The Molecular Mechanisms of HLA-G Regulatory Function on Immune Cells during Early Pregnancy
Abstract
:1. Introduction
2. The General Expression and Immune-Suppressive Function of HLA-G in Tumors and Autoimmune Diseases
2.1. The General Expression of HLA-G
2.2. The Immune-Suppressive Function of HLA-G in Tumors and Autoimmune Diseases
3. HLA-G and Immune Cells at the Maternal–Fetal Interface
3.1. Immune Cells at the Maternal–Fetal Interface
3.2. HLA-G and dNK Cells
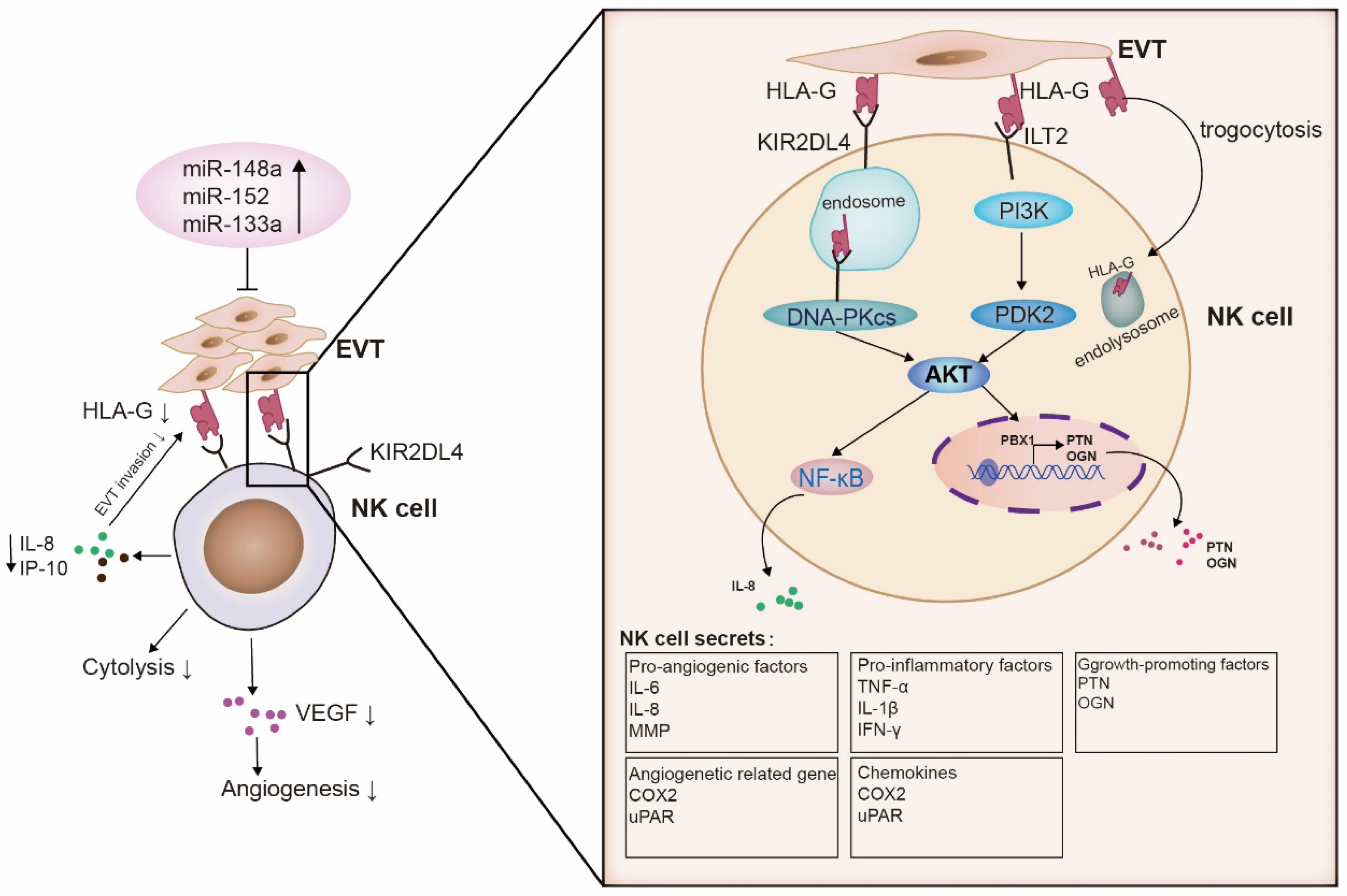
3.2.1. MiRNAs
3.2.2. Growth-Promoting Factors
3.2.3. Senescence Signal
3.2.4. Trogocytosis
3.3. HLA-G and T Cells
3.4. HLA-G and DCs
3.5. HLA-G and Macrophages
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Knofler, M.; Haider, S.; Saleh, L.; Pollheimer, J.; Gamage, T.; James, J. Human placenta and trophoblast development: Key molecular mechanisms and model systems. Cell. Mol. Life Sci. 2019, 76, 3479–3496. [Google Scholar] [PubMed] [Green Version]
- Turco, M.Y.; Gardner, L.; Kay, R.G.; Hamilton, R.S.; Prater, M.; Hollinshead, M.S.; Mcwhinnie, A.; Esposito, L.; Fernando, R.; Skelton, H.; et al. Trophoblast organoids as a model for maternal-fetal interactions during human placentation. Nature 2018, 564, 263–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, L.K.; Benagiano, M.; D’Elios, M.M.; Brosens, I.; Benagiano, G. Placental bed research: II. Functional and immunological investigations of the placental bed. Am. J. Obstet. Gynecol. 2019, 221, 457–469. [Google Scholar]
- Durgam, S.S.; Alegre, M.L.; Chong, A.S. Toward an understanding of allogeneic conflict in pregnancy and transplantation. J. Exp. Med. 2022, 219, e20211493. [Google Scholar] [CrossRef] [PubMed]
- Papuchova, H.; Kshirsagar, S.; Xu, L.; Bougleux Gomes, H.A.; Li, Q.; Iyer, V.; Norwitz, E.R.; Strominger, J.L.; Tilburgs, T. Three types of HLA-G+ extravillous trophoblasts that have distinct immune regulatory properties. Proc. Natl. Acad. Sci. USA 2020, 117, 15772–15777. [Google Scholar] [CrossRef]
- Ellis, S.A.; Sargent, I.L.; Redman, C.W.G.; Mcmichael, A.J. Evidence for a novel HLA antigen found on human extravillous trophoblast and a choriocarcinoma cell line. Immunology 1986, 59, 595–601. [Google Scholar]
- Kovats, S.; Main, E.K.; Librach, C.; Stubblebine, M.; Fisher, S.J.; Demars, R. A class I antigen, HLA, expressed in human trophoblasts. Science 1990, 248, 220–223. [Google Scholar] [CrossRef]
- Piekarska, K.; Radwan, P.; Tarnowska, A.; Wiśniewski, A.; Krasiński, R.; Radwan, M.; Wilczyński, J.R.; Malinowski, A.; Nowak, I. The association of HLA gene polymorphism and its soluble form with male infertility. Front. Immunol. 2021, 12, 791399. [Google Scholar]
- Schallmoser, A.; Raab, M.; Karn, T.; Königsberger, S.; Schmidt, E.; Breitenbach-Koller, H.; Sänger, N. Quantitative analysis of the sHLA-G protein in seminal plasma. Am. J. Reprod. Immunol. 2019, 82, e13152. [Google Scholar] [CrossRef]
- Melo-Lima, B.L.; Poras, I.; Passos, G.A.; Carosella, E.D.; Donadi, E.A.; Moreau, P. The autoimmune regulator (Aire) transactivates HLA-G gene expression in thymic epithelial cells. Immunology 2019, 158, 121–135. [Google Scholar]
- Le Discorde, M.; Moreau, P.; Sabatier, P.; Legeais, J.M.; Carosella, E.D. Expression of HLA-G in human cornea, an immune-privileged tissue. Hum. Immunol. 2003, 64, 1039–1044. [Google Scholar] [CrossRef] [Green Version]
- Lebreton, F.; Hanna, R.; Wassmer, C.H.; Bellofatto, K.; Perez, L.; Othenin-Girard, V.; de Tejada, B.M.; Cohen, M.; Berishvili, E. Mechanisms of immunomodulation and cytoprotection conferred to pancreatic islet by human amniotic epithelial cells. Stem Cell Rev. Rep. 2022, 18, 346–359. [Google Scholar] [CrossRef]
- Xu, Y.F.; Lu, Y.; Cheng, H.; Jiang, J.; Xu, J.; Long, J.; Liu, L.; Ni, Q.; Liu, C.; Yu, X.J. High expression of human leukocyte antigen-G is associated with a poor prognosis in patients with PDAC. Curr. Mol. Med. 2015, 15, 360–367. [Google Scholar] [CrossRef]
- Ullah, M.; Azazzen, D.; Kaci, R.; Benabbou, N.; Pujade, L.E.; Pocard, M.; Mirshahi, M. High expression of HLA-G in ovarian carcinomatosis: The role of interleukin-1β. Neoplasia 2019, 21, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Wang, P.; Dai, P.; Jin, B.; Tong, Y.; Lin, H.; Shi, G. Correlation between human leukocyte antigen-G expression and clinical parameters in oral squamous cell carcinoma. Indian J. Cancer 2018, 55, 340–343. [Google Scholar] [PubMed]
- Grille-Cancela, Z.; Barge-Caballero, E.; Suárez-Fuentetaja, N.; Domenech-García, N.; Paniagua-Martín, M.J.; Barge-Caballero, G.; Couto-Mallón, D.; Enríquez-Vázquez, D.; Blanco-Canosa, P.; Pombo-Otero, J.; et al. Soluble HLA-G levels in heart transplant recipients: Dynamics and correlation with clinical outcomes. Transpl. Immunol. 2023, 76, 101771. [Google Scholar] [CrossRef] [PubMed]
- Sommese, L.; Paolillo, R.; Cacciatore, F.; Grimaldi, V.; Sabia, C.; Esposito, A.; Sorriento, A.; Iannone, C.; Rupealta, N.; Sarno, G.; et al. HLA-G and anti-HCV in patients on the waiting list for kidney transplantation. Adv. Med. Sci. 2018, 63, 317–322. [Google Scholar] [CrossRef]
- Seliger, B.; Jasinski-Bergner, S.; Massa, C.; Mueller, A.; Biehl, K.; Yang, B.; Bachmann, M.; Jonigk, D.; Eichhorn, P.; Hartmann, A.; et al. Induction of pulmonary HLA-G expression by SARS-CoV-2 infection. Cell. Mol. Life Sci. 2022, 79, 582. [Google Scholar] [CrossRef]
- Zare, M.; Namavar, J.B.; Gharesi-Fard, B. Analysis of the frequencies and functions of CD4(+)CD25(+)CD127(low/neg), CD4(+)HLA-G (+), and CD8(+)HLA-G (+) regulatory T cells in pre-eclampsia. J. Reprod. Immunol. 2019, 133, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Pankratz, S.; Bittner, S.; Herrmann, A.M.; Schuhmann, M.K.; Ruck, T.; Meuth, S.G.; Wiendl, H. Human CD4+ HLA-G + regulatory t cells are potent suppressors of graft-versus-host disease in vivo. FASEB J. 2014, 28, 3435–3445. [Google Scholar] [CrossRef]
- Ostapchuk, Y.O.; Cetin, E.A.; Perfilyeva, Y.V.; Yilmaz, A.; Skiba, Y.A.; Chirkin, A.P.; Omarbaeva, N.A.; Talaeva, S.G.; Belyaev, N.N.; Deniz, G. Peripheral blood NK cells expressing HLA-G, IL-10 and TGF-β in healthy donors and breast cancer patients. Cell. Immunol. 2015, 298, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Lozano, J.M.; González, R.; Kindelán, J.M.; Rouas-Freiss, N.; Caballos, R.; Dausset, J.; Carosella, E.D.; Peña, J. Monocytes and T lymphocytes in HIV-1-positive patients express HLA-G molecule. AIDS 2002, 16, 347–351. [Google Scholar] [CrossRef]
- Huang, J.; Burke, P.; Yang, Y.; Seiss, K.; Beamon, J.; Cung, T.; Toth, I.; Pereyra, F.; Lichterfeld, M.; Yu, X.G. Soluble HLA-G inhibits myeloid dendritic cell function in HIV-1 infection by interacting with leukocyte immunoglobulin-like receptor b2. J. Virol. 2010, 84, 10784–10791. [Google Scholar] [CrossRef] [Green Version]
- Ostapchuk, E.O.; Perfi, L.Y.; Talaeva, S.; Omarbaeva, N.A.; Belyaev, N.N. Content of HLA-G (+) T cells in the peripheral blood from healthy women and breast cancer patients. Bull. Exp. Biol. Med. 2015, 159, 649–651. [Google Scholar] [CrossRef]
- Lu, N.; Zhang, Y.; Zou, X.; Yang, X.; Tian, J.; Zhen, J.; Zhou, Y.; Zhao, S.; Shi, W. HLA-G on peripheral blood CD4(+) T lymphocytes: A potential predictor for acute renal rejection. Transpl. Int. 2011, 24, 1103–1111. [Google Scholar] [CrossRef]
- Castellaneta, A.; Mazariegos, G.V.; Nayyar, N.; Zeevi, A.; Thomson, A.W. HLA-G level on monocytoid dendritic cells correlates with regulatory T-cell Foxp3 expression in liver transplant tolerance. Transplantation 2011, 91, 1132–1140. [Google Scholar] [CrossRef] [Green Version]
- Eikmans, M.; van der Keur, C.; Anholts, J.; Drabbels, J.; van Beelen, E.; de Sousa, L.S.; van der Hoorn, M.L. Primary trophoblast cultures: Characterization of HLA profiles and immune cell interactions. Front. Immunol. 2022, 13, 814019. [Google Scholar] [CrossRef]
- Gallegos, C.E.; Michelin, S.; Trasci, S.B.; Lobos, E.A.; Dubner, D.; Carosella, E.D. HLA-G1 increases the radiosensitivity of human tumoral cells. Cell. Immunol. 2014, 287, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Lemaoult, J.; Krawice-Radanne, I.; Dausset, J.; Carosella, E.D. HLA-G1-expressing antigen-presenting cells induce immunosuppressive CD4+ T cells. Proc. Natl. Acad. Sci. USA 2004, 101, 7064–7069. [Google Scholar] [CrossRef] [PubMed]
- Hunt, J.S.; Morales, P.J.; Pace, J.L.; Fazleabas, A.T.; Langat, D.K. A commentary on gestational programming and functions of HLA-G in pregnancy. Placenta 2007, 28 (Suppl. A), S57–S63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rouas-Freiss, N.; Moreau, P.; Ferrone, S.; Carosella, E.D. HLA-G proteins in cancer: Do they provide tumor cells with an escape mechanism? Cancer Res. 2005, 65, 10139–10144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouji-Sageshima, N.; Geraghty, D.E.; Ishitani, A.; Hatake, K.; Ito, T. Establishment of optimized ELISA system specific for HLA-G in body fluids. HLA 2016, 88, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.; Kook, H.; Kang, S.; Lee, J. Study of immune-tolerized cell lines and extracellular vesicles inductive environment promoting continuous expression and secretion of HLA-G from semiallograft immune tolerance during pregnancy. J. Extracell. Vesicles 2020, 9, 1795364. [Google Scholar] [CrossRef] [PubMed]
- Díaz, R.R.; Zamora, R.B.; Sánchez, R.V.; Pérez, J.G.; Bethencourt, J. Embryo sHLA-G secretion is related to pregnancy rate. Zygote 2019, 27, 78–81. [Google Scholar] [CrossRef]
- Giacomini, E.; Vago, R.; Sanchez, A.M.; Podini, P.; Zarovni, N.; Murdica, V.; Rizzo, R.; Bortolotti, D.; Candiani, M.; Viganò, P. Secretome of in vitro cultured human embryos contains extracellular vesicles that are uptaken by the maternal side. Sci. Rep. 2017, 7, 5210. [Google Scholar] [CrossRef]
- Lynge Nilsson, L.; Djurisic, S.; Hviid, T.V.F. Controlling the immunological crosstalk during conception and pregnancy: HLA-G in reproduction. Front. Immunol. 2014, 5, 198. [Google Scholar]
- Lila, N.; Amrein, C.; Guillemain, R.; Chevalier, P.; Latremouille, C.; Fabiani, J.N.; Dausset, J.; Carosella, E.D.; Carpentier, A. Human leukocyte antigen-G expression after heart transplantation is associated with a reduced incidence of rejection. Circulation 2002, 105, 1949–1954. [Google Scholar] [CrossRef] [Green Version]
- Paul, P.; Cabestre, F.A.; Ibrahim, E.C.; Lefebvre, S.; Carosella, E.D. Identification of HLA-G7 as a new splice variant of the HLA-G mRNA and expression of soluble HLA-G5, -G6, and -G7 transcripts in human transfected cells. Hum. Immunol. 2000, 61, 1138–1149. [Google Scholar] [CrossRef]
- Abediankenari, S.; Farzad, F.; Rahmani, Z.; Hashemi-Soteh, M.B. HLA-G5 and G7 isoforms in pregnant women. Iran. J. Allergy Asthma Immunol. 2015, 14, 217–221. [Google Scholar]
- Zhuang, B.; Shang, J.; Yao, Y. HLA-G: An important mediator of maternal-fetal immune-tolerance. Front. Immunol. 2021, 12, 744324. [Google Scholar]
- Radwan, P.; Tarnowska, A.; Piekarska, K.; Wiśniewski, A.; Krasiński, R.; Radwan, M.; Nowak, I. The impact of soluble HLA-G in IVF/ICSI embryo culture medium on implantation success. Front. Immunol. 2022, 13, 982518. [Google Scholar] [CrossRef]
- Feger, U.; Tolosa, E.; Huang, Y.H.; Waschbisch, A.; Biedermann, T.; Melms, A.; Wiendl, H. HLA-G expression defines a novel regulatory T-cell subset present in human peripheral blood and sites of inflammation. Blood 2007, 110, 568–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alegre, E.; Diaz-Lagares, A.; Lemaoult, J.; Lopez-Moratalla, N.; Carosella, E.D.; Gonzalez, A. Maternal antigen presenting cells are a source of plasmatic HLA-G during pregnancy: Longitudinal study during pregnancy. Hum. Immunol. 2007, 68, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Klitkou, L.; Dahl, M.; Hviid, T.V.; Djurisic, S.; Piosik, Z.M.; Skovbo, P.; Moller, A.M.; Steffensen, R.; Christiansen, O.B. Human leukocyte antigen (HLA)-G during pregnancy part I: Correlations between maternal soluble HLA-G at midterm, at term, and umbilical cord blood soluble HLA-G at term. Hum. Immunol. 2015, 76, 254–259. [Google Scholar] [CrossRef]
- Rizzo, R.; Fuzzi, B.; Stignani, M.; Criscuoli, L.; Melchiorri, L.; Dabizzi, S.; Campioni, D.; Lanza, F.; Marzola, A.; Branconi, F.; et al. Soluble HLA-G molecules in follicular fluid: A tool for oocyte selection in IVF? J. Reprod. Immunol. 2007, 74, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Ouji-Sageshima, N.; Yuui, K.; Nakanishi, M.; Takeda, N.; Odawara, Y.; Yamashita, M.; Iwayama, H.; Awai, K.; Hashimoto, H.; Geraghty, D.E.; et al. sHLA-G and s HLA-I levels in follicular fluid are not associated with successful implantation. J. Reprod. Immunol. 2016, 113, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Morandi, F.; Rizzo, R.; Fainardi, E.; Rouas-Freiss, N.; Pistoia, V.; Berzins, S. Recent advances in our understanding of HLA-G biology: Lessons from a wide spectrum of human diseases. J. Immunol. Res. 2016, 2016, 4326495. [Google Scholar] [CrossRef] [Green Version]
- Paul, P.; Rouas-Freiss, N.; Khalil-Daher, I.; Moreau, P.; Riteau, B.; Le Gal, F.A.; Avril, M.F.; Dausset, J.; Guillet, J.G.; Carosella, E.D. HLA-G expression in melanoma: A way for tumor cells to escape from immunosurveillance. Proc. Natl. Acad. Sci. USA 1998, 95, 4510–4515. [Google Scholar] [CrossRef]
- Wang, C.; Chen, J.; Zhang, Q.; Li, W.; Zhang, S.; Xu, Y.; Wang, F.; Zhang, B.; Zhang, Y.; Gao, W.Q. Elimination of CD4(low) HLA-G (+) T cells overcomes castration-resistance in prostate cancer therapy. Cell Res. 2018, 28, 1103–1117. [Google Scholar] [CrossRef] [Green Version]
- Fahim, N.M.; Shehata, I.H.; Taha, S.E.; Fahmy, R.A.; Elsayed, M.S. Human leukocyte antigen-G (HLA-G) expression in precancerous and cancerous cervical lesions: Association with human papilloma virus infection and host immune response. Egypt. J. Immunol. 2018, 25, 125–134. [Google Scholar]
- Liu, L.; Wang, L.; Zhao, L.; He, C.; Wang, G. The role of HLA-G in tumor escape: Manipulating the phenotype and function of immune cells. Front. Oncol. 2020, 10, 597468. [Google Scholar] [CrossRef] [PubMed]
- Chervonsky, A.V. Influence of microbial environment on autoimmunity. Nat. Immunol. 2010, 11, 28–35. [Google Scholar] [PubMed]
- Mckinney, E.F.; Cuthbertson, I.; Harris, K.M.; Smilek, D.E.; Connor, C.; Manferrari, G.; Carr, E.J.; Zamvil, S.S.; Smith, K. A CD8(+) NK cell transcriptomic signature associated with clinical outcome in relapsing remitting multiple sclerosis. Nat. Commun. 2021, 12, 635. [Google Scholar] [CrossRef]
- Fainardi, E.; Bortolotti, D.; Bolzani, S.; Castellazzi, M.; Tamborino, C.; Roversi, G.; Baldi, E.; Caniatti, M.L.; Casetta, I.; Gentili, V.; et al. Cerebrospinal fluid amounts of HLA-G in dimeric form are strongly associated to patients with MRI inactive multiple sclerosis. Mult. Scler. J. 2016, 22, 245–249. [Google Scholar] [CrossRef]
- Fainardi, E.; Rizzo, R.; Castellazzi, M.; Stignani, M.; Granieri, E.; Baricordi, O.R. Potential role of soluble human leukocyte antigen-G molecules in multiple sclerosis. Hum. Immunol. 2009, 70, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Contini, P.; Murdaca, G.; Puppo, F.; Negrini, S. HLA-G expressing immune cells in immune mediated diseases. Front. Immunol. 2020, 11, 1613. [Google Scholar] [CrossRef]
- Anna, F.; Bole-Richard, E.; Lemaoult, J.; Escande, M.; Lecomte, M.; Certoux, J.; Souque, P.; Garnache, F.; Adotevi, O.; Langlade-Demoyen, P.; et al. First immunotherapeutic CAR-T cells against the immune checkpoint protein HLA-G. J. Immunother. Cancer 2021, 9, e1998. [Google Scholar] [CrossRef]
- Liu, H.; Huang, X.; Mor, G.; Liao, A. Epigenetic modifications working in the decidualization and endometrial receptivity. Cell Mol. Life Sci. 2020, 77, 2091–2101. [Google Scholar] [CrossRef]
- Toth, B.; Vomstein, K.; Togawa, R.; Böttcher, B.; Hudalla, H.; Strowitzki, T.; Daniel, V.; Kuon, R.J. The impact of previous live births on peripheral and uterine natural killer cells in patients with recurrent miscarriage. Reprod. Biol. Endocrinol. 2019, 17, 72. [Google Scholar] [CrossRef] [Green Version]
- Santos, L.C.; de Souza, C.A.; Silva, J.F.; Ocarino, N.M.; Serakides, R. Maternal hyperthyroidism alters the immunological mediators profile and population of natural killers cells in decidua of rats. Acta Histochem. 2023, 125, 152026. [Google Scholar] [CrossRef]
- Lysakova-Devine, T.; O’Farrelly, C. Tissue-specific NK cell populations and their origin. J. Leukoc. Biol. 2014, 96, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Del, Z.G.; Marcenaro, E.; Vacca, P.; Sivori, S.; Pende, D.; Della, C.M.; Moretta, F.; Ingegnere, T.; Mingari, M.C.; Moretta, A.; et al. Markers and function of human NK cells in normal and pathological conditions. Cytom. Part B-Clin. Cytom. 2017, 92, 100–114. [Google Scholar]
- Montaldo, E.; Del, Z.G.; Della, C.M.; Mingari, M.C.; Moretta, A.; De Maria, A.; Moretta, L. Human NK cell receptors/markers: A tool to analyze NK cell development, subsets and function. Cytom. Part A 2013, 83, 702–713. [Google Scholar]
- Zhang, J.; Lye, S.J. The immune potential of decidua-resident CD16(+) CD56(+) NK cells in human pregnancy. Hum. Immunol. 2021, 82, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Mei, C.; Yang, W.; Wei, X.; Wu, K.; Huang, D. The unique microbiome and innate immunity during pregnancy. Front. Immunol. 2019, 10, 2886. [Google Scholar] [CrossRef] [PubMed]
- Salvany-Celades, M.; van der Zwan, A.; Benner, M.; Setrajcic-Dragos, V.; Bougleux Gomes, H.A.; Iyer, V.; Norwitz, E.R.; Strominger, J.L.; Tilburgs, T. Three types of functional regulatory T cells control T cell responses at the human maternal-fetal interface. Cell Rep. 2019, 27, 2537–2547. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Lin, Y.; Li, Y.; Zhao, D.; Du, M. Mesenchymal stem cells enhance Treg immunosuppressive function at the fetal-maternal interface. J. Reprod. Immunol. 2021, 148, 103366. [Google Scholar] [CrossRef]
- Gomez-Lopez, N.; Garcia-Flores, V.; Chin, P.Y.; Groome, H.M.; Bijland, M.T.; Diener, K.R.; Romero, R.; Robertson, S.A. Macrophages exert homeostatic actions in pregnancy to protect against preterm birth and fetal inflammatory injury. JCI Insight 2021, 6, e146089. [Google Scholar] [CrossRef]
- Thomas, J.R.; Appios, A.; Zhao, X.; Dutkiewicz, R.; Donde, M.; Lee, C.; Naidu, P.; Lee, C.; Cerveira, J.; Liu, B.; et al. Phenotypic and functional characterization of first-trimester human placental macrophages, hofbauer cells. J. Exp. Med. 2021, 218, e20200891. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, D.; Xu, L.; Dong, L.; Zheng, J.; Lin, Y.; Huang, J.; Zhang, Y.; Tao, Y.; Zang, X.; et al. Cell-cell contact with proinflammatory macrophages enhances the immunotherapeutic effect of mesenchymal stem cells in two abortion models. Cell. Mol. Immunol. 2019, 16, 908–920. [Google Scholar] [CrossRef]
- Co, E.C.; Gormley, M.; Kapidzic, M.; Rosen, D.B.; Scott, M.A.; Stolp, H.A.; Mcmaster, M.; Lanier, L.L.; Bárcena, A.; Fisher, S.J. Maternal decidual macrophages inhibit NK cell killing of invasive cytotrophoblasts during human pregnancy. Biol. Reprod. 2013, 88, 155. [Google Scholar] [CrossRef]
- Gori, S.; Soczewski, E.; Fernández, L.; Grasso, E.; Gallino, L.; Merech, F.; Colado, A.; Borge, M.; Pérez, L.C.; Salamone, G.; et al. Decidualization process induces maternal monocytes to tolerogenic IL-10-producing dendritic cells (DC-10). Front. Immunol. 2020, 11, 1571. [Google Scholar] [CrossRef]
- Yang, H.L.; Lai, Z.Z.; Shi, J.W.; Zhou, W.J.; Mei, J.; Ye, J.F.; Zhang, T.; Wang, J.; Zhao, J.Y.; Li, D.J.; et al. A defective lysophosphatidic acid-autophagy axis increases miscarriage risk by restricting decidual macrophage residence. Autophagy 2022, 18, 2459–2480. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zheng, Q.; Jin, L. Dynamic function and composition changes of immune cells during normal and pathological pregnancy at the maternal-fetal interface. Front. Immunol. 2019, 10, 2317. [Google Scholar] [PubMed] [Green Version]
- Tarca, A.L.; Romero, R.; Xu, Z.; Gomez-Lopez, N.; Erez, O.; Hsu, C.D.; Hassan, S.S.; Carey, V.J. Targeted expression profiling by RNA-seq improves detection of cellular dynamics during pregnancy and identifies a role for t cells in term parturition. Sci. Rep. 2019, 9, 848. [Google Scholar] [PubMed] [Green Version]
- Pique-Regi, R.; Romero, R.; Tarca, A.L.; Sendler, E.D.; Xu, Y.; Garcia-Flores, V.; Leng, Y.; Luca, F.; Hassan, S.S.; Gomez-Lopez, N. Single cell transcriptional signatures of the human placenta in term and preterm parturition. Elife 2019, 8, e52004. [Google Scholar] [CrossRef] [PubMed]
- Arenas-Hernandez, M.; Romero, R.; Xu, Y.; Panaitescu, B.; Garcia-Flores, V.; Miller, D.; Ahn, H.; Done, B.; Hassan, S.S.; Hsu, C.D.; et al. Effector and activated T cells induce preterm labor and birth that is prevented by treatment with progesterone. J. Immunol. 2019, 202, 2585–2608. [Google Scholar]
- Lemaoult, J.; Zafaranloo, K.; Le Danff, C.; Carosella, E.D. HLA-G up-regulates ILT2, ILT3, ILT4, and KIR2DL4 in antigen presenting cells, NK cells, and T cells. FASEB J. 2005, 19, 662–664. [Google Scholar] [CrossRef]
- Rajagopalan, S.; Long, E.O. KIR2DL4 (CD158d): An activation receptor for HLA-G. Front. Immunol. 2012, 3, 258. [Google Scholar] [CrossRef] [Green Version]
- Sim, M.; Stotz, Z.; Lu, J.; Brennan, P.; Long, E.O.; Sun, P.D. T cells discriminate between groups C1 and C2 HLA-C. Elife 2022, 11, e75670. [Google Scholar] [CrossRef]
- Xiong, S.; Sharkey, A.M.; Kennedy, P.R.; Gardner, L.; Farrell, L.E.; Chazara, O.; Bauer, J.; Hiby, S.E.; Colucci, F.; Moffett, A. Maternal uterine NK cell-activating receptor KIR2DS1 enhances placentation. J. Clin. Investig. 2013, 123, 4264–4272. [Google Scholar] [CrossRef]
- Moffett, A.; Chazara, O.; Colucci, F.; Johnson, M.H. Variation of maternal KIR and fetal HLA-C genes in reproductive failure: Too early for clinical intervention. Reprod. Biomed. Online 2016, 33, 763–769. [Google Scholar] [PubMed] [Green Version]
- Tilburgs, T.; Meissner, T.B.; Ferreira, L.; Mulder, A.; Musunuru, K.; Ye, J.; Strominger, J.L. NLRP2 is a suppressor of NF-ƙB signaling and HLA-C expression in human trophoblasts. Biol. Reprod. 2017, 96, 831–842. [Google Scholar] [CrossRef] [Green Version]
- Hanna, J.; Goldman-Wohl, D.; Hamani, Y.; Avraham, I.; Greenfield, C.; Natanson-Yaron, S.; Prus, D.; Cohen-Daniel, L.; Arnon, T.I.; Manaster, I.; et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat. Med. 2006, 12, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Tilburgs, T.; Evans, J.H.; Crespo, Â.C.; Strominger, J.L. The HLA-G cycle provides for both NK tolerance and immunity at the maternal–fetal interface. Proc. Natl. Acad. Sci. USA 2015, 112, 13312. [Google Scholar] [CrossRef] [PubMed]
- Menez, S.; Moledina, D.G.; Garg, A.X.; Thiessen-Philbrook, H.; Mcarthur, E.; Jia, Y.; Liu, C.; Obeid, W.; Mansour, S.G.; Koyner, J.L.; et al. Results from the TRIBE-AKI study found associations between post-operative blood biomarkers and risk of chronic kidney disease after cardiac surgery. Kidney Int. 2021, 99, 716–724. [Google Scholar] [CrossRef]
- Favier, B.; Lemaoult, J.; Lesport, E.; Carosella, E.D. ILT2/HLA-G interaction impairs NK-cell functions through the inhibition of the late but not the early events of the NK-cell activating synapse. FASEB J. 2010, 24, 689–699. [Google Scholar] [CrossRef]
- Naji, A.; Durrbach, A.; Carosella, E.D.; Rouas-Freiss, N. Soluble HLA-G and HLA-G1 expressing antigen-presenting cells inhibit t-cell alloproliferation through ILT-2/ILT-4/FasL-mediated pathways. Hum. Immunol. 2007, 68, 233–239. [Google Scholar] [CrossRef]
- Naji, A.; Menier, C.; Morandi, F.; Agaugué, S.; Maki, G.; Ferretti, E.; Bruel, S.; Pistoia, V.; Carosella, E.D.; Rouas-Freiss, N. Binding of HLA-G to ITIM-bearing Ig-like transcript 2 receptor suppresses B cell responses. J. Immunol. 2014, 192, 1536–1546. [Google Scholar] [CrossRef] [Green Version]
- Liang, S.; Ristich, V.; Arase, H.; Dausset, J.; Carosella, E.D.; Horuzsko, A. Modulation of dendritic cell differentiation by HLA-G and ILT4 requires the IL-6—STAT3 signaling pathway. Proc. Natl. Acad. Sci. USA 2008, 105, 8357–8362. [Google Scholar] [CrossRef]
- Rajagopalan, S.; Moyle, M.W.; Joosten, I.; Long, E.O. DNA-PKcs controls an endosomal signaling pathway for a proinflammatory response by natural killer cells. Sci. Signal. 2010, 3, ra14. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Wang, Y.; Feng, M. Human leukocyte antigen–G1 inhibits natural killer cytotoxicity through blocking the activating signal transduction pathway and formation of activating immunologic synapse. Hum. Immunol. 2008, 69, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Lindaman, A.; Dowden, A.; Zavazava, N. Soluble HLA-G molecules induce apoptosis in natural killer cells. Am. J. Reprod. Immunol. 2006, 56, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Ketroussi, F.; Giuliani, M.; Bahri, R.; Azzarone, B.; Charpentier, B.; Durrbach, A. Lymphocyte cell-cycle inhibition by HLA-G is mediated by phosphatase SHP-2 and acts on the mtor pathway. PLoS ONE 2011, 6, e22776. [Google Scholar] [CrossRef]
- Amodio, G.; Mugione, A.; Sanchez, A.M.; Viganò, P.; Candiani, M.; Somigliana, E.; Roncarolo, M.G.; Panina-Bordignon, P.; Gregori, S. HLA-G expressing DC-10 and CD4(+) T cells accumulate in human decidua during pregnancy. Hum. Immunol. 2013, 74, 406–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Djurisic, S.; Skibsted, L.; Hviid, T.V. A phenotypic analysis of regulatory T cells and uterine NK cells from first trimester pregnancies and associations with HLA-G. Am. J. Reprod. Immunol. 2015, 74, 427–444. [Google Scholar] [CrossRef]
- Prašnikar, E.; Perdih, A.; Borišek, J. What a difference an amino acid makes: An all-atom simulation study of nonameric peptides in inhibitory HLA-E/NKG2A/CD94 immune complexes. Front. Pharmacol. 2022, 13, 925427. [Google Scholar] [CrossRef]
- Guo, W.; Fang, L.; Li, B.; Xiao, X.; Chen, S.; Wang, J.; Yang, F.; Chen, L.; Wang, X. Decreased human leukocyte antigen-G expression by miR-133a contributes to impairment of proinvasion and proangiogenesis functions of decidual NK cells. Front. Immunol. 2017, 8, 741. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Han, T.; Wang, X.; Li, Y.; Yang, H.; Luo, Y.; Yin, G.; Yao, Y. Overexpression of miR-152 leads to reduced expression of human leukocyte antigen-G and increased natural killer cell mediated cytolysis in JEG-3 cells. Am. J. Obstet. Gynecol. 2010, 202, 591–592. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, L.; Ding, Y. Human leukocyte antigen G and miR-148a are associated with the pathogenesis of intrahepatic cholestasis of pregnancy. Exp. Ther. Med. 2014, 8, 1701–1706. [Google Scholar] [CrossRef] [Green Version]
- Kaminski, V.; Ellwanger, J.H.; Chies, J. Down-regulation of HLA-G gene expression as an immunogenetic contraceptive therapy. Med. Hypotheses 2017, 102, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Ball, M.; Carmody, M.; Wynne, F.; Dockery, P.; Aigner, A.; Cameron, I.; Higgins, J.; Smith, S.D.; Aplin, J.D.; Moore, T. Expression of pleiotrophin and its receptors in human placenta suggests roles in trophoblast life cycle and angiogenesis. Placenta 2009, 30, 649–653. [Google Scholar] [CrossRef]
- Jee, Y.H.; Lebenthal, Y.; Chaemsaithong, P.; Yan, G.; Peran, I.; Wellstein, A.; Romero, R.; Baron, J. Midkine and pleiotrophin concentrations in amniotic fluid in healthy and complicated pregnancies. PLoS ONE 2016, 11, e153325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nulali, J.; Zhan, M.; Zhang, K.; Tu, P.; Liu, Y.; Song, H. Osteoglycin: An ECM factor regulating fibrosis and tumorigenesis. Biomolecules 2022, 12, 1674. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Fu, B.; Xu, X.; Zhang, J.; Tong, X.; Wang, Y.; Dong, Z.; Zhang, X.; Shen, N.; Zhai, Y.; et al. PBX1 expression in uterine natural killer cells drives fetal growth. Sci. Transl. Med. 2020, 12, eaax1798. [Google Scholar] [CrossRef]
- An, X.; Qin, J.; Hu, X.; Zhou, Y.; Fu, B.; Wei, H. Overexpression of lipocalin 2 in PBX1-deficient decidual NK cells promotes inflammation at the maternal-fetal interface. Am. J. Reprod. Immunol. 2023, 89, e13676. [Google Scholar] [CrossRef]
- Ni, X.; Fu, B.; Zhang, J.; Sun, R.; Tian, Z.; Wei, H. Cytokine-based generation of CD49a(+)eomes(-/+) natural killer cell subsets. Front. Immunol. 2018, 9, 2126. [Google Scholar] [CrossRef]
- Fu, B.; Zhou, Y.; Ni, X.; Tong, X.; Xu, X.; Dong, Z.; Sun, R.; Tian, Z.; Wei, H. Natural killer cells promote fetal development through the secretion of growth-promoting factors. Immunity 2017, 47, 1100–1113. [Google Scholar] [CrossRef] [Green Version]
- Du, X.; Zhu, H.; Jiao, D.; Nian, Z.; Zhang, J.; Zhou, Y.; Zheng, X.; Tong, X.; Wei, H.; Fu, B. Human-induced CD49a(+) NK cells promote fetal growth. Front. Immunol. 2022, 13, 821542. [Google Scholar] [CrossRef]
- Saul, D.; Kosinsky, R.L.; Atkinson, E.J.; Doolittle, M.L.; Zhang, X.; Lebrasseur, N.K.; Pignolo, R.J.; Robbins, P.D.; Niedernhofer, L.J.; Ikeno, Y.; et al. A new gene set identifies senescent cells and predicts senescence-associated pathways across tissues. Nat. Commun. 2022, 13, 4827. [Google Scholar] [CrossRef]
- Kawagoe, Y.; Kawashima, I.; Sato, Y.; Okamoto, N.; Matsubara, K.; Kawamura, K. CXCL5-CXCR2 signaling is a senescence-associated secretory phenotype in preimplantation embryos. Aging Cell 2020, 19, e13240. [Google Scholar] [CrossRef]
- Rajagopalan, S.; Long, E.O. Cellular senescence induced by CD158d reprograms natural killer cells to promote vascular remodeling. Proc. Natl. Acad. Sci. USA 2012, 109, 20596–20601. [Google Scholar] [CrossRef]
- Rajagopalan, S.; Lee, E.C.; Duprie, M.L.; Long, E.O. TNFR-associated factor 6 and TGF-β-activated kinase 1 control signals for a senescence response by an endosomal NK cell receptor. J. Immunol. 2014, 192, 714–721. [Google Scholar] [CrossRef] [Green Version]
- Pollheimer, J.; Vondra, S.; Baltayeva, J.; Beristain, A.G.; Knofler, M. Regulation of placental extravillous trophoblasts by the maternal uterine environment. Front. Immunol. 2018, 9, 2597. [Google Scholar]
- Shukla, V.; Kaushal, J.B.; Sankhwar, P.; Manohar, M.; Dwivedi, A. Inhibition of TPPP3 attenuates β-catenin/NF-κB/COX-2 signaling in endometrial stromal cells and impairs decidualization. J. Endocrinol. 2019, 240, 417–429. [Google Scholar]
- Li, X.; Ballantyne, L.L.; Crawford, M.C.; Fitzgerald, G.A.; Funk, C.D. Isoform-specific compensation of cyclooxygenase (Ptgs) genes during implantation and late-stage pregnancy. Sci. Rep. 2018, 8, 12097. [Google Scholar] [CrossRef] [Green Version]
- Pereira, B.I.; Devine, O.P.; Vukmanovic-Stejic, M.; Chambers, E.S.; Subramanian, P.; Patel, N.; Virasami, A.; Sebire, N.J.; Kinsler, V.; Valdovinos, A.; et al. Senescent cells evade immune clearance via HLA-E-mediated NK and CD8(+) T cell inhibition. Nat. Commun. 2019, 10, 2387. [Google Scholar] [PubMed] [Green Version]
- Song, Z.; Li, B.; Li, M.; Luo, J.; Hong, Y.; He, Y.; Chen, S.; Yang, Z.; Liang, C.; Yang, Z. Caveolin-1 regulation and function in mouse uterus during early pregnancy and under human in vitro decidualization. Int. J. Mol. Sci. 2022, 23, 3699. [Google Scholar] [CrossRef] [PubMed]
- Brighton, P.J.; Maruyama, Y.; Fishwick, K.; Vrljicak, P.; Tewary, S.; Fujihara, R.; Muter, J.; Lucas, E.S.; Yamada, T.; Woods, L.; et al. Clearance of senescent decidual cells by uterine natural killer cells in cycling human endometrium. Elife 2017, 6, e31274. [Google Scholar] [CrossRef] [PubMed]
- Gal, H.; Lysenko, M.; Stroganov, S.; Vadai, E.; Youssef, S.A.; Tzadikevitch-Geffen, K.; Rotkopf, R.; Biron-Shental, T.; de Bruin, A.; Neeman, M.; et al. Molecular pathways of senescence regulate placental structure and function. EMBO J. 2019, 38, e100849. [Google Scholar] [CrossRef]
- Kong, C.S.; Ordoñez, A.A.; Turner, S.; Tremaine, T.; Muter, J.; Lucas, E.S.; Salisbury, E.; Vassena, R.; Tiscornia, G.; Fouladi-Nashta, A.A.; et al. Embryo biosensing by uterine natural killer cells determines endometrial fate decisions at implantation. FASEB J. 2021, 35, e21336. [Google Scholar] [CrossRef] [PubMed]
- Deryabin, P.I.; Ivanova, J.S.; Borodkina, A.V. Senescence of stromal cells contributes to endometrium dysfunction and embryo implantation failure. Hum. Reprod. 2022, 37, 1505–1524. [Google Scholar] [CrossRef] [PubMed]
- Caumartin, J.; Favier, B.; Daouya, M.; Guillard, C.; Moreau, P.; Carosella, E.D.; Lemaoult, J. Trogocytosis-based generation of suppressive NK cells. EMBO J. 2007, 26, 1423–1433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zidi, I.; Rizzo, R.; Bouaziz, A.; Laaribi, A.B.; Zidi, N.; Di Luca, D.; Tlili, H.; Bortolotti, D. sHLA-G 1 and HLA-G5 levels are decreased in tunisian women with multiple abortion. Hum. Immunol. 2016, 77, 342–345. [Google Scholar] [CrossRef] [PubMed]
- Craenmehr, M.; Nederlof, I.; Cao, M.; Drabbels, J.; Spruyt-Gerritse, M.J.; Anholts, J.; Kapsenberg, H.M.; Stegehuis, J.A.; van der Keur, C.; Fasse, E.; et al. Increased HLA-G expression in term placenta of women with a history of recurrent miscarriage despite their genetic predisposition to decreased HLA-G levels. Int. J. Mol. Sci. 2019, 20, 652. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, L.; Meissner, T.B.; Tilburgs, T.; Strominger, J.L. HLA-G: At the interface of maternal-fetal tolerance. Trends Immunol. 2017, 38, 272–286. [Google Scholar] [PubMed]
- Green, S.; Politis, M.; Rallis, K.S.; Saenz, D.V.C.A.; Efthymiou, A.; Mureanu, N.; Dalrymple, K.V.; Scottà, C.; Lombardi, G.; Tribe, R.M.; et al. Regulatory T cells in pregnancy adverse outcomes: A systematic review and meta-analysis. Front. Immunol. 2021, 12, 737862. [Google Scholar]
- Gomez-Lopez, N.; Arenas-Hernandez, M.; Romero, R.; Miller, D.; Garcia-Flores, V.; Leng, Y.; Xu, Y.; Galaz, J.; Hassan, S.S.; Hsu, C.D.; et al. Regulatory T cells play a role in a subset of idiopathic preterm labor/birth and adverse neonatal outcomes. Cell Rep. 2020, 32, 107874. [Google Scholar]
- Erlebacher, A. Immunology of the maternal-fetal interface. Annu. Rev. Immunol. 2013, 31, 387–411. [Google Scholar] [CrossRef]
- Lila, N.; Rouas-Freiss, N.; Dausset, J.; Carpentier, A.; Carosella, E.D. Soluble HLA-G protein secreted by allo-specific CD4+ T cells suppresses the allo-proliferative response: A CD4+ T cell regulatory mechanism. Proc. Natl. Acad. Sci. USA 2001, 98, 12150–12155. [Google Scholar] [CrossRef]
- Mestrallet, G.; Auvré, F.; Schenowitz, C.; Carosella, E.D.; Lemaoult, J.; Martin, M.T.; Rouas-Freiss, N.; Fortunel, N.O. Human keratinocytes inhibit CD4(+) T-cell proliferation through TGFB1 secretion and surface expression of HLA-G 1 and PD-L1 immune checkpoints. Cells 2021, 10, 1438. [Google Scholar] [CrossRef] [PubMed]
- Dumont, C.; Jacquier, A.; Verine, J.; Noel, F.; Goujon, A.; Wu, C.L.; Hung, T.M.; Desgrandchamps, F.; Culine, S.; Carosella, E.D.; et al. CD8(+)PD-1(-)ILT2(+) T cells are an intratumoral cytotoxic population selectively inhibited by the immune-checkpoint HLA-G. Cancer Immunol. Res. 2019, 7, 1619–1632. [Google Scholar] [CrossRef] [PubMed]
- Jacquier, A.; Lambert, T.; Delattre, J.F.; Djouadou, M.; Vérine, J.; Dumont, C.; Desgrandchamps, F.; Carosella, E.D.; Lemaoult, J.; Rouas-Freiss, N. Tumor infiltrating and peripheral CD4(+)ILT2(+) T cells are a cytotoxic subset selectively inhibited by HLA-G in clear cell renal cell carcinoma patients. Cancer Lett. 2021, 519, 105–116. [Google Scholar] [CrossRef]
- Reed, J.; Wetzel, S.A. Trogocytosis-mediated intracellular signaling in CD4(+) T cells drives T(h)2-associated effector cytokine production and differentiation. J. Immunol. 2019, 202, 2873–2887. [Google Scholar] [CrossRef]
- Vianna, P.; Mondadori, A.G.; Bauer, M.E.; Dornfeld, D.; Chies, J.A. HLA-G and CD8+ regulatory T cells in the inflammatory environment of pre-eclampsia. Reproduction 2016, 152, 741–751. [Google Scholar] [CrossRef] [Green Version]
- Wienke, J.; Brouwers, L.; van der Burg, L.M.; Mokry, M.; Scholman, R.C.; Nikkels, P.G.; van Rijn, B.B.; van Wijk, F. Human Tregs at the materno-fetal interface show site-specific adaptation reminiscent of tumor Tregs. JCI Insight. 2020, 5, e137926. [Google Scholar] [CrossRef]
- Gregori, S.; Tomasoni, D.; Pacciani, V.; Scirpoli, M.; Battaglia, M.; Magnani, C.F.; Hauben, E.; Roncarolo, M. Differentiation of type 1 T regulatory cells (Tr1) by tolerogenic DC-10 requires the IL-10–dependent ILT4/HLA-G pathway. Blood 2010, 116, 935–944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, M.R.; Guo, P.F.; Piao, H.L.; Wang, S.C.; Sun, C.; Jin, L.P.; Tao, Y.; Li, Y.H.; Zhang, D.; Zhu, R.; et al. Embryonic trophoblasts induce decidual regulatory T cell differentiation and maternal-fetal tolerance through thymic stromal lymphopoietin instructing dendritic cells. J. Immunol. 2014, 192, 1502–1511. [Google Scholar] [CrossRef] [Green Version]
- Comi, M.; Avancini, D.; Santoni, D.S.F.; Villa, M.; Uyeda, M.J.; Floris, M.; Tomasoni, D.; Bulfone, A.; Roncarolo, M.G.; Gregori, S. Coexpression of CD163 and CD141 identifies human circulating IL-10-producing dendritic cells (DC-10). Cell Mol. Immunol. 2020, 17, 95–107. [Google Scholar] [CrossRef] [Green Version]
- Amodio, G.; Comi, M.; Tomasoni, D.; Gianolini, M.E.; Rizzo, R.; Lemaoult, J.; Roncarolo, M.G.; Gregori, S. HLA-G expression levels influence the tolerogenic activity of human DC-10. Haematologica 2015, 100, 548–557. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Wei, H.; Li, Y.; Diao, L.; Lian, R.; Zhang, X.; Zeng, Y. Characterization of dendritic cell (DC)-10 in recurrent miscarriage and recurrent implantation failure. Reproduction 2019, 158, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Zhong, Y.; Shen, W.; Chen, Y.; Shi, J.; Di, J.; Zeng, S.; Saito, S. TSLP-induced placental DC activation and IL-10(+) NK cell expansion: Comparative study based on BALB/c x C57BL/6 and NOD/SCID x C57BL/6 pregnant models. Clin. Immunol. 2008, 126, 104–117. [Google Scholar] [CrossRef] [PubMed]
- Blois, S.M.; Freitag, N.; Tirado-González, I.; Cheng, S.B.; Heimesaat, M.M.; Bereswill, S.; Rose, M.; Conrad, M.L.; Barrientos, G.; Sharma, S. NK cell-derived IL-10 is critical for DC-NK cell dialogue at the maternal-fetal interface. Sci. Rep. 2017, 7, 2189. [Google Scholar] [CrossRef] [Green Version]
- Plaks, V.; Birnberg, T.; Berkutzki, T.; Sela, S.; Benyashar, A.; Kalchenko, V.; Mor, G.; Keshet, E.; Dekel, N.; Neeman, M.; et al. Uterine DCs are crucial for decidua formation during embryo implantation in mice. J. Clin. Investig. 2008, 118, 3954–3965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, P.; Santner-Nanan, B.; Dahlstrom, J.E.; Fadia, M.; Chandra, A.; Peek, M.; Nanan, R. Altered decidual DC-SIGN+ antigen-presenting cells and impaired regulatory T-cell induction in preeclampsia. Am. J. Pathol. 2012, 181, 2149–2160. [Google Scholar] [CrossRef]
- Hsu, P.; Santner-Nanan, B.; Joung, S.; Peek, M.J.; Nanan, R. Expansion of CD4(+) HLA-G(+) T cell in human pregnancy is impaired in pre-eclampsia. Am. J. Reprod. Immunol. 2014, 71, 217–228. [Google Scholar] [CrossRef]
- Houser, B.L.; Tilburgs, T.; Hill, J.; Nicotra, M.L.; Strominger, J.L. Two unique human decidual macrophage populations. J. Immunol. 2011, 186, 2633–2642. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Houser, B.L.; Nicotra, M.L.; Strominger, J.L. HLA-G homodimer-induced cytokine secretion through HLA-G receptors on human decidual macrophages and natural killer cells. Proc. Natl. Acad. Sci. USA 2009, 106, 5767. [Google Scholar] [CrossRef]
- Lombardelli, L.; Aguerre-Girr, M.; Logiodice, F.; Kullolli, O.; Casart, Y.; Polgar, B.; Berrebi, A.; Romagnani, S.; Maggi, E.; Le Bouteiller, P.; et al. HLA-G5 induces IL-4 secretion critical for successful pregnancy through differential expression of ILT2 receptor on decidual CD4⁺ T cells and macrophages. J. Immunol. 2013, 191, 3651–3662. [Google Scholar] [CrossRef] [Green Version]
- Eguchi, H.; Maeda, A.; Lo, P.C.; Matsuura, R.; Esquivel, E.L.; Asada, M.; Sakai, R.; Nakahata, K.; Yamamichi, T.; Umeda, S.; et al. HLA-G1, but not HLA-G3, suppresses human monocyte/macrophage-mediated swine endothelial cell lysis. Transplant. Proc. 2016, 48, 1285–1287. [Google Scholar] [CrossRef]
- Wu, Z.M.; Yang, H.; Li, M.; Yeh, C.C.; Schatz, F.; Lockwood, C.J.; Di, W.; Huang, S.J. Pro-inflammatory cytokine-stimulated first trimester decidual cells enhance macrophage-induced apoptosis of extravillous trophoblasts. Placenta 2012, 33, 188–194. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.L.; Guo, Y.; So, K.H.; Vijayan, M.; Guo, Y.; Wong, V.H.; Yao, Y.; Lee, K.F.; Chiu, P.C.; Yeung, W.S. Soluble human leukocyte antigen G5 polarizes differentiation of macrophages toward a decidual macrophage-like phenotype. Hum. Reprod. 2015, 30, 2263–2274. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Q.; Zhou, W.J.; Hou, X.X.; Fu, Q.; Li, D.J. Trophoblast-derived CXCL16 induces M2 macrophage polarization that in turn inactivates NK cells at the maternal-fetal interface. Cell. Mol. Immunol. 2018, 15, 1038–1046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Songcun, W.; Xiaoyong, Z.; Yuanyuan, X.; Di, Z.; Yanhong, L.; Tao, Y.; Hailan, P.; Dajin, L.; Meirong, D. Programmed cell death-1 (PD-1) and T-cell immunoglobulin mucin-3 (Tim-3) regulate CD4+ T cells to induce type 2 helper Tcell (Th2) bias at the maternal-fetal interface. Hum. Reprod. 2016, 31, 700–711. [Google Scholar]
- Li, Z.H.; Wang, L.L.; Liu, H.; Muyayalo, K.P.; Huang, X.B.; Mor, G.; Liao, A.H. Galectin-9 alleviates LPS-induced preeclampsia-like impairment in rats via switching decidual macrophage polarization to M2 subtype. Front. Immunol. 2019, 9, 3142. [Google Scholar]
- Niu, Z.; Wang, L.; Pang, R.T.K.; Guo, Y.; Yeung, W.S.B.; Yao, Y. A meta-analysis of the impact of human leukocyte antigen-G on the outcomes of IVF/ICSI. Reprod. Biomed. Online 2017, 34, 611–618. [Google Scholar] [CrossRef] [Green Version]
- Taylor, S.K.; Houshdaran, S.; Robinson, J.F.; Gormley, M.J.; Kwan, E.Y.; Kapidzic, M.; Schilling, B.; Giudice, L.C.; Fisher, S.J. Cytotrophoblast extracellular vesicles enhance decidual cell secretion of immune modulators via TNFα. Development 2020, 14, dev187013. [Google Scholar] [CrossRef] [PubMed]

| HLA-G Isoforms | Types | Protein Isoforms | Expressed Cells |
|---|---|---|---|
| HLA-G1 | Membrane-bound | 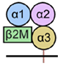 | Trophoblasts [27] Tumor cell lines [28] Antigen-presenting cells (APC) [29] |
| HLA-G2 HLA-G3 HLA-G4 | Membrane-bound |  | Cytotrophoblast [30] Tumor cell lines [31] |
| HLA-G5 | Soluble | 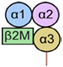 | Body fluids (such as amniotic fluid and serum) [32] Trophoblasts [33] Thymus [10] Oocytes [34] Pre-implantation embryo [35] |
| HLA-G6 | Soluble |  | Maternal blood during pregnancy [36] Serum from heart-transplanted patients [37] |
| HLA-G7 | Soluble |  | Transfected cell supernatant [38] Maternal blood during pregnancy [39] |
| HLA-G Receptors | Immune Cells | Signal Pathway | Function |
|---|---|---|---|
| ILT-2 | NK cell | Cytoskeleton rearrangement | Inhibition of NK cytolysis [87] |
| T cell | ILT-2/ILT-4/FasL | Inhibition of T-cell alloproliferation [88] | |
| B cell | SHP-1/AKT, mTOR, c-Raf, GSK3β, and Foxo pathways | Regulating B cell fate decision, such as cell proliferation and differentiation [89] | |
| ILT-4 | T cell | IL4/SHP-1/2-IL-6--STAT3 | Modulation of dendritic cell differentiation [90] |
| KIR2DL4 | NK cell | (DNA-PKcs)-Akt-NF-κB | Pro-inflammatory response or proangiogenesis [91] |
| Blocking MAPK and DNA-PKcs | Inhibition of killing activity of NK and promotion of inflammatory cytokines secretion [91] | ||
| Syk/MAPK/ERK | Inhibition of NK cytotoxicity [92] | ||
| Uncertain | Fas/FasL | Apoptosis of NK cells [93] | |
| SHP-2/mTOR | Inhibition of lymphocyte cell cycle [94] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, J.; Feng, Y.; Zhu, X.; Ma, F. The Molecular Mechanisms of HLA-G Regulatory Function on Immune Cells during Early Pregnancy. Biomolecules 2023, 13, 1213. https://doi.org/10.3390/biom13081213
Mao J, Feng Y, Zhu X, Ma F. The Molecular Mechanisms of HLA-G Regulatory Function on Immune Cells during Early Pregnancy. Biomolecules. 2023; 13(8):1213. https://doi.org/10.3390/biom13081213
Chicago/Turabian StyleMao, Jia, Ying Feng, Xiaofeng Zhu, and Fang Ma. 2023. "The Molecular Mechanisms of HLA-G Regulatory Function on Immune Cells during Early Pregnancy" Biomolecules 13, no. 8: 1213. https://doi.org/10.3390/biom13081213





