Bacterial Decontamination of Water-Containing Objects Using Piezoelectric Direct Discharge Plasma and Plasma Jet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plasma Source and Treatment Methods
2.2. Methods for Determination of the Concentration of Chemical Compounds in Liquid
2.3. Bacterial Culture and Cultivation Protocol
2.4. Protocol for the Treatment of Bacterial Suspension Cultures
2.5. Protocol for the Treatment of Bacterial Cells on Agar
3. Results
3.1. Reactive Oxygen and Nitrogen Species Generation in Phosphate-Buffered Saline
3.2. Plasma Treatment of Bacterial Culture
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adamovich, I.; Agarwal, S.; Ahedo, E.; Alves, L.L.; Baalrud, S.; Babaeva, N.; Bogaerts, A.; Bourdon, A.; Bruggeman, P.J.; Canal, C.; et al. The 2022 Plasma Roadmap: Low Temperature Plasma Science and Technology. J. Phys. D Appl. Phys. 2022, 55, 373001. [Google Scholar] [CrossRef]
- Yan, S.; Komasa, S.; Agariguchi, A.; Pezzotti, G.; Okazaki, J.; Maekawa, K. Osseointegration Properties of Titanium Implants Treated by Nonthermal Atmospheric-Pressure Nitrogen Plasma. Int. J. Mol. Sci. 2022, 23, 15420. [Google Scholar] [CrossRef]
- Korzec, D.; Hoppenthaler, F.; Andres, T.; Guentner, S.; Lerach, S. Application of Nitrogen Piezoelectric Direct Discharge for Increase in Surface Free Energy of Polymers. Plasma 2022, 5, 111–129. [Google Scholar] [CrossRef]
- Korzec, D.; Hoffmann, M.; Nettesheim, S. Application of Plasma Bridge for Grounding of Conductive Substrates Treated by Transferred Pulsed Atmospheric Arc. Plasma 2023, 6, 139–161. [Google Scholar] [CrossRef]
- Lebedev, Y.A.; Shakhatov, V.A. Decomposition of Carbon Dioxide in Microwave Discharges (an Analytical Review). Russ. J. Appl. Chem. 2022, 95, 1–20. [Google Scholar] [CrossRef]
- Babaeva, N.Y.; Naidis, G.V.; Tereshonok, D.V.; Chernyshev, T.V.; Volkov, L.S.; Vasiliev, M.M.; Petrov, O.F. CO2 Conversion in a Microwave Plasma Torch: 2D vs 1D Approaches. Plasma Sources Sci. Technol. 2023, 32, 054001. [Google Scholar] [CrossRef]
- Małajowicz, J.; Khachatryan, K.; Kozłowska, M. Properties of Water Activated with Low-Temperature Plasma in the Context of Microbial Activity. Beverages 2022, 8, 63. [Google Scholar] [CrossRef]
- Veerana, M.; Yu, N.; Ketya, W.; Park, G. Application of Non-Thermal Plasma to Fungal Resources. J. Fungi 2022, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.M.; Robinson Junior, N.A.; Mok, Y.S.; Wu, S. Investigation of Silver Nanoparticle Synthesis with Various Nonthermal Plasma Reactor Configurations. Arab. J. Chem. 2023, 16, 105174. [Google Scholar] [CrossRef]
- Nonnenmacher, L.; Fischer, M.; Haralambiev, L.; Bekeschus, S.; Schulze, F.; Wassilew, G.I.; Schoon, J.; Reichert, J.C. Orthopaedic Applications of Cold Physical Plasma. EFORT Open Rev. 2023, 8, 409–423. [Google Scholar] [CrossRef] [PubMed]
- Koga-Ito, C.Y.; Kostov, K.G.; Miranda, F.S.; Milhan, N.V.M.; Azevedo Neto, N.F.; Nascimento, F.; Pessoa, R.S. Cold Atmospheric Plasma as a Therapeutic Tool in Medicine and Dentistry. Plasma Chem. Plasma Process 2023. [Google Scholar] [CrossRef]
- Lotfi, M.; Khani, M.; Shokri, B. A Review of Cold Atmospheric Plasma Applications in Dermatology and Aesthetics. Plasma Med. 2023, 13, 39–63. [Google Scholar] [CrossRef]
- Moszczyńska, J.; Roszek, K.; Wiśniewski, M. Non-Thermal Plasma Application in Medicine—Focus on Reactive Species Involvement. Int. J. Mol. Sci. 2023, 24, 12667. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.; Gromov, M.; Nikiforov, A.; Smits, E.; Bogaerts, A. Characterization of Non-Thermal Dielectric Barrier Discharges for Plasma Medicine: From Plastic Well Plates to Skin Surfaces. Plasma Chem. Plasma Process 2023, 43, 1587. [Google Scholar] [CrossRef]
- Konchekov, E.M.; Gusein-zade, N.; Burmistrov, D.E.; Kolik, L.V.; Dorokhov, A.S.; Izmailov, A.Y.; Shokri, B.; Gudkov, S.V. Advancements in Plasma Agriculture: A Review of Recent Studies. Int. J. Mol. Sci. 2023, 24, 15093. [Google Scholar] [CrossRef]
- Ranieri, P.; Sponsel, N.; Kizer, J.; Rojas-Pierce, M.; Hernández, R.; Gatiboni, L.; Grunden, A.; Stapelmann, K. Plasma Agriculture: Review from the Perspective of the Plant and Its Ecosystem. Plasma Process Polym. 2021, 18, 2000162. [Google Scholar] [CrossRef]
- Leti, L.-I.; Gerber, I.C.; Mihaila, I.; Galan, P.-M.; Strajeru, S.; Petrescu, D.-E.; Cimpeanu, M.-M.; Topala, I.; Gorgan, D.-L. The Modulatory Effects of Non-Thermal Plasma on Seed’s Morphology, Germination and Genetics—A Review. Plants 2022, 11, 2181. [Google Scholar] [CrossRef]
- Mildaziene, V.; Ivankov, A.; Sera, B.; Baniulis, D. Biochemical and Physiological Plant Processes Affected by Seed Treatment with Non-Thermal Plasma. Plants 2022, 11, 856. [Google Scholar] [CrossRef] [PubMed]
- Pańka, D.; Jeske, M.; Łukanowski, A.; Baturo-Cieśniewska, A.; Prus, P.; Maitah, M.; Maitah, K.; Malec, K.; Rymarz, D.; Muhire, J.D.D.; et al. Can Cold Plasma Be Used for Boosting Plant Growth and Plant Protection in Sustainable Plant Production? Agronomy 2022, 12, 841. [Google Scholar] [CrossRef]
- Kuzin, A.; Solovchenko, A.; Khort, D.; Filippov, R.; Lukanin, V.; Lukina, N.; Astashev, M.; Konchekov, E. Effects of Plasma-Activated Water on Leaf and Fruit Biochemical Composition and Scion Growth in Apple. Plants 2023, 12, 385. [Google Scholar] [CrossRef] [PubMed]
- Soni, A.; Choi, J.; Brightwell, G. Plasma-Activated Water (PAW) as a Disinfection Technology for Bacterial Inactivation with a Focus on Fruit and Vegetables. Foods 2021, 10, 166. [Google Scholar] [CrossRef]
- Gavahian, M.; Sarangapani, C.; Misra, N.N. Cold Plasma for Mitigating Agrochemical and Pesticide Residue in Food and Water: Similarities with Ozone and Ultraviolet Technologies. Food Res. Int. 2021, 141, 110138. [Google Scholar] [CrossRef]
- Laroussi, M.; Bekeschus, S.; Keidar, M.; Bogaerts, A.; Fridman, A.; Lu, X.; Ostrikov, K.; Hori, M.; Stapelmann, K.; Miller, V.; et al. Low-Temperature Plasma for Biology, Hygiene, and Medicine: Perspective and Roadmap. IEEE Trans. Radiat. Plasma Med. Sci. 2022, 6, 127–157. [Google Scholar] [CrossRef]
- Zhou, R.; Zhou, R.; Wang, P.; Xian, Y.; Mai-Prochnow, A.; Lu, X.; Cullen, P.J.; Ostrikov, K.; Bazaka, K. Plasma-Activated Water: Generation, Origin of Reactive Species and Biological Applications. J. Phys. D Appl. Phys. 2020, 53, 303001. [Google Scholar] [CrossRef]
- Shaji, M.; Rabinovich, A.; Surace, M.; Sales, C.; Fridman, A. Physical Properties of Plasma-Activated Water. Plasma 2023, 6, 45–57. [Google Scholar] [CrossRef]
- Abduvokhidov, D.; Yusupov, M.; Shahzad, A.; Attri, P.; Shiratani, M.; Oliveira, M.C.; Razzokov, J. Unraveling the Transport Properties of RONS across Nitro-Oxidized Membranes. Biomolecules 2023, 13, 1043. [Google Scholar] [CrossRef] [PubMed]
- Ghasemitarei, M.; Ghorbi, T.; Yusupov, M.; Zhang, Y.; Zhao, T.; Shali, P.; Bogaerts, A. Effects of Nitro-Oxidative Stress on Biomolecules: Part 1—Non-Reactive Molecular Dynamics Simulations. Biomolecules 2023, 13, 1371. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Thompson, E.W.; Ostrikov, K. Receptor-Mediated Redox Imbalance: An Emerging Clinical Avenue against Aggressive Cancers. Biomolecules 2022, 12, 1880. [Google Scholar] [CrossRef] [PubMed]
- Biryukov, M.; Semenov, D.; Kryachkova, N.; Polyakova, A.; Patrakova, E.; Troitskaya, O.; Milakhina, E.; Poletaeva, J.; Gugin, P.; Ryabchikova, E.; et al. The Molecular Basis for Selectivity of the Cytotoxic Response of Lung Adenocarcinoma Cells to Cold Atmospheric Plasma. Biomolecules 2023, 13, 1672. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Gong, F.; Jin, T.; Liu, Q.; Fang, H.; Chen, Y.; Wang, G.; Chu, P.K.; Wu, Z.; Ostrikov, K. Dose-Dependent Effects in Plasma Oncotherapy: Critical In Vivo Immune Responses Missed by In Vitro Studies. Biomolecules 2023, 13, 707. [Google Scholar] [CrossRef] [PubMed]
- Danileyko, Y.K.; Belov, S.V.; Egorov, A.B.; Lukanin, V.I.; Apasheva, L.M.; Ovcharenko, E.N.; Lobanov, A.V.; Astashev, M.E.; Simakin, A.V.; Shkirin, A.V.; et al. Portable Technology for Obtaining Plasma-Activated Water to Stimulate the Growth of Spruce and Strawberry Plants. Horticulturae 2023, 9, 1142. [Google Scholar] [CrossRef]
- Ashurov, M.K.; Ashurov, E.M.; Astashev, M.E.; Baimler, I.V.; Gudkov, S.V.; Konchekov, E.M.; Lednev, V.N.; Lukina, N.A.; Matveeva, T.A.; Markendudis, A.G.; et al. Development of an Environmentally Friendly Technology for the Treatment of Aqueous Solutions with High-Purity Plasma for the Cultivation of Cotton, Wheat and Strawberries. ChemEngineering 2022, 6, 91. [Google Scholar] [CrossRef]
- Korzec, D.; Hoppenthaler, F.; Nettesheim, S. Piezoelectric Direct Discharge: Devices and Applications. Plasma 2020, 4, 1–41. [Google Scholar] [CrossRef]
- Korzec, D.; Hoppenthaler, F.; Burger, D.; Andres, T.; Nettesheim, S. Atmospheric Pressure Plasma Jet Powered by Piezoelectric Direct Discharge. Plasma Process. Polym. 2020, 17, 2000053. [Google Scholar] [CrossRef]
- Johnson, M.J.; Boris, D.R.; Petrova, T.B.; Walton, S.G. Characterization of a Compact, Low-Cost Atmospheric-Pressure Plasma Jet Driven by a Piezoelectric Transformer. IEEE Trans. Plasma Sci. 2019, 47, 434–444. [Google Scholar] [CrossRef]
- Akopdzhanov, A.G.; Sveshnikova, E.D.; Guseyn-zade, N.G.; Kolik, L.V.; Konchekov, E.M.; Shimanovskiy, N.L. Enhancement of the Cytotoxic Effect of Doxorubicin on Tumor Cells Upon Exposure to Atmospheric Cold Plasma. Pharm. Chem. J. 2021, 55, 11–13. [Google Scholar] [CrossRef]
- Pavlik, T.; Gusein-Zade, N. Characterizing the Biological Effects of Plasma-Activated Physiological Saline. Plasma Med. 2022, 12, 1–11. [Google Scholar] [CrossRef]
- Pavlik, T.I.; Gusein-zade, N.G.; Kolik, L.V.; Shimanovskii, N.L. Comparison of the Biological Properties of Plasma-Treated Solution and Solution of Chemical Reagents. Appl. Sci. 2022, 12, 3704. [Google Scholar] [CrossRef]
- Pavlik, T.; Gudkova, V.; Razvolyaeva, D.; Pavlova, M.; Kostukova, N.; Miloykovich, L.; Kolik, L.; Konchekov, E.; Shimanovskii, N. The Role of Autophagy and Apoptosis in the Combined Action of Plasma-Treated Saline, Doxorubicin, and Medroxyprogesterone Acetate on K562 Myeloid Leukaemia Cells. Int. J. Mol. Sci. 2023, 24, 5100. [Google Scholar] [CrossRef] [PubMed]
- Konchekov, E.M.; Kolik, L.V.; Danilejko, Y.K.; Belov, S.V.; Artem’ev, K.V.; Astashev, M.E.; Pavlik, T.I.; Lukanin, V.I.; Kutyrev, A.I.; Smirnov, I.G.; et al. Enhancement of the Plant Grafting Technique with Dielectric Barrier Discharge Cold Atmospheric Plasma and Plasma-Treated Solution. Plants 2022, 11, 1373. [Google Scholar] [CrossRef]
- Astashev, M.E.; Konchekov, E.M.; Kolik, L.V.; Gudkov, S.V. Electric Impedance Spectroscopy in Trees Condition Analysis: Theory and Experiment. Sensors 2022, 22, 8310. [Google Scholar] [CrossRef] [PubMed]
- Izmailov, A.; Khort, D.; Filippov, R.; Pishchalnikov, R.Y.; Simakin, A.V.; Shogenov, Y. Improvement of Winter Graft Techniques Using Cold Plasma and Plasma-Treated Solution on Cherry Cultures. Appl. Sci. 2022, 12, 4953. [Google Scholar] [CrossRef]
- Stekke, J.; Tendero, C.; Tierce, P.; Courtois, C.; Rauwel, G.; Lo, J.; Guillot, P.; Pigache, F. Low-Voltage Plasma Jet With Piezoelectric Generator: Preliminary Evaluation of Decontamination Capabilities. IEEE Trans. Plasma Sci. 2020, 48, 1264–1270. [Google Scholar] [CrossRef]
- Herianto, S.; Shih, M.-K.; Lin, C.-M.; Hung, Y.-C.; Hsieh, C.-W.; Wu, J.-S.; Chen, M.-H.; Chen, H.-L.; Hou, C.-Y. The Effects of Glazing with Plasma-Activated Water Generated by a Piezoelectric Direct Discharge Plasma System on Whiteleg Shrimp (Litopenaeus Vannamei). LWT 2022, 154, 112547. [Google Scholar] [CrossRef]
- Artem’ev, K.V.; Bogachev, N.N.; Gusein-zade, N.G.; Dolmatov, T.V.; Kolik, L.V.; Konchekov, E.M.; Andreev, S.E. Study of Characteristics of the Cold Atmospheric Plasma Source Based on a Piezo Transformer. Russ. Phys. J. 2020, 62, 2073–2080. [Google Scholar] [CrossRef]
- Konchekov, E.M.; Glinushkin, A.P.; Kalinitchenko, V.P.; Artem’ev, K.V.; Burmistrov, D.E.; Kozlov, V.A.; Kolik, L.V. Properties and Use of Water Activated by Plasma of Piezoelectric Direct Discharge. Front. Phys. 2021, 8, 616385. [Google Scholar] [CrossRef]
- Gudkova, V.V.; Razvolyaeva, D.A.; Borzosekov, V.D.; Konchekov, E.M. Features of the FOX and Griess Method for Assessing the Biological Activity of Plasma Treated Solutions. Plasma Chem. Plasma Process 2024, 44, 305. [Google Scholar] [CrossRef]
- Wolff, S.P. [18] Ferrous Ion Oxidation in Presence of Ferric Ion Indicator Xylenol Orange for Measurement of Hydroperoxides. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1994; Volume 233, pp. 182–189. ISBN 978-0-12-182134-0. [Google Scholar]
- Tarabová, B.; Lukeš, P.; Janda, M.; Hensel, K.; Šikurová, L.; Machala, Z. Specificity of Detection Methods of Nitrites and Ozone in Aqueous Solutions Activated by Air Plasma. Plasma Process. Polym. 2018, 15, 1800030. [Google Scholar] [CrossRef]
- Nicol, M.J.; Brubaker, T.R.; Honish, B.J.; Simmons, A.N.; Kazemi, A.; Geissel, M.A.; Whalen, C.T.; Siedlecki, C.A.; Bilén, S.G.; Knecht, S.D.; et al. Antibacterial Effects of Low-Temperature Plasma Generated by Atmospheric-Pressure Plasma Jet Are Mediated by Reactive Oxygen Species. Sci. Rep. 2020, 10, 3066. [Google Scholar] [CrossRef]
- Klämpfl, T.G.; Isbary, G.; Shimizu, T.; Li, Y.-F.; Zimmermann, J.L.; Stolz, W.; Schlegel, J.; Morfill, G.E.; Schmidt, H.-U. Cold Atmospheric Air Plasma Sterilization against Spores and Other Microorganisms of Clinical Interest. Appl. Environ. Microbiol. 2012, 78, 5077–5082. [Google Scholar] [CrossRef]
- Yusupov, M.; Bogaerts, A.; Huygh, S.; Snoeckx, R.; Van Duin, A.C.T.; Neyts, E.C. Plasma-Induced Destruction of Bacterial Cell Wall Components: A Reactive Molecular Dynamics Simulation. J. Phys. Chem. C 2013, 117, 5993–5998. [Google Scholar] [CrossRef]
- Yusupov, M.; Neyts, E.C.; Khalilov, U.; Snoeckx, R.; Van Duin, A.C.T.; Bogaerts, A. Atomic-Scale Simulations of Reactive Oxygen Plasma Species Interacting with Bacterial Cell Walls. New J. Phys. 2012, 14, 093043. [Google Scholar] [CrossRef]
- Mai-Prochnow, A.; Murphy, A.B.; McLean, K.M.; Kong, M.G.; Ostrikov, K. Atmospheric Pressure Plasmas: Infection Control and Bacterial Responses. Int. J. Antimicrob. Agents 2014, 43, 508–517. [Google Scholar] [CrossRef]
- Mai-Prochnow, A.; Clauson, M.; Hong, J.; Murphy, A.B. Gram Positive and Gram Negative Bacteria Differ in Their Sensitivity to Cold Plasma. Sci. Rep. 2016, 6, 38610. [Google Scholar] [CrossRef]
- Bekeschus, S.; Schmidt, A.; Weltmann, K.-D.; Von Woedtke, T. The Plasma Jet kINPen—A Powerful Tool for Wound Healing. Clin. Plasma Med. 2016, 4, 19–28. [Google Scholar] [CrossRef]
- Čobanović, R.; Maletić, D.; Kocić-Tanackov, S.; Čabarkapa, I.; Kokić, B.; Kojić, P.; Milošević, S.; Stulić, V.; Pavičić, T.V.; Vukić, M. Comparison of the Bacterial Inactivation Efficiency of Water Activated by a Plasma Jet Source and a Pin-to-Pin Electrode Configuration Source. Processes 2023, 11, 3286. [Google Scholar] [CrossRef]
- Laroque, D.A.; Seó, S.T.; Valencia, G.A.; Laurindo, J.B.; Carciofi, B.A.M. Cold Plasma in Food Processing: Design, Mechanisms, and Application. J. Food Eng. 2022, 312, 110748. [Google Scholar] [CrossRef]
- Jodzis, S.; Baran, K. The Influence of Gas Temperature on Ozone Generation and Decomposition in Ozone Generator. How Is Ozone Decomposed? Vacuum 2022, 195, 110647. [Google Scholar] [CrossRef]
- Korzec, D.; Hoppenthaler, F.; Shestakov, A.; Burger, D.; Shapiro, A.; Andres, T.; Lerach, S.; Nettesheim, S. Multi-Device Piezoelectric Direct Discharge for Large Area Plasma Treatment. Plasma 2021, 4, 281–293. [Google Scholar] [CrossRef]




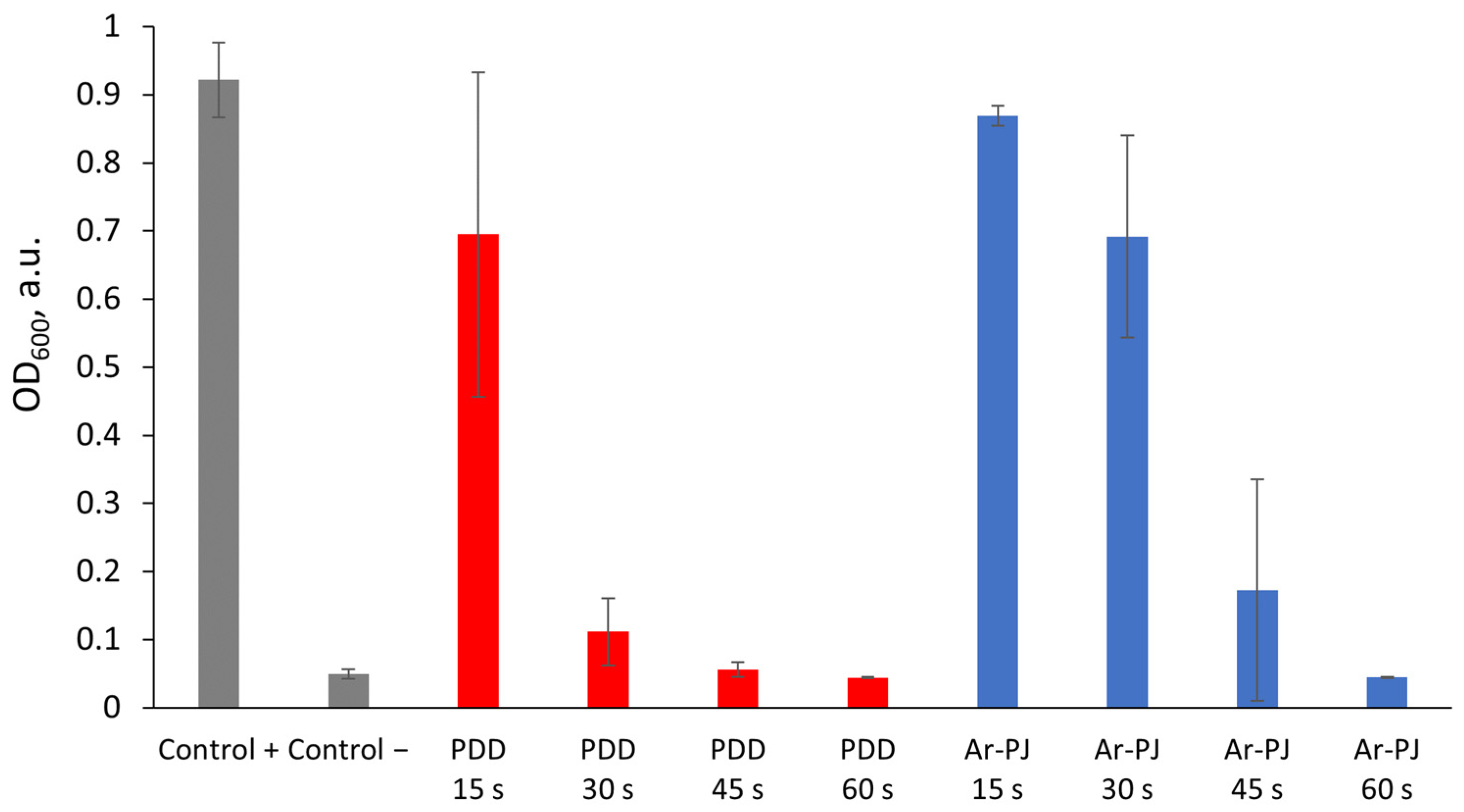

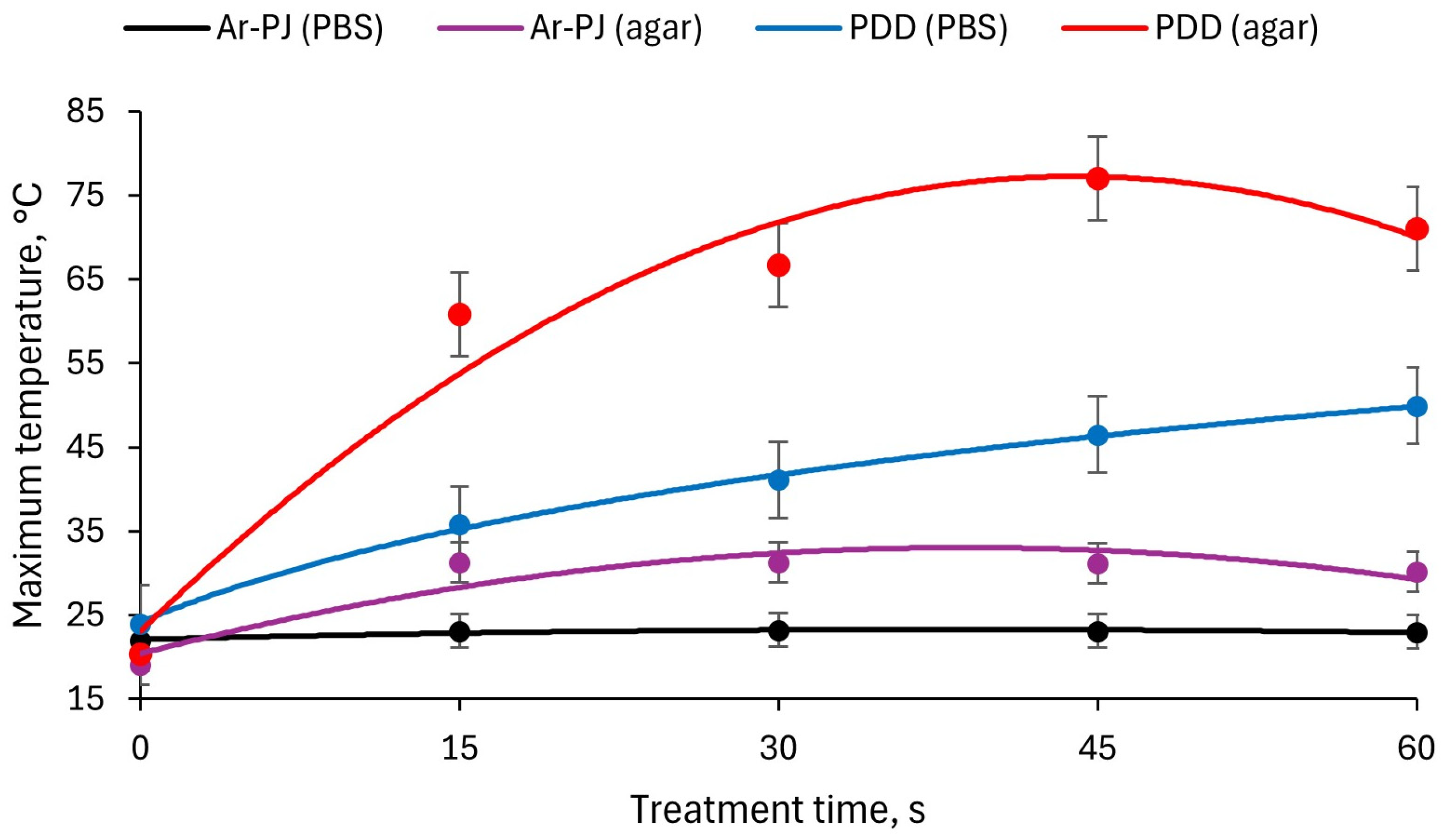
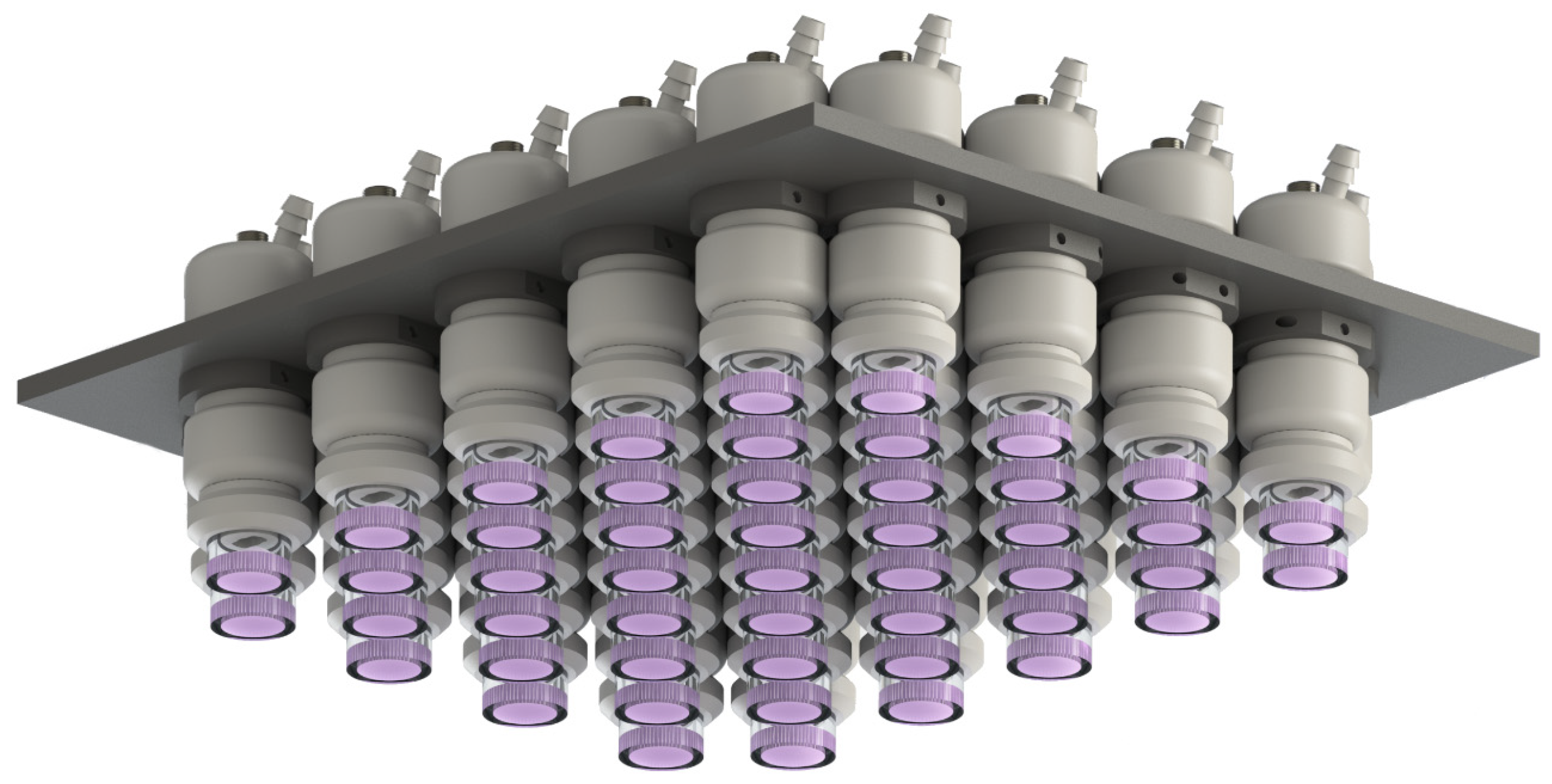
| CAP Type | Contaminated Object | Treatment Duration, s |
|---|---|---|
| PDD | PBS | 15 |
| PBS | 30 | |
| PBS | 45 | |
| PBS | 60 | |
| Agar | 45 | |
| Agar | 300 | |
| Ar-PJ | PBS | 15 |
| PBS | 30 | |
| PBS | 45 | |
| PBS | 60 | |
| Agar | 45 | |
| Agar | 300 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konchekov, E.M.; Gudkova, V.V.; Burmistrov, D.E.; Konkova, A.S.; Zimina, M.A.; Khatueva, M.D.; Polyakova, V.A.; Stepanenko, A.A.; Pavlik, T.I.; Borzosekov, V.D.; et al. Bacterial Decontamination of Water-Containing Objects Using Piezoelectric Direct Discharge Plasma and Plasma Jet. Biomolecules 2024, 14, 181. https://doi.org/10.3390/biom14020181
Konchekov EM, Gudkova VV, Burmistrov DE, Konkova AS, Zimina MA, Khatueva MD, Polyakova VA, Stepanenko AA, Pavlik TI, Borzosekov VD, et al. Bacterial Decontamination of Water-Containing Objects Using Piezoelectric Direct Discharge Plasma and Plasma Jet. Biomolecules. 2024; 14(2):181. https://doi.org/10.3390/biom14020181
Chicago/Turabian StyleKonchekov, Evgeny M., Victoria V. Gudkova, Dmitriy E. Burmistrov, Aleksandra S. Konkova, Maria A. Zimina, Mariam D. Khatueva, Vlada A. Polyakova, Alexandra A. Stepanenko, Tatyana I. Pavlik, Valentin D. Borzosekov, and et al. 2024. "Bacterial Decontamination of Water-Containing Objects Using Piezoelectric Direct Discharge Plasma and Plasma Jet" Biomolecules 14, no. 2: 181. https://doi.org/10.3390/biom14020181







