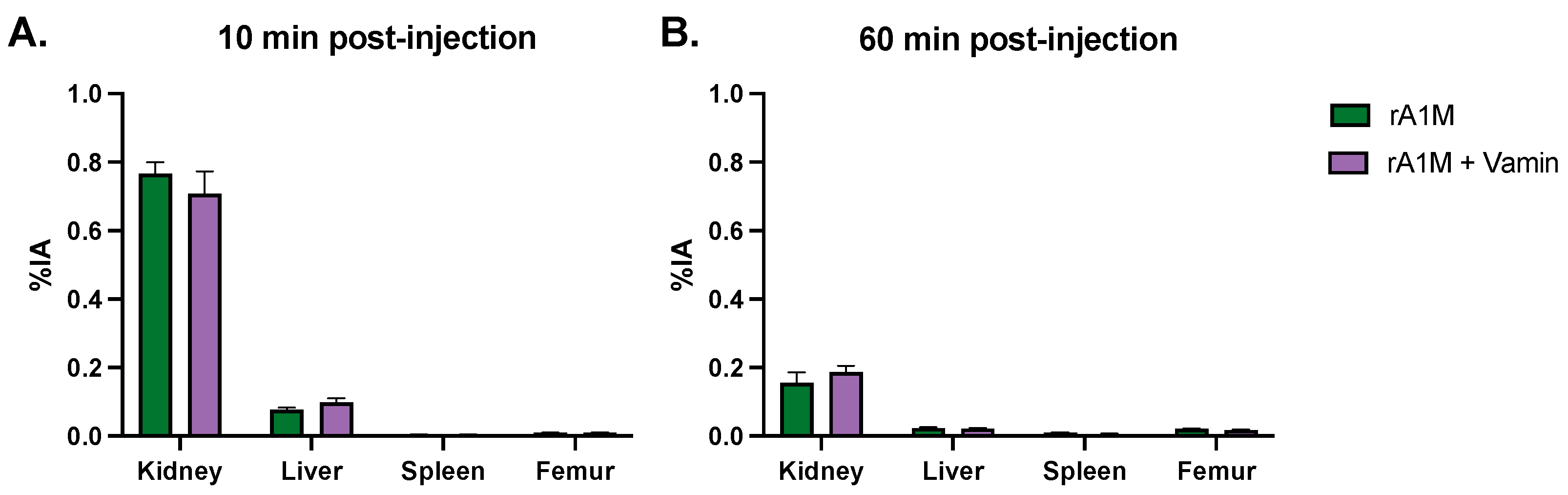
2.8. rA1M and Vamin
2.9. Blood and Bone Marrow Analysis
2.10. Functional Urine Markers
2.11. Plasma Markers
2.12. Statistical Analysis
3. Results
3.1. Pharmacokinetics
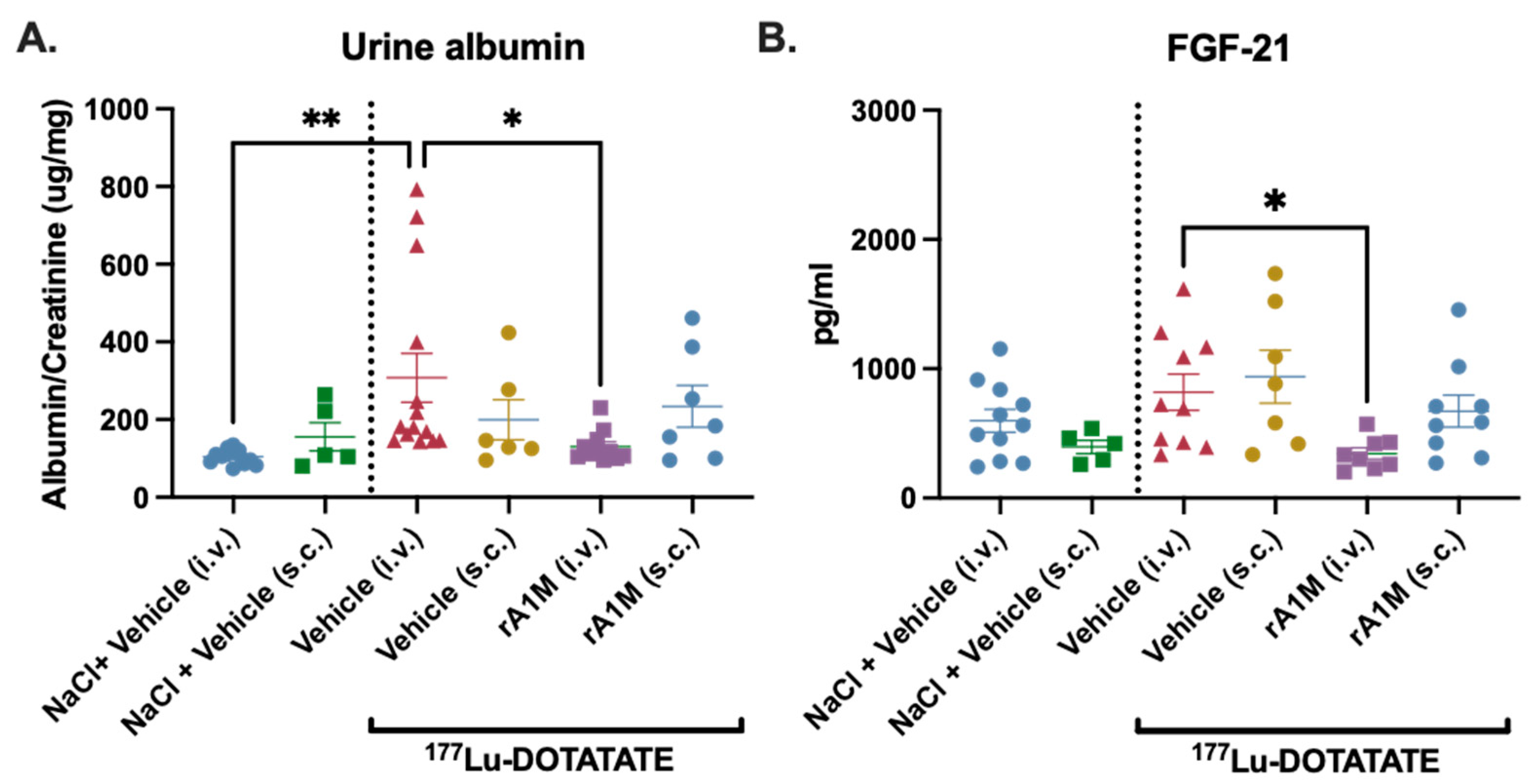
3.2. Comparision of Protective Effects of S.c. vs. I.v. Administered rA1M
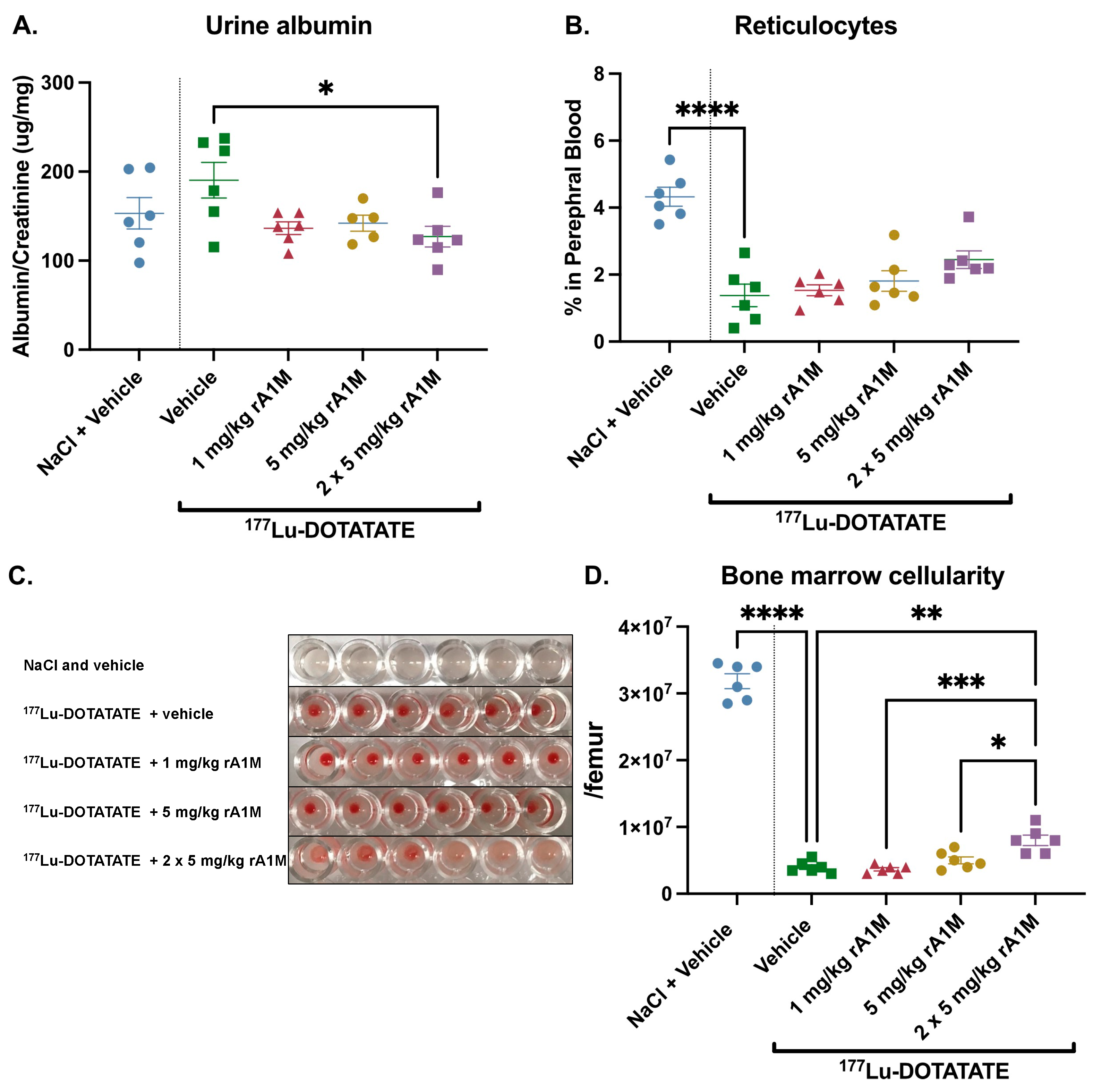
3.3. Dose Escalation of rA1M
3.4. rA1M in Combination with Vamin
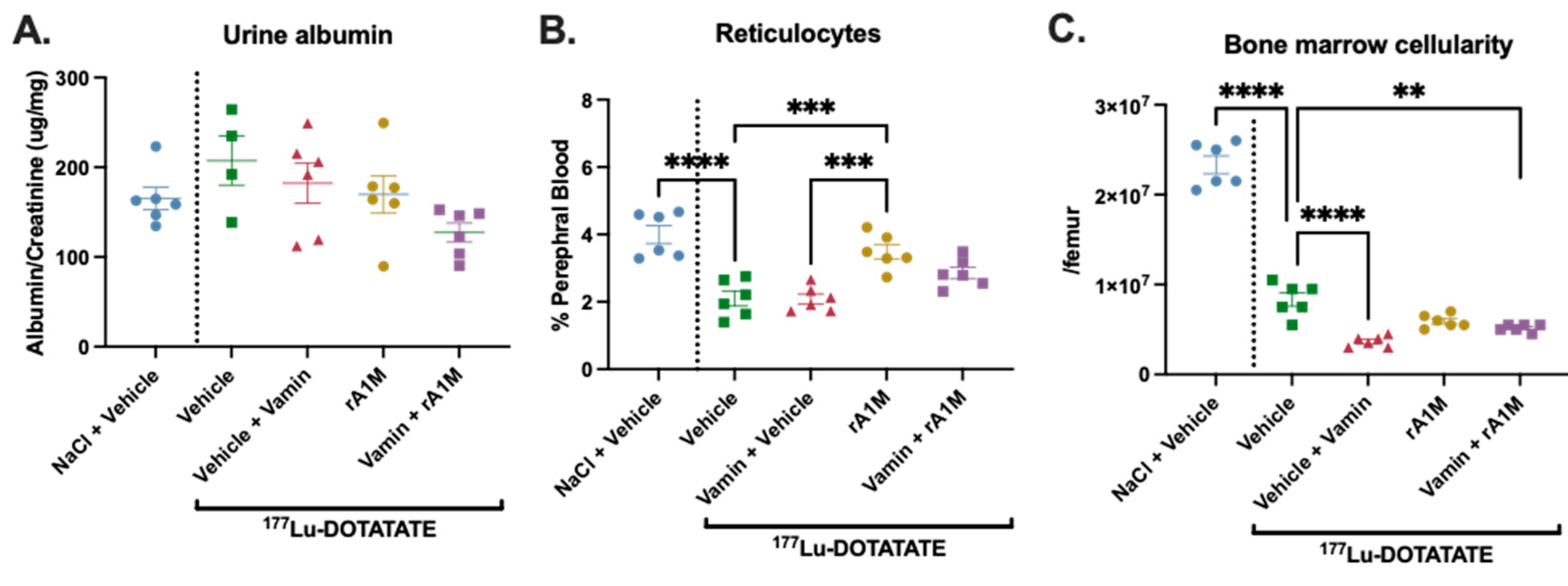
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kulke, M.H.; Shah, M.H.; Benson, A.B.; Bergsland, E.; Berlin, J.D.; Blaszkowsky, L.S.; Emerson, L.; Engstrom, P.F.; Fanta, P.; Giordano, T. Neuroendocrine tumors, version 1.2015. J. Natl. Compr. Cancer Netw. 2015, 13, 78–108. [Google Scholar] [CrossRef] [PubMed]
- Oronsky, B.; Ma, P.C.; Morgensztern, D.; Carter, C.A. Nothing But NET: A Review of Neuroendocrine Tumors and Carcinomas. Neoplasia 2017, 19, 991–1002. [Google Scholar] [CrossRef]
- Kaupp-Roberts, S.; Srirajaskanthan, R.; Ramage, J.K. Symptoms and quality of life in gastroenteropancreatic neuroendocrine tumours. EMJ Oncol. 2015, 3, 34–40. [Google Scholar] [CrossRef]
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients with Neuroendocrine Tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Camus, B.; Cottereau, A.S.; Palmieri, L.J.; Dermine, S.; Tenenbaum, F.; Brezault, C.; Coriat, R. Indications of Peptide Receptor Radionuclide Therapy (PRRT) in Gastroenteropancreatic and Pulmonary Neuroendocrine Tumors: An Updated Review. J. Clin. Med. 2021, 10, 1267. [Google Scholar] [CrossRef] [PubMed]
- Sorbye, H.; Kong, G.; Grozinsky-Glasberg, S. PRRT in high-grade gastroenteropancreatic neuroendocrine neoplasms (WHO G3). Endocr.-Relat. Cancer 2020, 27, R67–R77. [Google Scholar] [CrossRef]
- Reubi, J.C.; Schär, J.-C.; Waser, B.; Wenger, S.; Heppeler, A.; Schmitt, J.S.; Mäcke, H.R. Affinity profiles for human somatostatin receptor subtypes SST1–SST5 of somatostatin radiotracers selected for scintigraphic and radiotherapeutic use. Eur. J. Nucl. Med. 2000, 27, 273–282. [Google Scholar] [CrossRef]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef]
- Kwekkeboom, D.J.; de Herder, W.W.; Kam, B.L.; van Eijck, C.H.; van Essen, M.; Kooij, P.P.; Feelders, R.A.; van Aken, M.O.; Krenning, E.P. Treatment with the radiolabeled somatostatin analog [177Lu-DOTA0, Tyr3] octreotate: Toxicity, efficacy, and survival. J. Clin. Oncol. 2008, 26, 2124–2130. [Google Scholar] [CrossRef]
- Svensson, J.; Berg, G.; Wängberg, B.; Larsson, M.; Forssell-Aronsson, E.; Bernhardt, P. Renal function affects absorbed dose to the kidneys and haematological toxicity during 177Lu-DOTATATE treatment. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 947–955. [Google Scholar] [CrossRef]
- Brabander, T.; Teunissen, J.J.M.; Van Eijck, C.H.J.; Franssen, G.J.H.; Feelders, R.A.; de Herder, W.W.; Kwekkeboom, D.J. Peptide receptor radionuclide therapy of neuroendocrine tumours. Best Pract. Res. Clin. Endocrinol. Metab. 2016, 30, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Bergsma, H.; Konijnenberg, M.W.; Kam, B.L.; Teunissen, J.J.; Kooij, P.P.; de Herder, W.W.; Franssen, G.J.; van Eijck, C.H.; Krenning, E.P.; Kwekkeboom, D.J. Subacute haematotoxicity after PRRT with (177)Lu-DOTA-octreotate: Prognostic factors, incidence and course. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Bergsma, H.; van Lom, K.; Raaijmakers, M.H.; Konijnenberg, M.; Kam, B.B.L.; Teunissen, J.J.; de Herder, W.W.; Krenning, E.P.; Kwekkeboom, D.J. Persistent hematologic dysfunction after peptide receptor radionuclide therapy with 177Lu-DOTATATE: Incidence, course, and predicting factors in patients with gastroenteropancreatic neuroendocrine tumors. J. Nucl. Med. 2018, 59, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Stolniceanu, C.R.; Nistor, I.; Bilha, S.C.; Constantin, V.; Simona, V.; Matovic, M.; Stefanescu, C.; Covic, A. Nephrotoxicity/renal failure after therapy with 90Yttrium- and 177Lutetium-radiolabeled somatostatin analogs in different types of neuroendocrine tumors: A systematic review. Nucl. Med. Commun. 2020, 41, 601–617. [Google Scholar] [CrossRef]
- Bergwik, J.; Kristiansson, A.; Allhorn, M.; Gram, M.; Åkerström, B. Structure, Functions, and Physiological Roles of the Lipocalin α1-Microglobulin (A1M). Front. Physiol. 2021, 12, 251. [Google Scholar] [CrossRef] [PubMed]
- Kristiansson, A.; Ahlstedt, J.; Holmqvist, B.; Brinte, A.; Tran, T.A.; Forssell-Aronsson, E.; Strand, S.E.; Gram, M.; Åkerström, B. Protection of Kidney Function with Human Antioxidation Protein alpha1-Microglobulin in a Mouse (177)Lu-DOTATATE Radiation Therapy Model. Antioxid. Redox Signal. 2019, 30, 1746–1759. [Google Scholar] [CrossRef]
- Andersson, C.K.; Shubbar, E.; Schüler, E.; Åkerström, B.; Gram, M.; Forssell-Aronsson, E.B. Recombinant α1-Microglobulin Is a Potential Kidney Protector in 177Lu-Octreotate Treatment of Neuroendocrine Tumors. J. Nucl. Med. 2019, 60, 1600–1604. [Google Scholar] [CrossRef]
- Kristiansson, A.; Örbom, A.; Ahlstedt, J.; Karlsson, H.; Zedan, W.; Gram, M.; Åkerström, B.; Strand, S.E.; Altai, M.; Strand, J.; et al. (177)Lu-PSMA-617 Therapy in Mice, with or without the Antioxidant α(1)-Microglobulin (A1M), Including Kidney Damage Assessment Using (99m)Tc-MAG3 Imaging. Biomolecules 2021, 11, 263. [Google Scholar] [CrossRef]
- Åkerström, B.; Rosenlöf, L.; Hägerwall, A.; Rutardottir, S.; Ahlstedt, J.; Johansson, M.E.; Erlandsson, L.; Allhorn, M.; Gram, M. rA1M-035, a Physicochemically Improved Human Recombinant α(1)-Microglobulin, Has Therapeutic Effects in Rhabdomyolysis-Induced Acute Kidney Injury. Antioxid. Redox Signal. 2019, 30, 489–504. [Google Scholar] [CrossRef]
- Nääv, Å.; Erlandsson, L.; Axelsson, J.; Larsson, I.; Johansson, M.; Wester-Rosenlöf, L.; Mörgelin, M.; Casslén, V.; Gram, M.; Åkerström, B.; et al. A1M Ameliorates Preeclampsia-Like Symptoms in Placenta and Kidney Induced by Cell-Free Fetal Hemoglobin in Rabbit. PLoS ONE 2015, 10, e0125499. [Google Scholar] [CrossRef]
- Wester-Rosenlöf, L.; Casslén, V.; Axelsson, J.; Edström-Hägerwall, A.; Gram, M.; Holmqvist, M.; Johansson, M.E.; Larsson, I.; Ley, D.; Marsal, K.; et al. A1M/α1-Microglobulin Protects from Heme-Induced Placental and Renal Damage in a Pregnant Sheep Model of Preeclampsia. PLoS ONE 2014, 9, e86353. [Google Scholar] [CrossRef] [PubMed]
- Kristiansson, A.; Gram, M.; Flygare, J.; Hansson, S.R.; Åkerström, B.; Storry, J.R. The Role of α1-Microglobulin (A1M) in Erythropoiesis and Erythrocyte Homeostasis—Therapeutic Opportunities in Hemolytic Conditions. Int. J. Mol. Sci. 2020, 21, 7234. [Google Scholar] [CrossRef]
- Kristiansson, A.; Bergwik, J.; Alattar, A.G.; Flygare, J.; Gram, M.; Hansson, S.R.; Olsson, M.L.; Storry, J.R.; Allhorn, M.; Åkerström, B. Human radical scavenger α1-microglobulin protects against hemolysis in vitro and α1-microglobulin knockout mice exhibit a macrocytic anemia phenotype. Free Radic. Biol. Med. 2021, 162, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Ahlstedt, J.; Tran, T.A.; Strand, F.; Holmqvist, B.; Strand, S.-E.; Gram, M.; Åkerström, B. Biodistribution and pharmacokinetics of recombinant α1-microglobulin and its potential use in radioprotection of kidneys. Am. J. Nucl. Med. Mol. Imaging 2015, 5, 333–347. [Google Scholar]
- Roth, D.; Gustafsson, J.; Warfvinge, C.F.; Sundlöv, A.; Åkesson, A.; Tennvall, J.; Gleisner, K.S. Dosimetric quantities in neuroendocrine tumors over treatment cycles with 177Lu-DOTATATE. J. Nucl. Med. 2022, 63, 399–405. [Google Scholar] [CrossRef]
- Kristiansson, A.; Örbom, A.; Vilhelmsson Timmermand, O.; Ahlstedt, J.; Strand, S.-E.; Åkerström, B. Kidney Protection with the Radical Scavenger α1-Microglobulin (A1M) during Peptide Receptor Radionuclide and Radioligand Therapy. Antioxidants 2021, 10, 1271. [Google Scholar] [CrossRef] [PubMed]
- Åkerström, B.; Gram, M. A1M, an extravascular tissue cleaning and housekeeping protein. Free Radic. Biol. Med. 2014, 74, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Brieau, B.; Hentic, O.; Lebtahi, R.; Palazzo, M.; Ben Reguiga, M.; Rebours, V.; Maire, F.; Hammel, P.; Ruszniewski, P.; Fenaux, P. High risk of myelodysplastic syndrome and acute myeloid leukemia after 177Lu-octreotate PRRT in NET patients heavily pretreated with alkylating chemotherapy. Endocr.-Relat. Cancer 2016, 23, L17–L23. [Google Scholar] [CrossRef]
- Bodei, L.; Kidd, M.; Paganelli, G.; Grana, C.M.; Drozdov, I.; Cremonesi, M.; Lepensky, C.; Kwekkeboom, D.J.; Baum, R.P.; Krenning, E.P.; et al. Long-term tolerability of PRRT in 807 patients with neuroendocrine tumours: The value and limitations of clinical factors. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 5–19. [Google Scholar] [CrossRef]
- Minczeles, N.S.; de Herder, W.W.; Konijnenberg, M.W.; Feelders, R.A.; Brabander, T.; Hofland, J. Dose-Limiting Bone Marrow Toxicities After Peptide Receptor Radionuclide Therapy Are More Prevalent in Women Than in Men. Clin. Nucl. Med. 2022, 47, 599–605. [Google Scholar] [CrossRef]
- Trivigno, D.; Bornes, L.; Huber, S.M.; Rudner, J. Regulation of protein translation initiation in response to ionizing radiation. Radiat. Oncol. 2013, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Sjögren, S.E.; Chen, J.; Mattebo, A.; Alattar, A.G.; Karlsson, H.; Siva, K.; Soneji, S.; Tedgård, U.; Chen, J.J.; Gram, M.; et al. Targeting elevated heme levels to treat a mouse model for Diamond-Blackfan Anemia. Exp. Hematol. 2022, 105, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Olsson, M.G.; Nilsson, E.J.C.; Rutardóttir, S.; Paczesny, J.; Pallon, J.; Åkerström, B. Bystander Cell Death and Stress Response is Inhibited by the Radical Scavenger α1-Microglobulin in Irradiated Cell Cultures. Radiat. Res. 2010, 174, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Sámano, M.; Grajales-Gómez, M.; Zuarth-Vázquez, J.M.; Navarro-Flores, M.F.; Martínez-Saavedra, M.; Juárez-León, Ó.A.; Morales-García, M.G.; Enríquez-Estrada, V.M.; Gómez-Pérez, F.J.; Cuevas-Ramos, D. Fibroblast growth factor 21 and its novel association with oxidative stress. Redox Biol. 2017, 11, 335–341. [Google Scholar] [CrossRef]
- Lin, Z.; Zhou, Z.; Liu, Y.; Gong, Q.; Yan, X.; Xiao, J.; Wang, X.; Lin, S.; Feng, W.; Li, X. Circulating FGF21 Levels Are Progressively Increased from the Early to End Stages of Chronic Kidney Diseases and Are Associated with Renal Function in Chinese. PLoS ONE 2011, 6, e18398. [Google Scholar] [CrossRef]
- Olsson, M.G.; Rosenlof, L.W.; Kotarsky, H.; Olofsson, T.; Leanderson, T.; Morgelin, M.; Fellman, V.; Åkerström, B. The radical-binding lipocalin A1M binds to a Complex I subunit and protects mitochondrial structure and function. Antioxid. Redox Signal. 2013, 18, 2017–2028. [Google Scholar] [CrossRef]
- Kristiansson, A.; Davidsson, S.; Johansson, M.E.; Piel, S.; Elmér, E.; Hansson, M.J.; Åkerström, B.; Gram, M. α1-Microglobulin (A1M) protects human proximal tubule epithelial cells from heme-induced damage in vitro. Int. J. Mol. Sci. 2020, 21, 5825. [Google Scholar] [CrossRef]
- Walrand, S.; Jamar, F. Renal and Red Marrow Dosimetry in Peptide Receptor Radionuclide Therapy: 20 Years of History and Ahead. Int. J. Mol. Sci. 2021, 22, 8326. [Google Scholar] [CrossRef]
- Weiss, R.; Meersch, M.; Wempe, C.; von Groote, T.; Agervald, T.; Zarbock, A. Recombinant Alpha-1-Microglobulin (RMC-035) to Prevent Acute Kidney Injury in Cardiac Surgery Patients: Phase 1b Evaluation of Safety and Pharmacokinetics. Kidney Int. Rep. 2023, 8, 980–988. [Google Scholar] [CrossRef]

| Group | Route of Administration | Dose | AUC (95% CI) | t(max) |
|---|---|---|---|---|
| rA1M s.c. | s.c. | 20 mg/kg | 966.2 (559.1–1373) | 60 min |
| rA1M i.v. | i.v. | 5 mg/kg | 595.1 (458.7–731.6) | 1 min |
| rA1M i.p. | i.p. | 20 mg/kg | 2040 (1809–2272) | 30 min |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alattar, A.G.; Kristiansson, A.; Karlsson, H.; Vallius, S.; Ahlstedt, J.; Forssell-Aronsson, E.; Åkerström, B.; Strand, S.-E.; Flygare, J.; Gram, M. Recombinant α1-Microglobulin (rA1M) Protects against Hematopoietic and Renal Toxicity, Alone and in Combination with Amino Acids, in a 177Lu-DOTATATE Mouse Radiation Model. Biomolecules 2023, 13, 928. https://doi.org/10.3390/biom13060928
Alattar AG, Kristiansson A, Karlsson H, Vallius S, Ahlstedt J, Forssell-Aronsson E, Åkerström B, Strand S-E, Flygare J, Gram M. Recombinant α1-Microglobulin (rA1M) Protects against Hematopoietic and Renal Toxicity, Alone and in Combination with Amino Acids, in a 177Lu-DOTATATE Mouse Radiation Model. Biomolecules. 2023; 13(6):928. https://doi.org/10.3390/biom13060928
Chicago/Turabian StyleAlattar, Abdul Ghani, Amanda Kristiansson, Helena Karlsson, Suvi Vallius, Jonas Ahlstedt, Eva Forssell-Aronsson, Bo Åkerström, Sven-Erik Strand, Johan Flygare, and Magnus Gram. 2023. "Recombinant α1-Microglobulin (rA1M) Protects against Hematopoietic and Renal Toxicity, Alone and in Combination with Amino Acids, in a 177Lu-DOTATATE Mouse Radiation Model" Biomolecules 13, no. 6: 928. https://doi.org/10.3390/biom13060928







