Novel Apoplastic Antifreeze Proteins of Deschampsia antarctica as Enhancer of Common Cell Freezing Media for Cryobanking of Genetic Resources, a Preliminary Study
Abstract
Highlights
- Cryopreservation generates ice recrystallization.
- D. antarctica apoplastic proteins show antifreeze activity.
- PMI of S. salar sperm can be maintained with AFPs.
- High MMP of sperm increases with AFPs.
- D. antarctica apoplastic proteins act as nonpermeable cryoprotectants.
Abstract
1. Introduction
2. Material and Methods
2.1. Overview
- (a)
- Characterization of the antifreeze activity of the apoplastic extract, considering thermal hysteresis activity, ice recrystallization inhibition and ice crystal morphology;
- (b)
- Evaluation of antifreeze activity of the apoplastic extract in presence of a commonly used freezing media for cryopreservation;
- (c)
- Evaluation of cryoprotectant effect of the apoplastic extract in fish spermatozoa.
2.2. Extraction of Apoplastic Proteins from D. antarctica
2.3. Antifreeze Activity Assays
2.4. Broodstock
2.5. Semen Collection and Analysis
2.6. Cryopreservation Process
2.7. Frozen–Thawed Semen Analysis
2.8. Postthaw Motility Assessment
2.9. Statistical Analysis
3. Results
3.1. Antifreeze Activity in D. antarctica Apoplastic Extract
3.2. Effect of Freezing Medium on Antifreeze Activity in Apoplastic Extracts of D. antarctica
3.3. Cryoprotective Effect of Apoplastic Extracts of D. antarctica
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deng, J.; Apfelbaum, E.; Drori, R. Ice Growth Acceleration by Antifreeze Proteins Leads to Higher Thermal Hysteresis Activity. J. Phys. Chem. B 2020, 124, 11081–11088. [Google Scholar] [CrossRef]
- Baskaran, A.; Kaari, M.; Venugopal, G.; Manikkam, R.; Joseph, J.; Bhaskar, P. Anti freeze proteins (Afp): Properties, sources and applications—A review. Int. J. Biol. Macromol. 2021, 189, 292–305. [Google Scholar] [CrossRef]
- Farvardin, A.; González-Hernández, A.I.; Llorens, E.; García-Agustín, P.; Scalschi, L.; Vicedo, B. The Apoplast: A Key Player in Plant Survival. Antioxidants 2020, 9, 604. [Google Scholar] [CrossRef]
- Correia, L.F.L.; Espírito-Santo, C.G.; Braga, R.F.; Carvalho-de-Paula, C.J.; da Silva, A.A.; Brandão, F.Z.; Freitas, V.J.F.; Ungerfeld, R.; Souza-Fabjan, J.M.G. Addition of antifreeze protein type I or III to extenders for ram sperm cryopreservation. Cryobiology 2021, 98, 194–200. [Google Scholar] [CrossRef]
- Eskandari, A.; Leow, T.C.; Rahman, M.B.A.; Oslan, S.N. Antifreeze proteins and their practical utilization in industry, medicine, and agriculture. Biomolecules 2020, 10, 1649. [Google Scholar] [CrossRef] [PubMed]
- Robles, V.; Valcarce, D.G.; Riesco, M.F. The Use of Antifreeze Proteins in the Cryopreservation of Gametes and Embryos. Biomolecules 2019, 9, 181. [Google Scholar] [CrossRef]
- Niu, J.; Wang, X.; Liu, P.; Liu, H.; Li, R.; Li, Z.; He, Y.; Qi, J. Effects of Cryopreservation on Sperm with Cryodiluent in Viviparous Black Rockfish (Sebastes schlegelii). Int. J. Mol. Sci. 2022, 23, 3392. [Google Scholar] [CrossRef] [PubMed]
- Oliver, L.; Evavold, J.; Cain, K. Out-of-season spawning of burbot (Lota lota) through temperature and photoperiod manipulation. Aquaculture 2021, 543, 736917. [Google Scholar] [CrossRef]
- Clauss, M.; Zerbe, P.; Bingaman Lackey, L.; Codron, D.; Müller, D.W.H. Basic considerations on seasonal breeding in mammals including their testing by comparing natural habitats and zoos. Mamm. Biol. 2021, 101, 373–386. [Google Scholar] [CrossRef]
- Aygül, E.; Güneş, Y.; Menekşe Didem, D. Cryopreservation Studies in Aquaculture from Past to Present: Scientific Techniques and Quality Controls for Commercial Applications. In Cryopreservation—Applications and Challenges; Marian, Q., Ed.; IntechOpen: Rijeka, Croatia, 2022; Chapter 1. [Google Scholar]
- Zidni, I.; Lee, H.-B.; Yoon, J.-H.; Park, J.-Y.; Jang, H.-S.; Cho, Y.-S.; Seo, Y.-S.; Lim, H.-K. Cryopreservation of Roughscale Sole (Clidoderma asperrimum) Sperm: Effects of Cryoprotectant, Diluent, Dilution Ratio, and Thawing Temperature. Animals 2022, 12, 2553. [Google Scholar] [CrossRef] [PubMed]
- Judith Betsy, C.; Siva, C.; Stephen Sampath Kumar, J. Cryopreservation and Its Application in Aquaculture. In Animal Reproduction; Yusuf, B., Mustafa Numan, B., Eds.; IntechOpen: Rijeka, Croatia, 2021; Chapter 5. [Google Scholar]
- Xin, M.; Niksirat, H.; Shaliutina-Kolešová, A.; Siddique, M.A.M.; Sterba, J.; Boryshpolets, S.; Linhart, O. Molecular and subcellular cryoinjury of fish spermatozoa and approaches to improve cryopreservation. Rev. Aquac. 2020, 12, 909–924. [Google Scholar] [CrossRef]
- Yang, S.; Chen, X.; Fan, B.; Hua, Y.; Meng, Z. Successful short-term sperm cryopreservation in brown-marbled grouper (Epinephelus fuscoguttatus) with the utility of ultra-freezer (−80 °C). Reprod. Domest. Anim. 2022, 57, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, E.; Valdebenito, I.; Merino, O.; Ubilla, A.; Risopatrón, J.; Farias, J.G. Cryopreservation of Atlantic salmon Salmo salar sperm: Effects on sperm physiology. J. Fish Biol. 2016, 89, 1537–1550. [Google Scholar] [CrossRef] [PubMed]
- Vafaei, F.; Kohram, H.; Zareh-Shahne, A.; Ahmad, E.; Seifi-Jamadi, A. Influence of Different Combinations of Permeable and Nonpermeable Cryoprotectants on the Freezing Capacity of Equine Sperm. J. Equine Vet. Sci. 2019, 75, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Carro, M.; Luquez, J.; Peñalva, D.; Buschiazzo, J.; Hozbor, F.; Furland, N. PUFA-rich phospholipid classes and subclasses of ram spermatozoa are unevenly affected by cryopreservation with a soybean lecithin-based extender. Theriogenology 2022, 186, 122–134. [Google Scholar] [CrossRef]
- Liu, S.; Su, Y.; Yi, H.; Liu, X.; Chen, X.; Lai, H.; Bi, S.; Zhang, Y.; Zhao, X.; Li, G. Ultra-low temperature cryopreservation and −80 °C storage of sperm from normal-male and pseudo-male Siniperca chuatsi. Aquaculture 2022, 553, 738007. [Google Scholar] [CrossRef]
- Medeiros, C.M.O.; Forell, F.; Oliveira, A.T.D.; Rodrigues, J.L. Current status of sperm cryopreservation: Why isn’t it better? Theriogenology 2002, 57, 327–344. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Prasad, J.K.; Srivastava, N.; Ghosh, S.K. Strategies to Minimize Various Stress-Related Freeze–Thaw Damages During Conventional Cryopreservation of Mammalian Spermatozoa. Biopreserv. Biobank. 2019, 17, 603–612. [Google Scholar] [CrossRef]
- Yang, H.; Hu, E.; Buchanan, J.T.; Tiersch, T.R. A Strategy for Sperm Cryopreservation of Atlantic Salmon, Salmo salar, for Remote Commercial-scale High-throughput Processing. J. World Aquac. Soc. 2018, 49, 96–112. [Google Scholar] [CrossRef]
- Bravo, L.A.; Ulloa, N.; Zuñiga, G.E.; Casanova, A.; Corcuera, L.J.; Alberdi, M. Cold resistance in Antarctic angiosperms. Physiol. Plant. 2001, 111, 55–65. [Google Scholar] [CrossRef]
- Bravo, L.A.; Griffith, M. Characterization of antifreeze activity in Antarctic plants. J. Exp. Bot. 2005, 56, 1189–1196. [Google Scholar] [CrossRef]
- Doucet, C.J.; Byass, L.; Elias, L.; Worrall, D.; Smallwood, M.; Bowles, D.J. Distribution and Characterization of Recrystallization Inhibitor Activity in Plant and Lichen Species from the UK and Maritime Antarctic. Cryobiology 2000, 40, 218–227. [Google Scholar] [CrossRef]
- Short, S.; Díaz, R.; Quiñones, J.; Beltrán, J.; Farías, J.G.; Graether, S.P.; Bravo, L.A. Effect of in vitro cold acclimation of Deschampsia antarctica on the accumulation of proteins with antifreeze activity. J. Exp. Bot. 2020, 71, 2933–2942. [Google Scholar] [CrossRef]
- Ding, X.; Zhang, H.; Chen, H.; Wang, L.; Qian, H.; Qi, X. Extraction, purification and identification of antifreeze proteins from cold acclimated malting barley (Hordeum vulgare L.). Food Chem. 2015, 175, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Gorb, E.V.; Kozeretska, I.A.; Gorb, S.N. Hierachical epicuticular wax coverage on leaves of Deschampsia antarctica as a possible adaptation to severe environmental conditions. Beilstein J. Nanotechnol. 2022, 13, 807–816. [Google Scholar] [CrossRef]
- Cuadra, P.; Guajardo, J.; Carrasco-Orellana, C.; Stappung, Y.; Fajardo, V.; Herrera, R. Differential expression after UV-B radiation and characterization of chalcone synthase from the Patagonian hairgrass Deschampsia antarctica. Phytochemistry 2020, 169, 112–179. [Google Scholar] [CrossRef]
- Gruneberg, A.K.; Graham, L.A.; Eves, R.; Agrawal, P.; Oleschuk, R.D.; Davies, P.L. Ice recrystallization inhibition activity varies with ice-binding protein type and does not correlate with thermal hysteresis. Cryobiology 2021, 99, 28–39. [Google Scholar] [CrossRef]
- Davies, P.L. Ice-binding proteins: A remarkable diversity of structures for stopping and starting ice growth. Trends Biochem. Sci. 2014, 39, 548–555. [Google Scholar] [CrossRef]
- Griffith, M.; Yaish, M.W.F. Antifreeze proteins in overwintering plants: A tale of two activities. Trends Plant Sci. 2004, 9, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lee, J.H.; Hur, Y.B.; Lee, C.W.; Park, S.-H.; Koo, B.-W. Marine Antifreeze Proteins: Structure, Function, and Application to Cryopreservation as a Potential Cryoprotectant. Mar. Drugs 2017, 15, 27. [Google Scholar] [CrossRef] [PubMed]
- Prathalingam, N.S.; Holt, W.V.; Revell, S.G.; Mirczuk, S.; Fleck, R.A.; Watson, P.F. Impact of antifreeze proteins and antifreeze glycoproteins on bovine sperm during freeze-thaw. Theriogenology 2006, 66, 1894–1900. [Google Scholar] [CrossRef]
- Hon, W.C.; Griffith, M.; Chong, P.; Yang, D.S.C. Extraction and Isolation of Antifreeze Proteins from Winter Rye (Secale cereale L.) Leaves. Plant Physiol. 1994, 104, 971–980. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- DeVries, A.L. Antifreeze glycopeptides and peptides: Interactions with ice and water. Methods Enzymol. 1986, 127, 293–303. [Google Scholar] [PubMed]
- Kim, E.J.; Kim, J.E.; Hwang, J.S.; Kim, I.C.; Lee, S.G.; Kim, S.; Lee, J.H.; Han, S.J. Increased Productivity and Antifreeze Activity of Ice-binding Protein from Flavobacterium frigoris PS1 Produced using Escherichia coli as Bioreactor. Appl. Biochem. Microbiol. 2019, 55, 489–494. [Google Scholar] [CrossRef]
- Hughes, S.L.; Schart, V.; Malcolmson, J.; Hogarth, K.A.; Martynowicz, D.M.; Tralman-Baker, E.; Patel, S.N.; Graether, S.P. The Importance of Size and Disorder in the Cryoprotective Effects of Dehydrins. Plant Physiol. 2013, 163, 1376–1386. [Google Scholar] [CrossRef] [PubMed]
- Truscott, B.; Idler, D.R.; Hoyle, R.J.; Freeman, H.C. Sub-Zero Preservation of Atlantic Salmon Sperm. J. Fish. Res. Board Can. 1968, 25, 363–372. [Google Scholar] [CrossRef]
- Díaz, R.; Lee-Estevez, M.; Quiñones, J.; Dumorné, K.; Short, S.; Ulloa-Rodríguez, P.; Valdebenito, I.; Sepúlveda, N.; Farías, J.G. Changes in Atlantic salmon (Salmo salar) sperm morphology and membrane lipid composition related to cold storage and cryopreservation. Anim. Reprod. Sci. 2019, 204, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Magnotti, C.; Cerqueira, V.; Villasante, A.; Romero, J.; Watanabe, I.; Oliveira, R.P.S.; Farias, J.; Merino, O.; Valdebenito Figueroa, E. Spermatological characteristics and effects of cryopreservation in Lebranche mullet spermatozoa (Mugil liza Valenciennes, 1836): First report of ultra-rapid freezing. Anim. Reprod. Sci. 2022, 241, 106986. [Google Scholar] [CrossRef] [PubMed]
- Bravo, W.; Dumorné, K.; Beltrán Lissabet, J.; Jara-Seguel, P.; Romero, J.; Farías, J.G.; Risopatrón, J.; Valdebenito, I.; Figueroa, E. Effects of selection by the Percoll density gradient method on motility, mitochondrial membrane potential and fertility in a subpopulation of Atlantic salmon (Salmo salar) testicular spermatozoa. Anim. Reprod. Sci. 2020, 216, 106–344. [Google Scholar] [CrossRef]
- Lahnsteiner, F.; Mansour, N.; Kunz, F.A. The effect of antioxidants on the quality of cryopreserved semen in two salmonid fish, the brook trout (Salvelinus fontinalis) and the rainbow trout (Oncorhynchus mykiss). Theriogenology 2011, 76, 882–890. [Google Scholar] [CrossRef]
- Figueroa, E.; Risopatrón, J.; Sánchez, R.; Isachenko, E.; Merino, O.; Isachenko, V.; Valdebenito, I. Spermatozoa vitrification of sex-reversed rainbow trout (Oncorhynchus mykiss): Effect of seminal plasma on physiological parameters. Aquaculture 2013, 372–375, 119–126. [Google Scholar] [CrossRef]
- John, U.P.; Polotnianka, R.M.; Sivakumaran, K.A.; Chew, O.; Mackin, L.; Kuiper, M.J.; Talbot, J.P.; Nugent, G.D.; Mautord, J.; Schrauf, G.E.; et al. Ice recrystallization inhibition proteins (IRIPs) and freeze tolerance in the cryophilic Antarctic hair grass Deschampsia antarctica E. Desv. Plant Cell Environ. 2009, 32, 336–348. [Google Scholar] [CrossRef]
- Capicciotti, C.J.; Doshi, M.; Ben, R.N. Ice Recrystallization Inhibitors: From Biological Antifreezes to Small Molecules. In Recent Developments in the Study of Recrystallization; Peter, W., Ed.; IntechOpen: Rijeka, Croatia, 2013; Chapter 7. [Google Scholar]
- Tejo, B.A.; Asmawi, A.A.; Rahman, M.B.A. Antifreeze Proteins: Characteristics and Potential Applications. Makara J. Sci. 2020, 24, 8. [Google Scholar]
- Furukawa, Y.; Nagashima, K.; Nakatsubo, S.; Yoshizaki, I.; Tamaru, H.; Shimaoka, T.; Sone, T.; Yokoyama, E.; Zepeda, S.; Terasawa, T.; et al. Oscillations and accelerations of ice crystal growth rates in microgravity in presence of antifreeze glycoprotein impurity in supercooled water. Sci. Rep. 2017, 7, 43157. [Google Scholar] [CrossRef]
- Drori, R.; Davies, P.L.; Braslavsky, I. When Are Antifreeze Proteins in Solution Essential for Ice Growth Inhibition? Langmuir 2015, 31, 5805–5811. [Google Scholar] [CrossRef]
- Naing, A.H.; Kim, C.K. A brief review of applications of antifreeze proteins in cryopreservation and metabolic genetic engineering. 3 Biotech 2019, 9, 329. [Google Scholar] [CrossRef] [PubMed]
- Cabrita, E.; Sarasquete, C.; Martínez-Páramo, S.; Robles, V.; Beirão, J.; Pérez-Cerezales, S.; Herráez, M.P. Cryopreservation of fish sperm: Applications and perspectives. J. Appl. Ichthyol. 2010, 26, 623–635. [Google Scholar] [CrossRef]
- Zilli, L.; Beirão, J.; Schiavone, R.; Herraez, M.P.; Gnoni, A.; Vilella, S. Comparative Proteome Analysis of Cryopreserved Flagella and Head Plasma Membrane Proteins from Sea Bream Spermatozoa: Effect of Antifreeze Proteins. PLoS ONE 2014, 9, e99992. [Google Scholar] [CrossRef] [PubMed]
- Notman, R.; Noro, M.; O’Malley, B.; Anwar, J. Molecular Basis for Dimethylsulfoxide (DMSO) Action on Lipid Membranes. J. Am. Chem. Soc. 2006, 128, 13982–13983. [Google Scholar] [CrossRef] [PubMed]
- Rijsselaere, T.; Van Soom, A.; Maes, D.; Verberckmoes, S.; de Kruif, A. Effect of blood admixture on in vitro survival of chilled and frozen–thawed canine spermatozoa. Theriogenology 2004, 61, 1589–1602. [Google Scholar] [CrossRef]
- Beirão, J.; Zilli, L.; Vilella, S.; Cabrita, E.; Schiavone, R.; Herráez, M.P. Improving Sperm Cryopreservation with Antifreeze Proteins: Effect on Gilthead Seabream (Sparus aurata) Plasma Membrane Lipids. Biol. Reprod. 2012, 86, 59. [Google Scholar] [CrossRef]
- Saragusty, J.; Arav, A. Current progress in oocyte and embryo cryopreservation by slow freezing and vitrification. Reproduction 2011, 141, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Hossen, S.; Sharker, M.d.R.; Cho, Y.; Sukhan, Z.P.; Kho, K.H. Effects of Antifreeze Protein III on Sperm Cryopreservation of Pacific Abalone, Haliotis discus hannai. Int. J. Mol. Sci. 2021, 22, 3917. [Google Scholar] [CrossRef] [PubMed]
- Erraud, A.; Bonnard, M.; Cornet, V.; Ben Ammar, I.; Antipine, S.; Peignot, Q.; Lambert, J.; Mandiki, S.N.M.; Kestemont, P. How age, captivity and cryopreservation affect sperm quality and reproductive efficiency in precocious Atlantic salmon (Salmo salar L. 1758). Aquaculture 2021, 544, 737047. [Google Scholar] [CrossRef]
- Dziewulska, K.; Rzemieniecki, A.; Czerniawski, R.; Domagała, J. Post-thawed motility and fertility from Atlantic salmon (Salmo salar L.) sperm frozen with four cryodiluents in straws or pellets. Theriogenology 2011, 76, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Le François, N.R.; Lamarre, S.G.; Tveiten, H.; Blier, P.U.; Bailey, J. Sperm cryoconservation in Anarhichas sp., endangered cold-water aquaculture species with internal fertilization. Aquac. Int. 2008, 16, 273–279. [Google Scholar] [CrossRef]
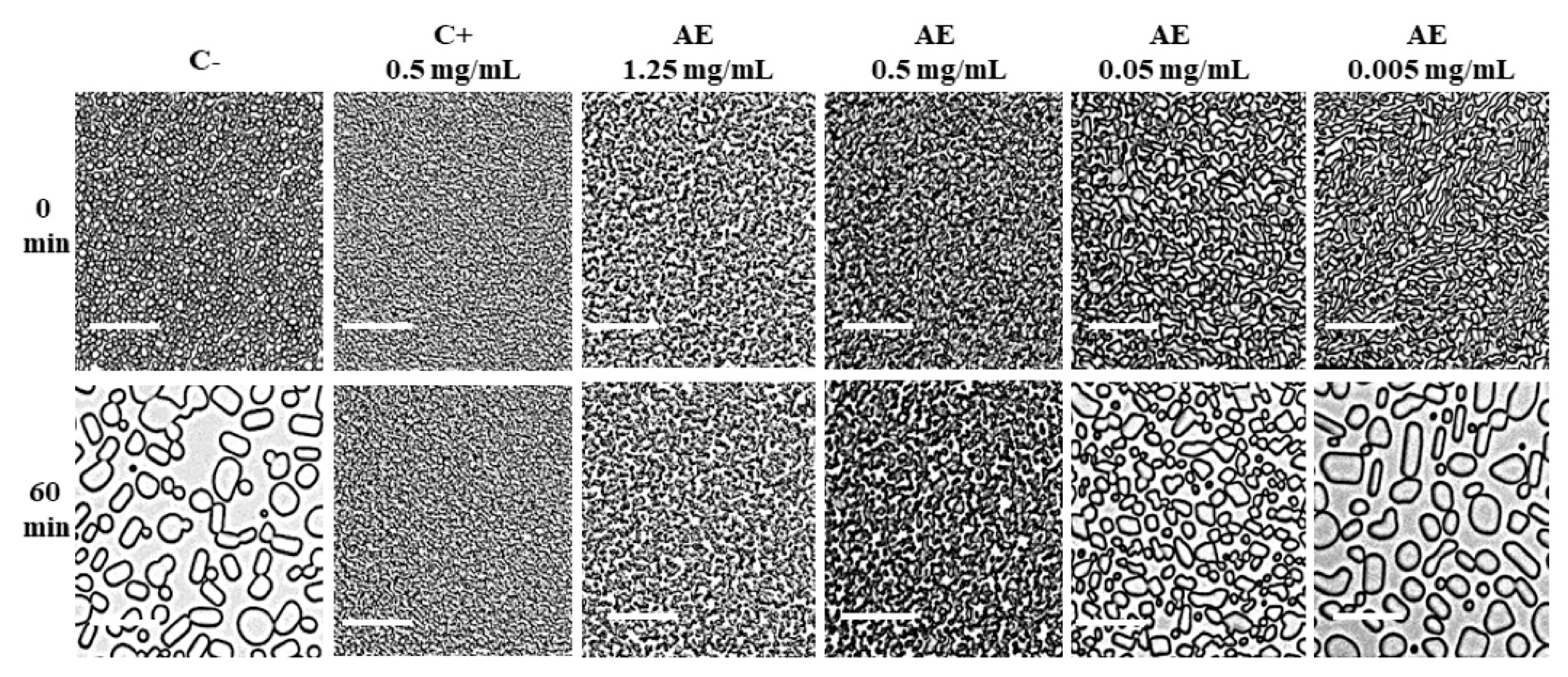
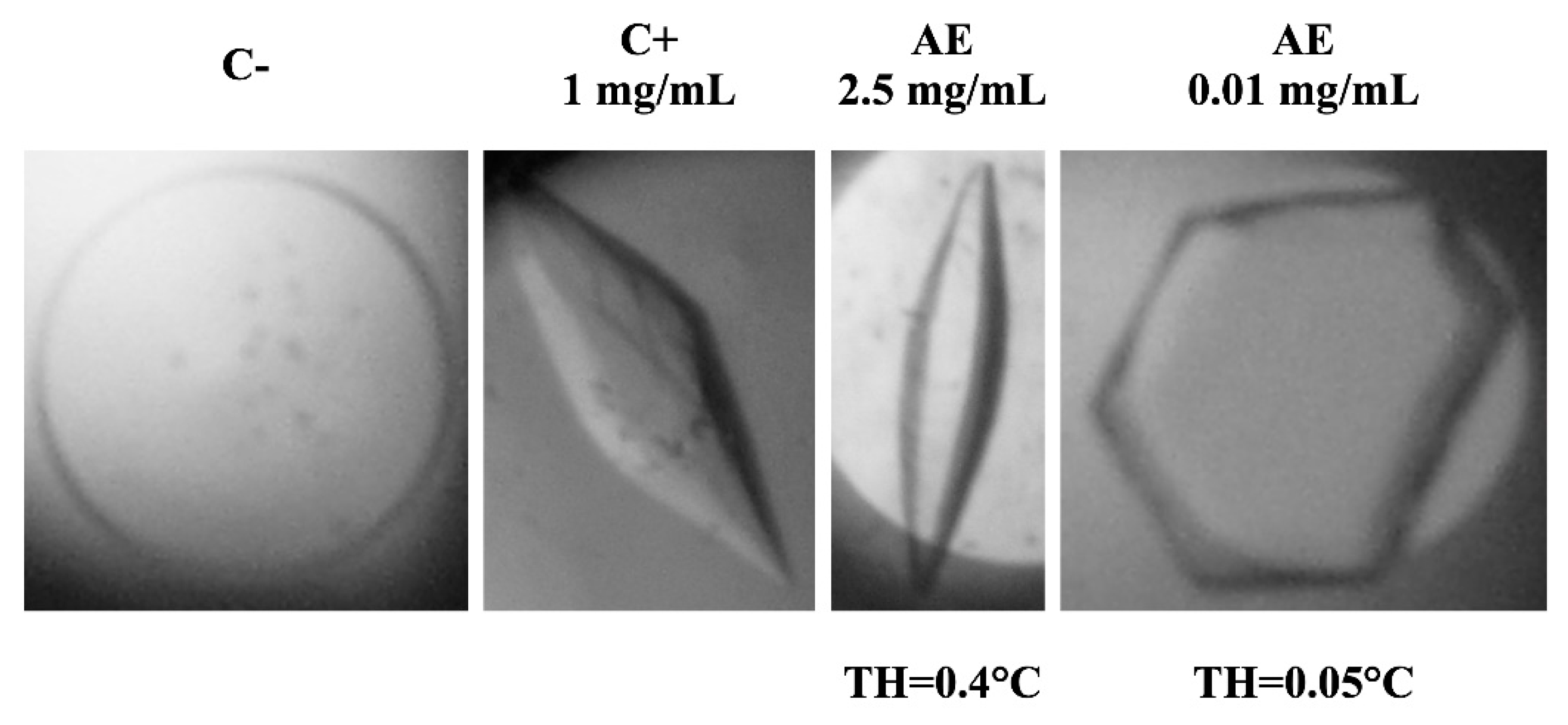
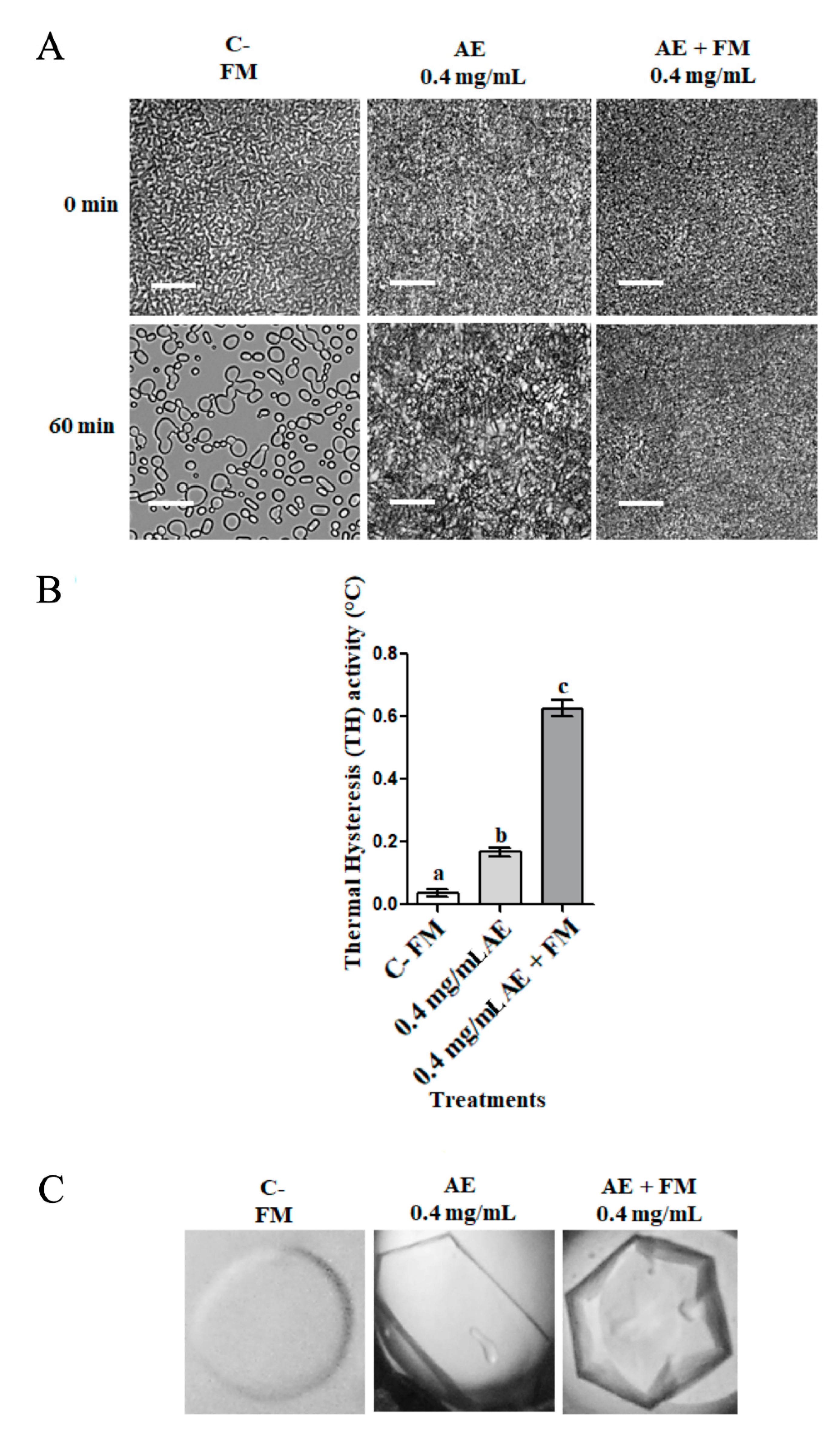
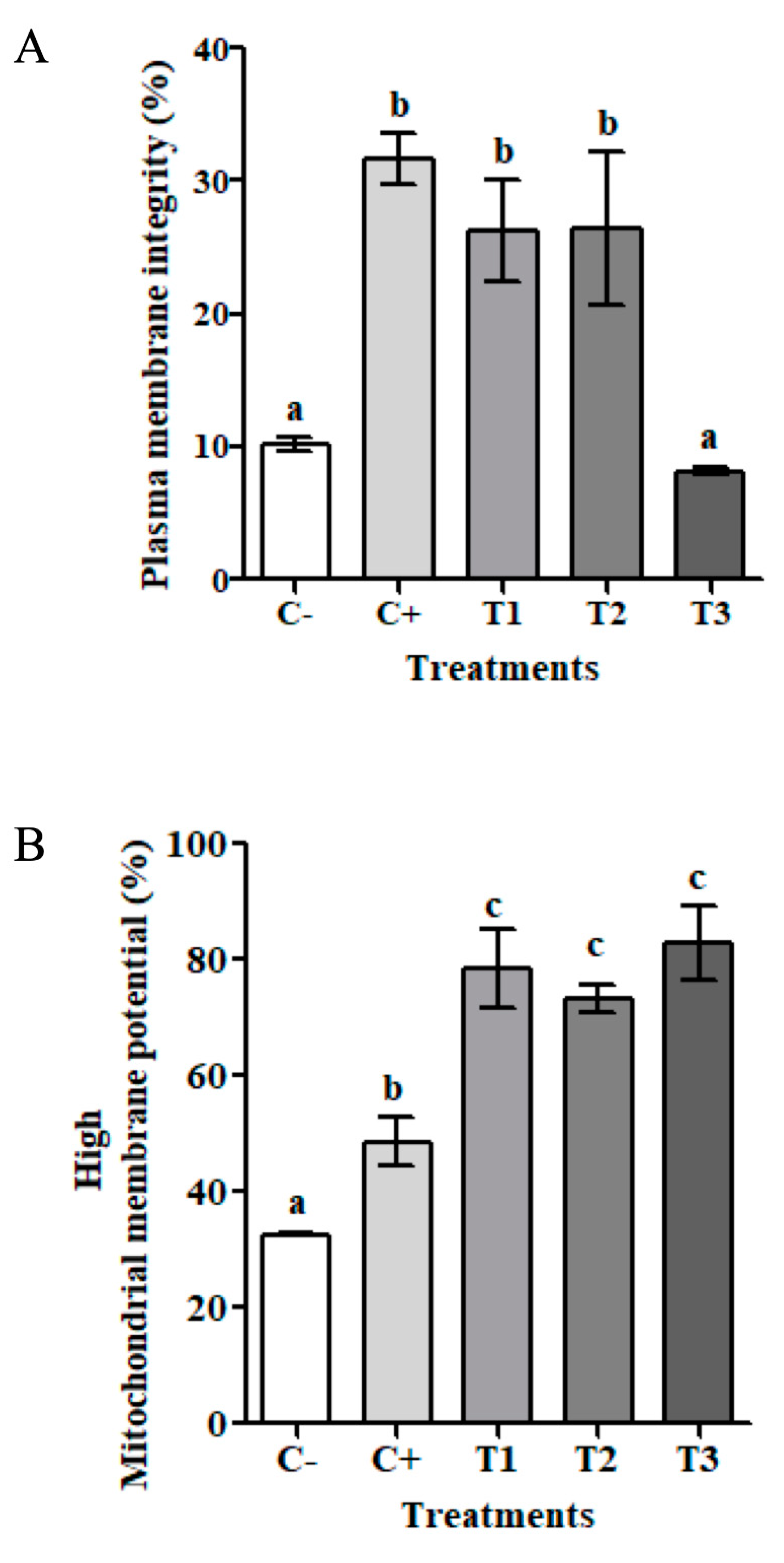
| Controls and Treatments | Description |
|---|---|
| C− | Semen diluted in Cortland® medium in the ratio 1:3 (semen: Cortland®) without cryoprotectants. |
| C+ | Semen diluted in Cortland® medium supplemented with 1.3 M Me2SO; 0.3 M glucose and 2% (w/v) BSA in the ratio 1:3 (semen: freezing medium). |
| T1 | Semen diluted in Cortland® medium supplemented with 1.3 M Me2SO; 0.3 M glucose, 2% (w/v) BSA and 0.05 mg/mL apoplastic extract in the ratio 1:3 (semen: freezing medium). |
| T2 | Semen diluted in Cortland® medium supplemented with 1.3 M Me2SO; 0.3 M glucose and 0.05 mg/mL of apoplastic extract in the ratio 1:3 (semen: freezing medium). |
| T3 | Semen diluted in Cortland® medium supplemented with 0.05 mg/mL apoplastic extract in the ratio 1:3 (semen: freezing medium). |
| Controls and Treatments | Total Motility (%) |
|---|---|
| C− | 100 (E) |
| C+ | 0–1 (L) |
| T1 | 0–1 (L) |
| T2 | 2.8 (L); 0.80 (M); 0.22 (R) |
| T3 | 100 (E) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Short, S.E.; Zamorano, M.; Aranzaez-Ríos, C.; Lee-Estevez, M.; Díaz, R.; Quiñones, J.; Ulloa-Rodríguez, P.; Villalobos, E.F.; Bravo, L.A.; Graether, S.P.; et al. Novel Apoplastic Antifreeze Proteins of Deschampsia antarctica as Enhancer of Common Cell Freezing Media for Cryobanking of Genetic Resources, a Preliminary Study. Biomolecules 2024, 14, 174. https://doi.org/10.3390/biom14020174
Short SE, Zamorano M, Aranzaez-Ríos C, Lee-Estevez M, Díaz R, Quiñones J, Ulloa-Rodríguez P, Villalobos EF, Bravo LA, Graether SP, et al. Novel Apoplastic Antifreeze Proteins of Deschampsia antarctica as Enhancer of Common Cell Freezing Media for Cryobanking of Genetic Resources, a Preliminary Study. Biomolecules. 2024; 14(2):174. https://doi.org/10.3390/biom14020174
Chicago/Turabian StyleShort, Stefania E., Mauricio Zamorano, Cristian Aranzaez-Ríos, Manuel Lee-Estevez, Rommy Díaz, John Quiñones, Patricio Ulloa-Rodríguez, Elías Figueroa Villalobos, León A. Bravo, Steffen P. Graether, and et al. 2024. "Novel Apoplastic Antifreeze Proteins of Deschampsia antarctica as Enhancer of Common Cell Freezing Media for Cryobanking of Genetic Resources, a Preliminary Study" Biomolecules 14, no. 2: 174. https://doi.org/10.3390/biom14020174
APA StyleShort, S. E., Zamorano, M., Aranzaez-Ríos, C., Lee-Estevez, M., Díaz, R., Quiñones, J., Ulloa-Rodríguez, P., Villalobos, E. F., Bravo, L. A., Graether, S. P., & Farías, J. G. (2024). Novel Apoplastic Antifreeze Proteins of Deschampsia antarctica as Enhancer of Common Cell Freezing Media for Cryobanking of Genetic Resources, a Preliminary Study. Biomolecules, 14(2), 174. https://doi.org/10.3390/biom14020174










