Exploring FDA-Approved Frontiers: Insights into Natural and Engineered Peptide Analogues in the GLP-1, GIP, GHRH, CCK, ACTH, and α-MSH Realms
Abstract
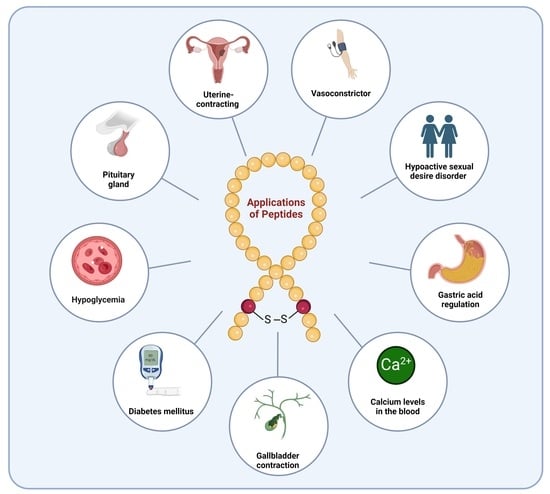
:1. Introduction
2. Natural Peptides
2.1. Insulin (Iletin)
2.2. Corticotropin (H.P. Acthar Gel)
2.3. Cyclosporine (Sandimmune)
2.4. Oxytocin (Syntocinon)
2.5. Glucagon (Baqsimi)
2.6. Secretin (ChiRhoStim)
2.7. Calcitonin (Miacalcin)
2.8. Vasopressin (Vasostrict)
2.9. Parathyroid Hormone (PTH) (Natpara)
2.10. Angiotensin II (Giapreza)
3. Diabetes Peptide-Based Drugs
3.1. Diabetes Type I
3.1.1. Insulin
3.1.2. Pramlintide (Symlin)
3.2. Diabetes Type 2
3.2.1. Exenatide (Byetta)
3.2.2. Liraglutide (Victoza)
3.2.3. Lixisenatide (Adlyxin)
3.2.4. Albiglutide (Tanzeum)
3.2.5. Dulaglutide (Trulicity)
3.2.6. Semaglutide (Ozempic)
3.2.7. Tirzepatide (Mounjaro)
4. Growth-Hormone-Releasing Hormone (GHRH) Analogues
4.1. Sermorelin (Geref)
4.2. Mecasermin (Increlex)
4.3. Tesamorelin (Egrifta)
4.4. Macimorelin (Macrilen)
5. Cholecystokinin Analogues (CCK)
Sincalide (Kinevac)
6. Adrenocorticotropic Hormone (ACTH) and Analogues
6.1. Corticorelin (Acthrel)
6.2. Corticotropin (Cosyntropin)
7. α-Melanocyte Stimulating Hormone (α-MSH) Analogues
7.1. Afamelanotide (Scenesse)
7.2. Bremelanotide (Vyleesi)
7.3. Setmelanotide (Imcivree)
8. Conclusions
Funding
Conflicts of Interest
References
- Al Musaimi, O.; Lombardi, L.; Williams, D.R.; Albericio, F. Strategies for Improving Peptide Stability and Delivery. Pharmaceuticals 2022, 15, 1283. [Google Scholar] [CrossRef] [PubMed]
- Tsomaia, N. Peptide therapeutics: Targeting the undruggable space. Eur. J. Med. Chem. 2015, 94, 459–470. [Google Scholar] [CrossRef]
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Therapeutic peptides: Current applications and future directions. Sig. Transduct. Target Ther. 2022, 7, 48. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Williams, P.; Albericio, F.; Giralt, E. Chemical Approaches to the Synthesis of Peptides and Proteins; CRC Press: Boca Raton, FL, USA, 1997. [Google Scholar]
- Eggen, I.F.; Bakelaar, F.T.; Petersen, A.; Ten Kortenaar, P.B.W.; Ankone, N.H.S.; Bijsterveld, H.E.J.M.; Bours, G.H.L.; Bellaj, F.E.L.; Hartsuiker, M.J.; Kuiper, G.J.; et al. A novel method for repetitive peptide synthesis in solution without isolation of intermediates. J. Pept. Sci. 2005, 11, 633–641. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, A.; de la Torre, B.G.; Albericio, F. Liquid-Phase Peptide Synthesis (LPPS): A Third Wave for the Preparation of Peptides. Chem. Rev. 2022, 122, 13516–13546. [Google Scholar] [CrossRef]
- Craik, D.J.; Fairlie, D.P.; Liras, S.; Price, D. The future of peptide-based drugs. Chem. Biol. Drug Des. 2013, 81, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Fosgerau, K.; Hoffmann, T. Peptide therapeutics: Current status and future directions. Drug Discov. 2015, 20, 122–128. [Google Scholar] [CrossRef]
- Fetse, J.; Kandel, S.; Mamani, U.-F.; Cheng, K. Recent advances in the development of therapeutic peptides. Trends Pharmacol. Sci. 2023, 44, 425–441. [Google Scholar] [CrossRef]
- White, C.J.; Yudin, A.K. Contemporary strategies for peptide macrocyclization. Nat. Chem. 2011, 3, 509–524. [Google Scholar] [CrossRef]
- Pelay-Gimeno, M.; Tulla-Puche, J.; Albericio, F. “Head-to-Side-Chain” Cyclodepsipeptides of Marine Origin. Mar. Drugs 2013, 11, 1693–1717. [Google Scholar] [CrossRef]
- Mourão, C.B.F.; Brand, G.D.; Fernandes, J.P.C.; Prates, M.V.; Bloch, C., Jr.; Barbosa, J.; Freitas, S.M.; Restano-Cassulini, R.; Possani, L.D.; Schwartz, E.F. Head-to-Tail Cyclization after Interaction with Trypsin: A Scorpion Venom Peptide that Resembles Plant Cyclotides. J. Med. Chem. 2020, 63, 9500–9511. [Google Scholar] [CrossRef] [PubMed]
- Werner, H.M.; Cabalteja, C.C.; Horne, W.S. Peptide Backbone Composition and Protease Susceptibility: Impact of Modification Type, Position, and Tandem Substitution. Chembiochem 2016, 17, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Zhan, C.; Chen, X.; Hou, J.; Xie, C.; Lu, W. Retro-Inverso Isomer of Angiopep-2: A Stable d-Peptide Ligand Inspires Brain-Targeted Drug Delivery. Mol. Pharm. 2014, 11, 3261–3268. [Google Scholar] [CrossRef]
- Chatterjee, J.; Rechenmacher, F.; Kessler, H. N-methylation of peptides and proteins: An important element for modulating biological functions. Angew Chem. Int. Ed. Engl. 2013, 52, 254–269. [Google Scholar] [CrossRef]
- Cheloha, R.W.; Watanabe, T.; Dean, T.; Gellman, S.H.; Gardella, T.J. Backbone Modification of a Parathyroid Hormone Receptor-1 Antagonist/Inverse Agonist. ACS Chem. Biol. 2016, 11, 2752–2762. [Google Scholar] [CrossRef]
- Vecchio, I.; Tornali, C.; Bragazzi, N.L.; Martini, M. The Discovery of Insulin: An Important Milestone in the History of Medicine. Front. Endocrinol. 2018, 9, 613. [Google Scholar] [CrossRef]
- Scott, D.A.; Best, C.H. The Preparation of Insulin. Ind. Eng. Chem. 1925, 17, 238–240. [Google Scholar] [CrossRef]
- Rosenfeld, L. Insulin: Discovery and controversy. Clin. Chem. 2002, 48, 2270–2288. Available online: https://pubmed.ncbi.nlm.nih.gov/12446492/ (accessed on 19 February 2024). [CrossRef]
- Quianzon, C.C.; Cheikh, I. History of insulin. J. Community Hosp. Intern. Med. Perspect. 2012, 2, 18701. [Google Scholar] [CrossRef]
- Insulin Drug Label. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2006/018344s026,018345s027lbl.pdf (accessed on 19 February 2024).
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef]
- Bedinger, D.H.; Adams, S.H. Metabolic, anabolic, and mitogenic insulin responses: A tissue-specific perspective for insulin receptor activators. Mol. Cell. Endocrinol. 2015, 415, 143–156. [Google Scholar] [CrossRef]
- McKay, M.M.; Morrison, D.K. Integrating signals from RTKs to ERK/MAPK. Oncogene 2007, 26, 3113–3121. [Google Scholar] [CrossRef]
- Pearson, G.; Robinson, F.; Beers Gibson, T.; Xu, B.-E.; Karandikar, M.; Berman, K.; Cobb, M.H. Mitogen-Activated Protein (MAP) Kinase Pathways: Regulation and Physiological Functions. Endocr. Rev. 2001, 22, 153–183. [Google Scholar] [CrossRef] [PubMed]
- Humalog Drug Label. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/020563s115lbl.pdf (accessed on 19 February 2024).
- NovoLog Drug Label. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020986s057lbl.pdf (accessed on 19 February 2024).
- Bell, P.H. Purification and Structure of β-Corticotropin. J. Am. Chem. Soc. 1954, 76, 5565–5567. [Google Scholar] [CrossRef]
- Howard, K.S.; Shepherd, R.G.; Eigner, E.A.; Davies, D.S.; Bell, P.H. Structure OF β-Corticotropin: Final Sequence Studies. J. Am. Chem. Soc. 1955, 77, 3419–3420. [Google Scholar] [CrossRef]
- Arborelius, L.; Owens, M.J.; Plotsky, P.M.; Nemeroff, C.B. The role of corticotropin-releasing factor in depression and anxiety disorders. J. Endocrinol. 1999, 160, 1–12. [Google Scholar] [CrossRef]
- Koob, G.F. Corticotropin-releasing factor, norepinephrine, and stress. Biol. Psychiatry 1999, 46, 1167–1180. [Google Scholar] [CrossRef] [PubMed]
- Corticotropin Drug Label. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2010/022432Orig1s000LBL.pdf (accessed on 19 February 2024).
- Corticotropin Approval Letter. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2010/022432Orig1s000Approv.pdf (accessed on 19 February 2024).
- Grammatopoulos, D.K. Insights into mechanisms of corticotropin-releasing hormone receptor signal transduction. Br. J. Pharmacol. 2012, 166, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Forsythe, P.; Paterson, S. Ciclosporin 10 years on: Indications and efficacy. Vet. Rec. 2014, 174 (Suppl. S2), 13–21. [Google Scholar] [CrossRef]
- Di Gioia, M.L.; Leggio, A.; Malagrinò, F.; Romio, E.; Siciliano, C.; Liguori, A. N-Methylated α-Amino Acids And Peptides: Synthesis And Biological Activity. Mini Rev. Med. Chem. 2016, 16, 683–690. [Google Scholar] [CrossRef]
- Cyclosporine Drug Label. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/050715s027,050716s028lbl.pdf (accessed on 19 February 2024).
- Faulds, D.; Goa, K.L.; Benfield, P. Cyclosporin. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in immunoregulatory disorders. Drugs 1993, 45, 953–1040. [Google Scholar] [CrossRef] [PubMed]
- Kapturczak, M.H.; Meier-Kriesche, H.U.; Kaplan, B. Pharmacology of calcineurin antagonists. Transplant. Proc. 2004, 36 (Suppl. S2), 25s–32s. [Google Scholar] [CrossRef] [PubMed]
- Colombo, D.; Ammirati, E. Cyclosporine in transplantation—A history of converging timelines. J. Biol. Regul. Homeost. Agents 2011, 25, 493–504. [Google Scholar] [PubMed]
- Stähelin, H.F. The history of cyclosporin A (Sandimmune®) revisited: Another point of view. Experientia 1996, 52, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Cyclosporine Approval Letter. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2000/65017a_Cyclosporine_Approv.pdf (accessed on 19 February 2024).
- du Vigneaud, V.; Ressler, C.; Trippett, S. The sequence of amino acids in oxytocin, with a proposal for the structure of oxytocin. J. Biol. Chem. 1953, 205, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Vigneaud, V.d.; Ressler, C.; Swan, C.J.M.; Roberts, C.W.; Katsoyannis, P.G.; Gordon, S. The synthesis of an octapeptide amide with the hormonal activity of oxytocin. J. Am. Chem. Soc. 1953, 75, 4879–4880. [Google Scholar] [CrossRef]
- Vigneaud, V.d.; Ressler, C.; Swan, J.M.; Roberts, C.W.; Katsoyannis, P.G. The synthesis of oxytocin. J. Am. Chem. Soc. 1954, 76, 3115–3121. [Google Scholar] [CrossRef]
- Bodanszky, M.; Du Vigneaud, V. Synthesis of oxytocin by the nitrophenyl ester method. Nature 1959, 183, 1324–1325. [Google Scholar] [CrossRef]
- du Vigneaud, V. Trail of Sulfur Research: From Insulin to Oxytocin. Science 1956, 123, 967–974. [Google Scholar] [CrossRef]
- Smyth, D.G. On the molecular mechanism of oxytocin action. Biochim. Biophys. Acta (BBA) Protein Struct. 1970, 200, 395–403. [Google Scholar] [CrossRef]
- Arias, F. Pharmacology of Oxytocin and Prostaglandins. Clin. Obstet. Gynecol. 2000, 43, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Arrowsmith, S.; Wray, S. Oxytocin: Its Mechanism of Action and Receptor Signalling in the Myometrium. J. Neuroendocrinol. 2014, 26, 356–369. [Google Scholar] [CrossRef]
- Mitchell, B.; Fang, X.; Wong, S. Oxytocin: A paracrine hormone in the regulation of parturition? Rev. Reprod. 1998, 3, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Oxytocin Approval Letter. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/97/18261-s011_pitocin.pdf (accessed on 19 February 2024).
- Oxytocin Drug Label. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/018248s049lbl.pdf (accessed on 19 February 2024).
- Glucagon Approval Letter. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/98/20928.pdf (accessed on 19 February 2024).
- Jiang, G.; Zhang, B.B. Glucagon and regulation of glucose metabolism. Am. J. Physiol. Endocrinol. Metab. 2003, 284, E671–E678. [Google Scholar] [CrossRef]
- Secretin Drug Label. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2004/21-256_Synthetic%20Human%20Secretin_Prntlbl.pdf (accessed on 19 February 2024).
- Afroze, S.; Meng, F.; Jensen, K.; McDaniel, K.; Rahal, K.; Onori, P.; Gaudio, E.; Alpini, G.; Glaser, S.S. The physiological roles of secretin and its receptor. Ann. Transl. Med. 2013, 1, 29. [Google Scholar] [CrossRef]
- Chu, J.Y.; Cheng, C.Y.; Lee, V.H.; Chan, Y.S.; Chow, B.K. Secretin and body fluid homeostasis. Kidney Int. 2011, 79, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Berg, P.; Svendsen, S.L.; Sorensen, M.V.; Schreiber, R.; Kunzelmann, K.; Leipziger, J. The molecular mechanism of CFTR- and secretin-dependent renal bicarbonate excretion. J. Physiol. 2021, 599, 3003–3011. [Google Scholar] [CrossRef]
- Secretin Approval Letter. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2004/21-256_Synthetic%20Human%20Secretin_Approv.pdf (accessed on 19 February 2024).
- Azria, M. Possible mechanisms of the analgesic action of calcitonin. Bone 2002, 30 (Suppl. S1), 80–83. [Google Scholar] [CrossRef]
- Braga, P.C. Calcitonin and its antinociceptive activity: Animal and human investigations 1975–1992. Agents Actions 1994, 41, 121–131. [Google Scholar] [CrossRef]
- Fabbri, A.; Fraioli, F.; Pert, C.B.; Pert, A. Calcitonin receptors in the rat mesencephalon mediate its analgesic actions: Autoradiographic and behavioral analysis. Brain Res. 1985, 343, 205–215. [Google Scholar] [CrossRef]
- Fischer, J.A.; Sagar, S.M.; Martin, J.B. Characterization and regional distribution of calcitonin binding sites in the rat brain. Life Sci. 1981, 29, 663–671. [Google Scholar] [CrossRef]
- Calcitonin Drug Label. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/017808s035lbl.pdf (accessed on 19 February 2024).
- Calcitonin Approval Letter. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2005/021406s000_APPROV.pdf (accessed on 19 February 2024).
- Du Vigneaud, V.; Gish, D.T.; Katsoyannis, P.G.; Hess, G.P. Synthesis of the Pressor-Antidiuretic Hormone, Arginine-Vasopressin. J. Am. Chem. Soc. 1958, 80, 3355–3358. [Google Scholar] [CrossRef]
- Vasopressin Drug Label. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/212593s000lbl.pdf (accessed on 19 February 2024).
- Fong, C.T.O.; Silver, L.; Christman, D.R.; Schwartz, I.L. On the mechanism of action of the antidiuretic hormone (Vasopressin). Proc. Natl. Acad. Sci. USA 1960, 46, 1273–1277. [Google Scholar] [CrossRef]
- Bendicksen, L.; Kesselheim, A.S.; Rome, B.N. The Vexing Voyage of Vasopressin: The Consequences of Granting Market Exclusivity to Unapproved Drugs. Chest 2022, 162, 433–435. [Google Scholar] [CrossRef]
- Pioszak, A.A.; Parker, N.R.; Gardella, T.J.; Xu, H.E. Structural basis for parathyroid hormone-related protein binding to the parathyroid hormone receptor and design of conformation-selective peptides. J. Biol. Chem. 2009, 284, 28382–28391. [Google Scholar] [CrossRef]
- Natpara Drug Label. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/125511Orig1s000Lbl.pdf (accessed on 19 February 2024).
- Natpara Approval Letter. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/125511Orig1s000Approv.pdf (accessed on 19 February 2024).
- Angiotensin II Drug Label. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/209360s000lbl.pdf (accessed on 19 February 2024).
- Tigerstedt, R.; Bergman, P.Q. Niere und Kreislauf1. Skand. Arch. Physiol. 1898, 8, 223–271. [Google Scholar] [CrossRef]
- Basso, N.; Terragno, N.A. History About the Discovery of the Renin-Angiotensin System. Hypertension 2001, 38, 1246–1249. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, H.; Bumpus, F.M.; Page, I.H. Synthesis of a Biologically Active Octapeptide Similar to Natural Isoleucine Angiotonin Octapeptide1. J. Am. Chem. Soc. 1957, 79, 5697–5703. [Google Scholar] [CrossRef]
- Rittel, W.; Iselin, B.; Kappeler, H.; Riniker, B.; Schwyzer, R. Synthese eines hochwirksamen Hypertensin II-amids (L-Asparaginyl-L-arginyl-L-valyl-L-tyrosyl-L-isoleucyl-L-histidyl-L-prolyl-L-phenylalanin). Helv. Chim. Acta 1957, 40, 614–624. [Google Scholar] [CrossRef]
- Al Musaimi, O.; Al Shaer, D.; de la Torre, B.G.; Albericio, F. 2017 FDA Peptide Harvest. Pharmaceuticals 2018, 11, 42. [Google Scholar] [CrossRef]
- FDA. Angiotensin II Aproval Letter. 2017. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/209360Orig1s000Approv.pdf (accessed on 19 February 2024).
- Pramlintide Drug Label. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2005/21-332_Symlin%20Injection_prntlbl.PDF (accessed on 19 February 2024).
- Pullman, J.; Darsow, T.; Frias, J.P. Pramlintide in the management of insulin-using patients with type 2 and type 1 diabetes. Vasc. Health Risk Manag. 2006, 2, 203–212. [Google Scholar] [CrossRef]
- Pramlintide Approval Letter. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2005/21-332_Symlin%20Injection_approv.PDF (accessed on 19 February 2024).
- Thorens, B. Expression cloning of the pancreatic beta cell receptor for the gluco-incretin hormone glucagon-like peptide 1. Proc. Natl. Acad. Sci. USA 1992, 89, 8641–8645. [Google Scholar] [CrossRef]
- Seino, Y.; Fukushima, M.; Yabe, D. GIP and GLP-1, the two incretin hormones: Similarities and differences. J. Diabetes Investig. 2010, 1, 8–23. [Google Scholar] [CrossRef] [PubMed]
- Drucker, D.J. Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1. Cell Metab. 2018, 27, 740–756. [Google Scholar] [CrossRef]
- Marso, S.P.; Daniels, G.H.; Brown-Frandsen, K.; Kristensen, P.; Mann, J.F.E.; Nauck, M.A.; Nissen, S.E.; Pocock, S.; Poulter, N.R.; Ravn, L.S.; et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 311–322. [Google Scholar] [CrossRef]
- Exenatide Drug Label. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/021773s9s11s18s22s25lbl.pdf (accessed on 19 February 2024).
- Exenatide Approval Letter. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2005/021773_Byetta_approv.PDF (accessed on 19 February 2024).
- Liraglutide Drug Label. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/022341s027lbl.pdf (accessed on 19 February 2024).
- Liraglutide Approval Letter. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2010/022341s000approv.pdf (accessed on 19 February 2024).
- de la Torre, B.G.; Albericio, F. The Pharmaceutical Industry in 2016. An Analysis of FDA Drug Approvals from a Perspective of the Molecule Type. Molecules 2017, 22, 368. [Google Scholar] [CrossRef]
- Bain, S.C. The Clinical Development Program of Lixisenatide: A Once-Daily Glucagon-Like Peptide-1 Receptor Agonist. Diabetes Ther. 2014, 5, 367–383. [Google Scholar] [CrossRef] [PubMed]
- Lixisenatide Drug Label. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/208471orig1s000lbl.pdf (accessed on 19 February 2024).
- Lixisenatide Approval Letter. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2021/208471Orig1s004ltr.pdf (accessed on 19 February 2024).
- Sanofi—Discontinuation of Adlyxin® (Lixisenatide). Available online: https://professionals.optumrx.com/content/dam/optum3/professional-optumrx/news/rxnews/drug-withdrawls/drugwithdrawal_adlyxin_2023-0117.pdf (accessed on 19 February 2024).
- Albiglutide Drug Label. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125431s019lbl.pdf (accessed on 19 February 2024).
- Abiglutide Discontinuation. Available online: https://www.spglobal.com/marketintelligence/en/news-insights/trending/uht9tdWAaWg6Kn6Xg_nF1w2#:~:text=In%20July%202017%2C%20GSK%20said,1%20billion%20cost%2Dcutting%20drive (accessed on 19 February 2024).
- Abiglutide Approval Letter. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/125431Orig1s000Approv.pdf (accessed on 19 February 2024).
- Dulaglutide Drug Label. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125469s036lbl.pdf (accessed on 19 February 2024).
- Smith, L.L.; Mosley, J.F., 2nd; Parke, C.; Brown, J.; Barris, L.S.; Phan, L.D. Dulaglutide (Trulicity): The Third Once-Weekly GLP-1 Agonist. Pharm. Ther. 2016, 41, 357–360. [Google Scholar]
- Dulaglutide Approval Letter. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2022/125469Orig1s051ltr.pdf (accessed on 19 February 2024).
- Elkinson, S.; Keating, G.M. Lixisenatide: First Global Approval. Drugs 2013, 73, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Lau, J.; Bloch, P.; Schaffer, L.; Pettersson, I.; Spetzler, J.; Kofoed, J.; Madsen, K.; Knudsen, L.B.; McGuire, J.; Steensgaard, D.B.; et al. Discovery of the Once-Weekly Glucagon-Like Peptide-1 (GLP-1) Analogue Semaglutide. J. Med. Chem. 2015, 58, 7370–7380. [Google Scholar] [CrossRef]
- Jensen, L.; Helleberg, H.; Roffel, A.; van Lier, J.J.; Bjornsdottir, I.; Pedersen, P.J.; Rowe, E.; Derving Karsbol, J.; Pedersen, M.L. Absorption, metabolism and excretion of the GLP-1 analogue semaglutide in humans and nonclinical species. Eur. J. Pharm. Sci. 2017, 104, 31–41. [Google Scholar] [CrossRef]
- Pratley, R.E.; Aroda, V.R.; Lingvay, I.; Lüdemann, J.; Andreassen, C.; Navarria, A.; Viljoen, A. Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): A randomised, open-label, phase 3b trial. Lancet Diabetes Endocrinol. 2018, 6, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Semaglutide Drug Label. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/209637lbl.pdf (accessed on 19 February 2024).
- Lee, Y.S.; Jun, H.S. Anti-diabetic actions of glucagon-like peptide-1 on pancreatic beta-cells. Metabolism 2014, 63, 9–19. [Google Scholar] [CrossRef] [PubMed]
- FDA. Semaglutide (Ozempic) Approval Letter. 2017. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2017/209637s000ltr.pdf (accessed on 19 February 2024).
- Mounjaro Drug Label. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215866s000lbl.pdf (accessed on 19 February 2024).
- Frías, J.P. Tirzepatide: A glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) dual agonist in development for the treatment of type 2 diabetes. Expert Rev. Endocrinol. Metab. 2020, 15, 379–394. [Google Scholar] [CrossRef]
- Mounjaro Approval Letter. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2022/215866Orig1s000ltr.pdf (accessed on 19 February 2024).
- Ishida, J.; Saitoh, M.; Ebner, N.; Springer, J.; Anker, S.D.; von Haehling, S. Growth hormone secretagogues: History, mechanism of action, and clinical development. JCSM Rapid Commun. 2020, 3, 25–37. [Google Scholar] [CrossRef]
- Sermorelin Drug Label. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/022505s012s013lbl.pdf (accessed on 19 February 2024).
- Sermorelin Approval Letter. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/pre96/019863_S001_GEREF.pdf (accessed on 19 February 2024).
- Mecasermin Drug Label. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2005/021839lbl.pdf (accessed on 19 February 2024).
- Rinderknecht, E.; Humbel, R.E. The amino acid sequence of human insulin-like growth factor I and its structural homology with proinsulin. J. Biol. Chem. 1978, 253, 2769–2776. [Google Scholar] [CrossRef]
- Hober, S.; Forsberg, G.; Palm, G.; Hartmanis, M.; Nilsson, B. Disulfide exchange folding of insulin-like growth factor I. Biochem. 1992, 31, 1749–1756. [Google Scholar] [CrossRef] [PubMed]
- Mecasermin Approval Letter. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2005/021839ltr.pdf (accessed on 19 February 2024).
- Kemp, S.F. Mecasermin rinfabate. Drugs Today 2007, 43, 149–155. [Google Scholar] [CrossRef]
- Kemp, S.F. Insulin-like growth factor-I deficiency in children with growth hormone insensitivity: Current and future treatment options. BioDrugs 2009, 23, 155–163. [Google Scholar] [CrossRef]
- Tesamorelin Drug Label. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2010/022505Orig1s000Lbl.pdf (accessed on 19 February 2024).
- Dhillon, S. Tesamorelin: A review of its use in the management of HIV-associated lipodystrophy. Drugs 2011, 71, 1071–1091. [Google Scholar] [CrossRef]
- Tesamorelin Approval Letter. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2010/022505Orig1s000Approv.pdf (accessed on 19 February 2024).
- Macimorelin Drug Label. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/205598s000lbl.pdf (accessed on 19 February 2024).
- Guerlavais, V.; Boeglin, D.; Mousseaux, D.; Oiry, C.; Heitz, A.; Deghenghi, R.; Locatelli, V.; Torsello, A.; Ghé, C.; Catapano, F.; et al. New Active Series of Growth Hormone Secretagogues. J. Med. Chem. 2003, 46, 1191–1203. [Google Scholar] [CrossRef] [PubMed]
- FDA. Macimorelin Approval Letter. 2017. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/205598Orig1s000Approv.pdf (accessed on 19 February 2024).
- Gibbs, J.; Young, R.C.; Smith, G.P. Cholecystokinin decreases food intake in rats. J. Comp. Physiol. Psychol. 1973, 84, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Sekiguchi, T. Subchapter 20B—Cholecystokinin. In Handbook of Hormones; Takei, Y., Ando, H., Tsutsui, K., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 177-178-e20B-3. [Google Scholar]
- Nilaweera, K.N.; Giblin, L.; Ross, R.P. Nutrient regulation of enteroendocrine cellular activity linked to cholecystokinin gene expression and secretion. J. Physiol. Biochem. 2010, 66, 85–92. [Google Scholar] [CrossRef] [PubMed]
- de La Serre, C.B.; Moran, T.H. Chapter 144—CCK. In Handbook of Biologically Active Peptides, 2nd ed.; Kastin, A.J., Ed.; Academic Press: Boston, MA, USA, 2013; pp. 1077–1083. [Google Scholar]
- Sincalide Drug Label. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/210850s000lbl.pdf (accessed on 19 February 2024).
- Sincalide Approval Letter. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2002/017697Orig1s012.pdf (accessed on 19 February 2024).
- Bhatt, N.P.; Patel, K.; Borchardt, R.T. Chemical pathways of peptide degradation. I. Deamidation of adrenocorticotropic hormone. Pharm. Res. 1990, 7, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, M.E. Chapter 10—Adrenocorticotropic Hormone. In Stress: Neuroendocrinology and Neurobiology; Fink, G., Ed.; Academic Press: San Diego, CA, USA, 2017; pp. 109–116. [Google Scholar]
- Corticorelin Drug Label. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/020162s010lbl.pdf (accessed on 19 February 2024).
- Corticorelin Approval Letter. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2015/020162Orig1s010ltr.pdf (accessed on 19 February 2024).
- D’Agostino, G.; Diano, S. Alpha-melanocyte stimulating hormone: Production and degradation. J. Mol. Med. 2010, 88, 1195–1201. [Google Scholar] [CrossRef]
- Singh, M.; Mukhopadhyay, K. Alpha-melanocyte stimulating hormone: An emerging anti-inflammatory antimicrobial peptide. Biomed. Res. Int. 2014, 2014, 874610. [Google Scholar] [CrossRef]
- Al Shaer, D.; Al Musaimi, O.; Albericio, F.; de la Torre, B.G. 2019 FDA TIDES (Peptides and Oligonucleotides) Harvest. Pharmaceuticals 2020, 13, 40. [Google Scholar] [CrossRef]
- Lane, A.M.; McKay, J.T.; Bonkovsky, H.L. Advances in the management of erythropoietic protoporphyria—Role of afamelanotide. Appl. Clin. Genet. 2016, 9, 179–189. [Google Scholar] [CrossRef]
- Afamelanotide Drug Label. 2019. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/210797s000lbl.pdf (accessed on 19 February 2024).
- Minder, E.I.; Barman-Aksoezen, J.; Schneider-Yin, X. Pharmacokinetics and Pharmacodynamics of Afamelanotide and its Clinical Use in Treating Dermatologic Disorders. Clin. Pharmacokinet. 2017, 56, 815–823. [Google Scholar] [CrossRef]
- Kim, E.S.; Garnock-Jones, K.P. Afamelanotide: A Review in Erythropoietic Protoporphyria. Am. J. Clin. Dermatol. 2016, 17, 179–185. [Google Scholar] [CrossRef]
- Afamelanotide Approval Letter. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2019/210797Orig1s000ltr.pdf (accessed on 19 February 2024).
- Sawyer, T.K.; Sanfilippo, P.J.; Hruby, V.J.; Engel, M.H.; Heward, C.B.; Burnett, J.B.; Hadley, M.E. 4-Norleucine, 7-D-phenylalanine-a-melanocyte-stimulating hormone: A highly potent a-melanotropin with ultralong biological activity. Proc. Natl. Acad. Sci. USA 1980, 77, 5754–5758. [Google Scholar] [CrossRef]
- Molinoff, P.B.; Shadiack, A.M.; Earle, D.; Diamond, L.E.; Quon, C.Y. PT-141: A Melanocortin Agonist for the Treatment of Sexual Dysfunction. Ann. N. Y. Acad. Sci. 2003, 994, 96–102. [Google Scholar] [CrossRef]
- Polevoda, B.; Sherman, F. The diversity of acetylated proteins. Genome. Biol. 2002, 3, reviews0006. [Google Scholar] [CrossRef] [PubMed]
- Bremelanotide Drug Label. 2019. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/210557s000lbl.pdf (accessed on 19 February 2024).
- Pfaus, J.G.; Sadiq, A.; Spana, C.; Clayton, A.H. The neurobiology of bremelanotide for the treatment of hypoactive sexual desire disorder in premenopausal women. CNS Spectr. 2022, 27, 281–289. [Google Scholar] [CrossRef]
- Sohita, D.; Susan, J.K. Bremelanotide: First Approval. Drugs 2019, 79, 1599–1606. [Google Scholar] [CrossRef]
- Bremelanotide Approval Letter. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2019/210557Orig1s000ltr.pdf (accessed on 19 February 2024).
- Imcivree Drug Label. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/213793s000lbl.pdf (accessed on 19 February 2024).
- Collet, T.H.; Dubern, B.; Mokrosinski, J.; Connors, H.; Keogh, J.M.; Mendes de Oliveira, E.; Henning, E.; Poitou-Bernert, C.; Oppert, J.M.; Tounian, P.; et al. Evaluation of a melanocortin-4 receptor (MC4R) agonist (Setmelanotide) in MC4R deficiency. Mol. Metab. 2017, 6, 1321–1329. [Google Scholar] [CrossRef] [PubMed]
- Clément, K.; Biebermann, H.; Farooqi, I.S.; Van der Ploeg, L.; Wolters, B.; Poitou, C.; Puder, L.; Fiedorek, F.; Gottesdiener, K.; Kleinau, G.; et al. MC4R agonism promotes durable weight loss in patients with leptin receptor deficiency. Nat. Med. 2018, 24, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Kühnen, P.; Clément, K.; Wiegand, S.; Blankenstein, O.; Gottesdiener, K.; Martini, L.L.; Mai, K.; Blume-Peytavi, U.; Grüters, A.; Krude, H. Proopiomelanocortin Deficiency Treated with a Melanocortin-4 Receptor Agonist. N. Eng. J. Med. 2016, 375, 240–246. [Google Scholar] [CrossRef]
- Ramos-Molina, B.; Martin, M.G.; Lindberg, I. PCSK1 Variants and Human Obesity. Prog. Mol. Biol. Transl. Sci. 2016, 140, 47–74. [Google Scholar] [CrossRef] [PubMed]
- Imcivree Approval Letter. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2020/213793Orig1s000ltr.pdf (accessed on 19 February 2024).
- Al Shaer, D.; Al Musaimi, O.; Albericio, F.; de la Torre, B.G. 2023 FDA TIDES (Peptides and Oligonucleotides) Harvest. Pharmaceuticals 2024, 17, 243. [Google Scholar] [CrossRef]









































| Peptide (Trade Name) | Indication | Therapeutic Target | Route | FDA Approval Year |
|---|---|---|---|---|
| Insulin (Iletin) | To treat diabetes mellitus | Insulin receptors | SC | 1923 |
| Corticotropin (H.P. Acthar) | To treat a variety of specific and inadequately defined steroid responsive disorders, multiple sclerosis, and infantile spasms in infants and children under 2 years of age | CRH-R1 and CRH-R2 | SC, IM | 1952 |
| Cyclosporine (Sandimmune) | Immunosuppressant agent | Receptor cyclophilin-1 | Orally | 1983 |
| Oxytocin (Syntocinon) | Uterine-contracting and milk-ejecting hormone | Protein G | IV | 1996 |
| Glucagon (Baqsimi) | To manage and treat hypoglycemia as an antidote to beta-blocker and calcium channel blocker overdose, as an anaphylaxis refractory to epinephrine, and to aid in passing food boluses | Glucagon receptor | IV, IM, or SC | 1998 |
| Secretin (ChiRhoStim) | Regulation of gastric acid, regulation of pancreatic bicarbonate, and osmoregulation | CFTR | IV | 2002 |
| Calcitonin (Miacalcin) | To control the level of calcium in the blood | CNS receptors | IV | 2005 |
| Vasopressin (Vasostrict) | To increase the blood pressure in adults with vasodilatory shock who remain hypotensive after fluids and catecholamine | Vasopressin receptor | IV | 2014 |
| Parathyroid hormone (PTH) (Natpara) | An adjunct to calcium and vitamin D to control hypocalcemia in patients with hypoparathyroidism | PTH receptor | SC | 2015 |
| Angiotensin II (Giapreza) | A vasoconstrictor to increase blood pressure in adults with septic or other distributive shock | Angiotensin II Receptor type I | IV | 2017 |
| Peptide (Trade Name) | Indication | Therapeutic Target | Route | FDA Approval Year |
|---|---|---|---|---|
| Insulin | To treat diabetes mellitus. | Insulin receptor | 1923 | |
| Pramlintide (Symlin) | To treat diabetic patients treated with insulin (type I and II). | Calcitonin receptor, plus one of three receptor activity-modifying proteins, RAMP1, RAMP2, or RAMP3 | 2005 | |
| Exenatide (Byetta) | An adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. | GLP-1 receptor | SC | 2005 |
| Liraglutide (Victoza) | 1. An adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. 2. To reduce the risk of major adverse cardiovascular events in adults with type 2 diabetes mellitus and established cardiovascular disease. | 2010 | ||
| Lixisenatide (Adlyxin) | An adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. | 2013 | ||
| Albiglutide (Tanzeum) | To treat type 2 diabetes mellitus. | 2014 * | ||
| Dulaglutide (Trulicity) | 1. An adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. 2. To reduce the risk of major adverse cardiovascular events in adults with type 2 diabetes mellitus who have established cardiovascular disease or multiple cardiovascular risk factors. | 2014 | ||
| Semaglutide (Ozempic) | An adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. | 2017 | ||
| Tirzepatide (Mounjaro) | An adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. | GLP-1 and GIP receptors | 2022 |
| Peptide (Trade Name) | Indication | Therapeutic Target | Route | FDA Approval Year |
|---|---|---|---|---|
| Sermorelin (Geref) | To reduce the excess abdominal fat in human immunodeficiency virus (HIV)-infected adult patients with lipodystrophy. | GHRH | SC | 1991 |
| Mecasermin (Increlex) | To treat growth failure in children with severe primary IGF-1 deficiency (primary IGFD) or with GH gene deletion who have developed neutralizing antibodies to GH. | 2005 | ||
| Tesamorelin (Egrifta) | To reduce the excess abdominal fat in HIV-infected patients with lipodystrophy. | 2010 | ||
| Macimorelin (Macrilen) | To diagnose adult GH deficiency. | Orally | 2017 |
| Peptide (Trade Name) | Indication | Therapeutic Target | Route | FDA Approval Year |
|---|---|---|---|---|
| Corticorelin (Acthrel) | To evaluate the status of the pituitary–adrenal axis. | Anterior pituitary | IV | 1996 |
| Corticotropin (Cosyntropin) | To diagnose patients presumed to have adrenocortical insufficiency. | Receptor in the adrenal cell plasma membrane | IV | 2008 |
| Peptide (Trade Name) | Indication | Therapeutic Target | Route | FDA Approval Year |
|---|---|---|---|---|
| Afamelanotide (Scenesse) | To increase pain-free light exposure in adult patients with a history of phototoxic reactions from erythropoietic protoporphyria (EPP). | MC1R | SC | 2019 |
| Bremelanotide (Vyleesi) | To treat premenopausal women with acquired, generalized hypoactive sexual desire disorder (HSDD). | MC1R and MC4R | 2019 | |
| Setmelanotide (Imcivree) | To treat chronic weight management in adult and pediatric patients. | MC4R | 2020 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Musaimi, O. Exploring FDA-Approved Frontiers: Insights into Natural and Engineered Peptide Analogues in the GLP-1, GIP, GHRH, CCK, ACTH, and α-MSH Realms. Biomolecules 2024, 14, 264. https://doi.org/10.3390/biom14030264
Al Musaimi O. Exploring FDA-Approved Frontiers: Insights into Natural and Engineered Peptide Analogues in the GLP-1, GIP, GHRH, CCK, ACTH, and α-MSH Realms. Biomolecules. 2024; 14(3):264. https://doi.org/10.3390/biom14030264
Chicago/Turabian StyleAl Musaimi, Othman. 2024. "Exploring FDA-Approved Frontiers: Insights into Natural and Engineered Peptide Analogues in the GLP-1, GIP, GHRH, CCK, ACTH, and α-MSH Realms" Biomolecules 14, no. 3: 264. https://doi.org/10.3390/biom14030264