Navigating the Evolving Landscape of Primary Hyperoxaluria: Traditional Management Defied by the Rise of Novel Molecular Drugs
Abstract
:1. Introduction
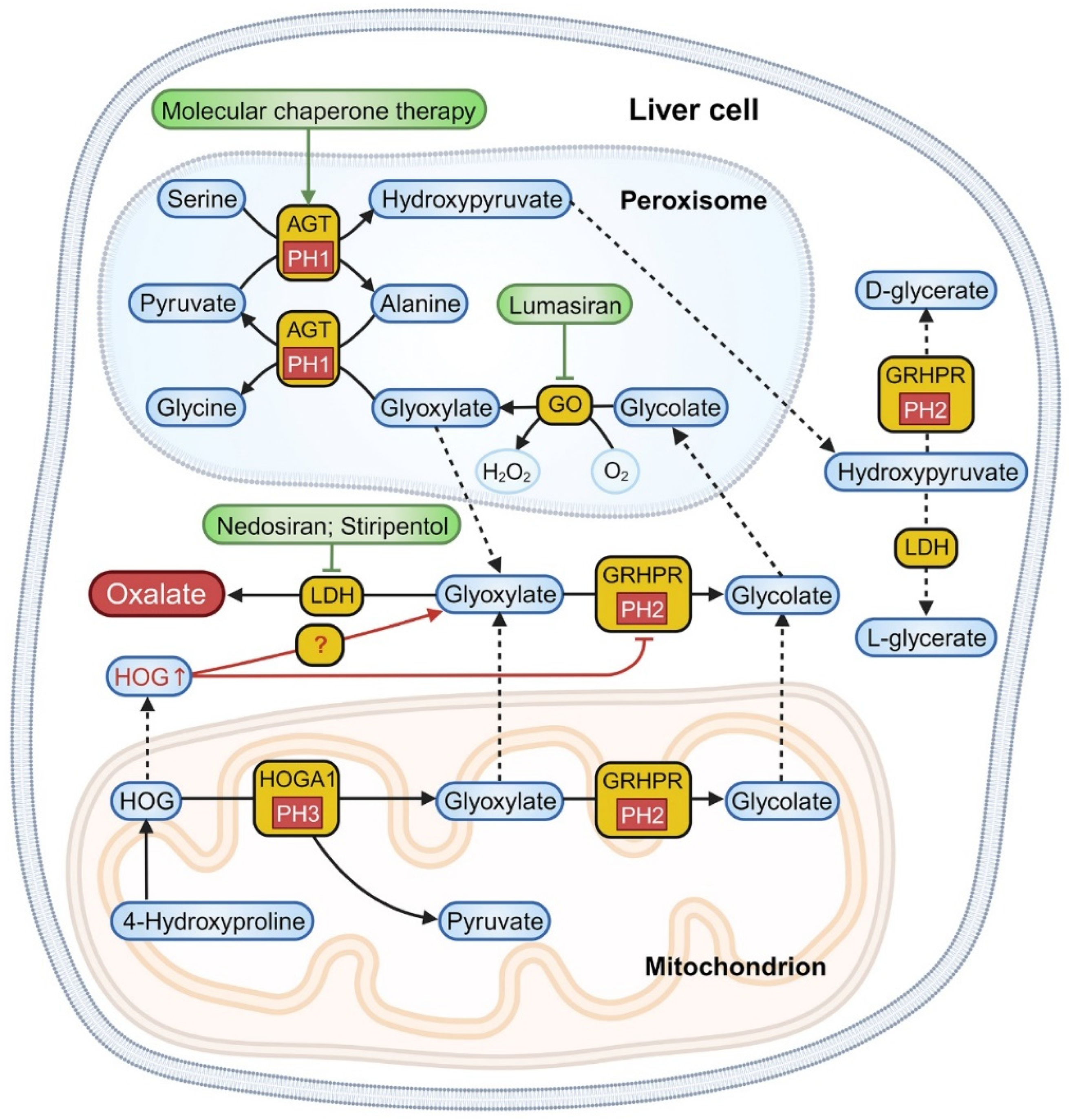
2. Biochemical Mechanism
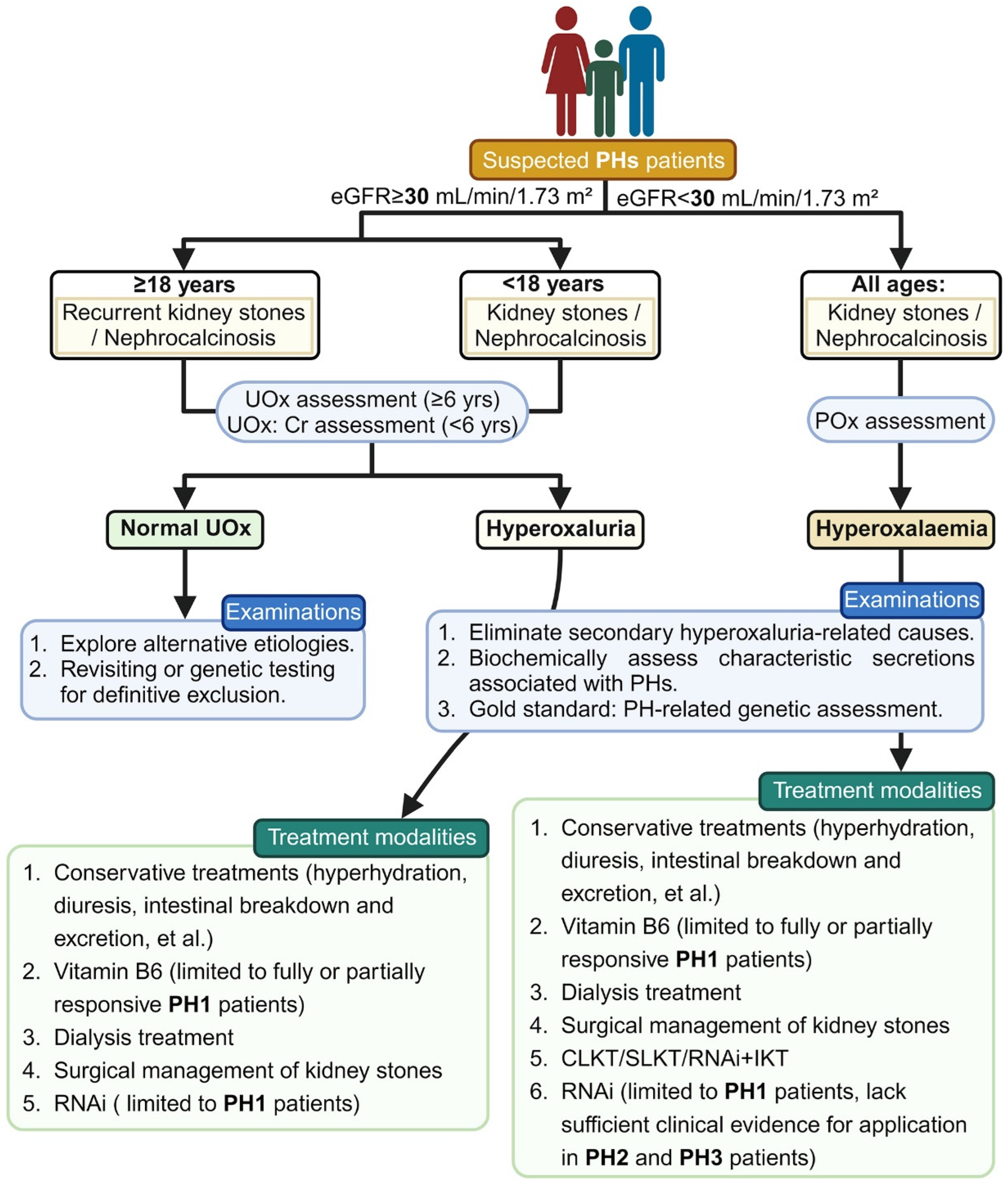
3. Diagnosis and Genetic Characterization
4. Treatment
4.1. Related Conservative Treatments
4.2. Chaperone Therapy
4.3. Dialysis Treatment
4.4. Surgical Interventions
4.5. Substrate Reduction Therapy (SRT)
4.6. Preclinical Therapeutic Explorations
4.6.1. CRISPR/Cas9
4.6.2. ERT
4.6.3. Immunoregulation
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AGT | alanine-glyoxylate aminotransferase |
| AI | artificial intelligence |
| CaOx | calcium oxalate |
| CKD | chronic kidney disease |
| CLKT | combined liver–kidney transplantation |
| COM | calcium oxalate monohydrate |
| Cpf1 | Prevotella and Francisella 1 |
| CRISPR | clustered regularly interspaced palindromic repeats |
| DECA | dequalinium chloride |
| EAU | European Association of Urology |
| eGFR | estimated glomerular filtration rate |
| EMA | European Medicines Agency |
| ERKNet | European Rare Kidney Disease Reference Network |
| ERT | enzyme replacement therapy |
| ESRD | end-stage renal disease |
| FDA | Food and Drug Administration |
| GO | glycolate oxidase |
| GRHPR | glyoxylate reductase/hydroxypyruvate reductase |
| HAO1 | hydroxy acid oxidase 1 |
| HD | hemodialysis |
| HOG | 4-hydroxy-2-oxoglutarate |
| HOGA1 | 4-hydroxy-2-oxoglutarate aldolase |
| KT | kidney transplantation |
| LaC | lanthanum carbonate |
| LDH | lactate dehydrogenase |
| LT | liver transplantation |
| PCNL | percutaneous nephrolithotomy |
| PH1 | primary hyperoxaluria type 1 |
| PH2 | primary hyperoxaluria type 2 |
| PH3 | primary hyperoxaluria type 3 |
| PHs | primary hyperoxalurias |
| PL | pyridoxal |
| PLP | pyridoxal 5′-phosphate |
| PLT | pre-emptive liver transplantation |
| PM | pyridoxamine |
| POx | plasma oxalate |
| RNAi | RNA interference |
| SLKT | sequential liver–kidney transplantation |
| SWL | shock wave lithotripsy |
| UOx | urinary oxalate |
| URS | ureteroscopy |
References
- Duranton, F.; Cohen, G.; De Smet, R.; Rodriguez, M.; Jankowski, J.; Vanholder, R.; Argiles, A. Normal and Pathologic Concentrations of Uremic Toxins. J. Am. Soc. Nephrol. 2012, 23, 1258–1270. [Google Scholar] [CrossRef] [PubMed]
- Lepoutre, C. Calculs Multiples Chez Un Enfant: Infiltration Du Parenchyme Rénal Par Des Dépots Cristallins. J. Urol. 1925, 20, 424. [Google Scholar]
- Ermer, T.; Eckardt, K.-U.; Aronson, P.S.; Knauf, F. Oxalate, Inflammasome, and Progression of Kidney Disease. Curr. Opin. Nephrol. Hypertens. 2016, 25, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Elder, T.D.; Wyngaarden, J.B. The Biosynthesis and Turnover of Oxalate in Normal and Hyperoxaluric Subjects. J. Clin. Investig. 1960, 39, 1337–1344. [Google Scholar] [CrossRef] [PubMed]
- Hautmann, R.; Osswald, H. Pharmacokinetic Studies of Oxalate in Man. Investig. Urol. 1979, 16, 395–398. [Google Scholar]
- Dill, H.; Martin-Higueras, C.; Hoppe, B. Diet-Related Urine Collections: Assistance in Categorization of Hyperoxaluria. Urolithiasis 2022, 50, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Ermer, T.; Nazzal, L.; Tio, M.C.; Waikar, S.; Aronson, P.S.; Knauf, F. Oxalate Homeostasis. Nat. Rev. Nephrol. 2023, 19, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Groothoff, J.W.; Metry, E.; Deesker, L.; Garrelfs, S.; Acquaviva, C.; Almardini, R.; Beck, B.B.; Boyer, O.; Cerkauskiene, R.; Ferraro, P.M.; et al. Clinical Practice Recommendations for Primary Hyperoxaluria: An Expert Consensus Statement from ERKNet and OxalEurope. Nat. Rev. Nephrol. 2023, 19, 194–211. [Google Scholar] [CrossRef] [PubMed]
- Cochat, P.; Rumsby, G. Primary Hyperoxaluria. N. Engl. J. Med. 2013, 369, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Hopp, K.; Cogal, A.G.; Bergstralh, E.J.; Seide, B.M.; Olson, J.B.; Meek, A.M.; Lieske, J.C.; Milliner, D.S.; Harris, P.C. Rare Kidney Stone Consortium Phenotype-Genotype Correlations and Estimated Carrier Frequencies of Primary Hyperoxaluria. J. Am. Soc. Nephrol. 2015, 26, 2559–2570. [Google Scholar] [CrossRef]
- Talati, J.J.; Hulton, S.-A.; Garrelfs, S.F.; Aziz, W.; Rao, S.; Memon, A.; Nazir, Z.; Biyabani, R.; Qazi, S.; Azam, I.; et al. Primary Hyperoxaluria in Populations of Pakistan Origin: Results from a Literature Review and Two Major Registries. Urolithiasis 2018, 46, 187–195. [Google Scholar] [CrossRef]
- Shee, K.; Stoller, M.L. Perspectives in Primary Hyperoxaluria—Historical, Current and Future Clinical Interventions. Nat. Rev. Urol. 2022, 19, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Danpure, C.J.; Jennings, P.R. Peroxisomal Alanine:Glyoxylate Aminotransferase Deficiency in Primary Hyperoxaluria Type I. FEBS Lett. 1986, 201, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Purdue, P.E.; Lumb, M.J.; Fox, M.; Griffo, G.; Hamon-Benais, C.; Povey, S.; Danpure, C.J. Characterization and Chromosomal Mapping of a Genomic Clone Encoding Human Alanine: Glyoxylate Aminotransferase. Genomics 1991, 10, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Danpure, C.J. Molecular and Clinical Heterogeneity in Primary Hyperoxaluria Type 1. Am. J. Kidney Dis. 1991, 17, 366–369. [Google Scholar] [CrossRef]
- Williams, H.E.; Smith, L.H. L-Glyceric Aciduria. N. Engl. J. Med. 1968, 278, 233–239. [Google Scholar] [CrossRef]
- Belostotsky, R.; Seboun, E.; Idelson, G.H.; Milliner, D.S.; Becker-Cohen, R.; Rinat, C.; Monico, C.G.; Feinstein, S.; Ben-Shalom, E.; Magen, D.; et al. Mutations in DHDPSL Are Responsible for Primary Hyperoxaluria Type III. Am. J. Hum. Genet. 2010, 87, 392–399. [Google Scholar] [CrossRef]
- Primary Hyperoxaluria Type III—A Model for Studying Perturbations in Glyoxylate Metabolism—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/22729392/ (accessed on 2 November 2023).
- Riedel, T.J.; Knight, J.; Murray, M.S.; Milliner, D.S.; Holmes, R.P.; Lowther, W.T. 4-Hydroxy-2-Oxoglutarate Aldolase Inactivity in Primary Hyperoxaluria Type 3 and Glyoxylate Reductase Inhibition. Biochim. Biophys. Acta 2012, 1822, 1544–1552. [Google Scholar] [CrossRef]
- Huang, A.; Burke, J.; Bunker, R.D.; Mok, Y.-F.; Griffin, M.D.; Baker, E.N.; Loomes, K.M. Regulation of Human 4-Hydroxy-2-Oxoglutarate Aldolase by Pyruvate and α-Ketoglutarate: Implications for Primary Hyperoxaluria Type-3. Biochem. J. 2019, 476, 3369–3383. [Google Scholar] [CrossRef]
- Huang, L.; Qi, C.; Zhu, G.; Ding, J.; Yuan, L.; Sun, J.; He, X.; Wang, X. Genetic Testing Enables a Precision Medicine Approach for Nephrolithiasis and Nephrocalcinosis in Pediatrics: A Single-Center Cohort. Mol. Genet. Genom. MGG 2022, 297, 1049–1061. [Google Scholar] [CrossRef]
- Lumlertgul, N.; Siribamrungwong, M.; Jaber, B.L.; Susantitaphong, P. Secondary Oxalate Nephropathy: A Systematic Review. Kidney Int. Rep. 2018, 3, 1363–1372. [Google Scholar] [CrossRef] [PubMed]
- van Woerden, C.S.; Groothoff, J.W.; Wanders, R.J.A.; Davin, J.-C.; Wijburg, F.A. Primary Hyperoxaluria Type 1 in the Netherlands: Prevalence and Outcome. Nephrol. Dial. Transplant. 2003, 18, 273–279. [Google Scholar] [CrossRef]
- Dindo, M.; Mandrile, G.; Conter, C.; Montone, R.; Giachino, D.; Pelle, A.; Costantini, C.; Cellini, B. The ILE56 Mutation on Different Genetic Backgrounds of Alanine:Glyoxylate Aminotransferase: Clinical Features and Biochemical Characterization. Mol. Genet. Metab. 2020, 131, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Song, J.; He, Y.; Zhong, C.; Yang, Q.; Li, Q.; Wang, M. Case Series and Literature Review of Primary Hyperoxaluria Type 1 in Chinese Patients. Urolithiasis 2023, 51, 123. [Google Scholar] [CrossRef] [PubMed]
- Harambat, J.; Fargue, S.; Acquaviva, C.; Gagnadoux, M.-F.; Janssen, F.; Liutkus, A.; Mourani, C.; Macher, M.-A.; Abramowicz, D.; Legendre, C.; et al. Genotype-Phenotype Correlation in Primary Hyperoxaluria Type 1: The p.Gly170Arg AGXT Mutation Is Associated with a Better Outcome. Kidney Int. 2010, 77, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Purdue, P.E.; Allsop, J.; Isaya, G.; Rosenberg, L.E.; Danpure, C.J. Mistargeting of Peroxisomal L-Alanine:Glyoxylate Aminotransferase to Mitochondria in Primary Hyperoxaluria Patients Depends upon Activation of a Cryptic Mitochondrial Targeting Sequence by a Point Mutation. Proc. Natl. Acad. Sci. USA 1991, 88, 10900–10904. [Google Scholar] [CrossRef] [PubMed]
- Rumsby, G.; Williams, E.; Coulter-Mackie, M. Evaluation of Mutation Screening as a First Line Test for the Diagnosis of the Primary Hyperoxalurias. Kidney Int. 2004, 66, 959–963. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, B.; Beck, B.B.; Milliner, D.S. The Primary Hyperoxalurias. Kidney Int. 2009, 75, 1264–1271. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Viehman, J.K.; Mehta, R.A.; Cogal, A.G.; Hasadsri, L.; Oglesbee, D.; Olson, J.B.; Seide, B.M.; Sas, D.J.; Harris, P.C.; et al. Clinical Characterization of Primary Hyperoxaluria Type 3 in Comparison with Types 1 and 2. Nephrol. Dial. Transplant. 2022, 37, 869–875. [Google Scholar] [CrossRef]
- Harambat, J.; Van Stralen, K.J.; Espinosa, L.; Groothoff, J.W.; Hulton, S.-A.; Cerkauskiene, R.; Schaefer, F.; Verrina, E.; Jager, K.J.; Cochat, P. Characteristics and Outcomes of Children with Primary Oxalosis Requiring Renal Replacement Therapy. Clin. J. Am. Soc. Nephrol. 2012, 7, 458–465. [Google Scholar] [CrossRef]
- Garrelfs, S.F.; Rumsby, G.; Peters-Sengers, H.; Erger, F.; Groothoff, J.W.; Beck, B.B.; Oosterveld, M.J.S.; Pelle, A.; Neuhaus, T.; Adams, B.; et al. Patients with Primary Hyperoxaluria Type 2 Have Significant Morbidity and Require Careful Follow-Up. Kidney Int. 2019, 96, 1389–1399. [Google Scholar] [CrossRef] [PubMed]
- Milliner, D.S.; Wilson, D.M.; Smith, L.H. Phenotypic Expression of Primary Hyperoxaluria: Comparative Features of Types I and II. Kidney Int. 2001, 59, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Cregeen, D.P.; Williams, E.L.; Hulton, S.; Rumsby, G. Molecular Analysis of the Glyoxylate Reductase (GRHPR) Gene and Description of Mutations Underlying Primary Hyperoxaluria Type 2. Hum. Mutat. 2003, 22, 497. [Google Scholar] [CrossRef]
- Martin-Higueras, C.; Garrelfs, S.F.; Groothoff, J.W.; Jacob, D.E.; Moochhala, S.H.; Bacchetta, J.; Acquaviva, C.; Zaniew, M.; Sikora, P.; Beck, B.B.; et al. A Report from the European Hyperoxaluria Consortium (OxalEurope) Registry on a Large Cohort of Patients with Primary Hyperoxaluria Type 3. Kidney Int. 2021, 100, 621–635. [Google Scholar] [CrossRef] [PubMed]
- Beck, B.B.; Baasner, A.; Buescher, A.; Habbig, S.; Reintjes, N.; Kemper, M.J.; Sikora, P.; Mache, C.; Pohl, M.; Stahl, M.; et al. Novel Findings in Patients with Primary Hyperoxaluria Type III and Implications for Advanced Molecular Testing Strategies. Eur. J. Hum. Genet. 2013, 21, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; He, L.; Xu, G.; Lin, H.; Xu, M.; Geng, H. Nine Novel HOGA1 Gene Mutations Identified in Primary Hyperoxaluria Type 3 and Distinct Clinical and Biochemical Characteristics in Chinese Children. Pediatr. Nephrol. 2019, 34, 1785–1790. [Google Scholar] [CrossRef] [PubMed]
- Richard, E.; Blouin, J.-M.; Harambat, J.; Llanas, B.; Bouchet, S.; Acquaviva, C.; de la Faille, R. Late Diagnosis of Primary Hyperoxaluria Type III. Ann. Clin. Biochem. 2017, 54, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Granberg, C.F.; Harris, P.C.; Lieske, J.C.; Licht, J.H.; Weiss, A.; Milliner, D.S. Primary Hyperoxaluria Type 3 Can Also Result in Kidney Failure: A Case Report. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2022, 79, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Cornière, N.; Thomson, R.B.; Thauvin, S.; Villoutreix, B.O.; Karp, S.; Dynia, D.W.; Burlein, S.; Brinkmann, L.; Badreddine, A.; Dechaume, A.; et al. Dominant Negative Mutation in Oxalate Transporter SLC26A6 Associated with Enteric Hyperoxaluria and Nephrolithiasis. J. Med. Genet. 2022, 59, 1035–1043. [Google Scholar] [CrossRef]
- Knauf, F.; Velazquez, H.; Pfann, V.; Jiang, Z.; Aronson, P.S. Characterization of Renal NaCl and Oxalate Transport in Slc26a6−/− Mice. Am. J. Physiol. Ren. Physiol. 2019, 316, F128–F133. [Google Scholar] [CrossRef]
- Méaux, M.-N.; Sellier-Leclerc, A.-L.; Acquaviva-Bourdain, C.; Harambat, J.; Allard, L.; Bacchetta, J. The Effect of Lumasiran Therapy for Primary Hyperoxaluria Type 1 in Small Infants. Pediatr. Nephrol. 2022, 37, 907–911. [Google Scholar] [CrossRef]
- Marchetti, F.; Corsello, G. Genetics and “democracy”. Ital. J. Pediatr. 2022, 48, 202. [Google Scholar] [CrossRef]
- Gedefaw, L.; Liu, C.-F.; Ip, R.K.L.; Tse, H.-F.; Yeung, M.H.Y.; Yip, S.P.; Huang, C.-L. Artificial Intelligence-Assisted Diagnostic Cytology and Genomic Testing for Hematologic Disorders. Cells 2023, 12, 1755. [Google Scholar] [CrossRef] [PubMed]
- Porowski, T.; Kirejczyk, J.K.; Mrozek, P.; Protas, P.; Kozerska, A.; Łabieniec, Ł.; Szymański, K.; Wasilewska, A. Upper Metastable Limit Osmolality of Urine as a Predictor of Kidney Stone Formation in Children. Urolithiasis 2019, 47, 155–163. [Google Scholar] [CrossRef]
- Skolarikos, A.; Straub, M.; Knoll, T.; Sarica, K.; Seitz, C.; Petřík, A.; Türk, C. Metabolic Evaluation and Recurrence Prevention for Urinary Stone Patients: EAU Guidelines. Eur. Urol. 2015, 67, 750–763. [Google Scholar] [CrossRef]
- Reilly, R.F.; Peixoto, A.J.; Desir, G.V. The Evidence-Based Use of Thiazide Diuretics in Hypertension and Nephrolithiasis. Clin. J. Am. Soc. Nephrol. 2010, 5, 1893–1903. [Google Scholar] [CrossRef] [PubMed]
- Cheungpasitporn, W.; Erickson, S.B.; Rule, A.D.; Enders, F.; Lieske, J.C. Short-Term Tolvaptan Increases Water Intake and Effectively Decreases Urinary Calcium Oxalate, Calcium Phosphate and Uric Acid Supersaturations. J. Urol. 2016, 195, 1476–1481. [Google Scholar] [CrossRef] [PubMed]
- Milliner, D.S.; Eickholt, J.T.; Bergstralh, E.J.; Wilson, D.M.; Smith, L.H. Results of Long-Term Treatment with Orthophosphate and Pyridoxine in Patients with Primary Hyperoxaluria. N. Engl. J. Med. 1994, 331, 1553–1558. [Google Scholar] [CrossRef]
- Cochat, P.; Hulton, S.-A.; Acquaviva, C.; Danpure, C.J.; Daudon, M.; De Marchi, M.; Fargue, S.; Groothoff, J.; Harambat, J.; Hoppe, B.; et al. Primary Hyperoxaluria Type 1: Indications for Screening and Guidance for Diagnosis and Treatment. Nephrol. Dial. Transplant. 2012, 27, 1729–1736. [Google Scholar] [CrossRef]
- Leumann, E.; Hoppe, B.; Neuhaus, T. Management of Primary Hyperoxaluria: Efficacy of Oral Citrate Administration. Pediatr. Nephrol. 1993, 7, 207–211. [Google Scholar] [CrossRef]
- Chung, J.; Granja, I.; Taylor, M.G.; Mpourmpakis, G.; Asplin, J.R.; Rimer, J.D. Molecular Modifiers Reveal a Mechanism of Pathological Crystal Growth Inhibition. Nature 2016, 536, 446–450. [Google Scholar] [CrossRef]
- Ruggenenti, P.; Caruso, M.R.; Cortinovis, M.; Perna, A.; Peracchi, T.; Giuliano, G.A.; Rota, S.; Brambilla, P.; Invernici, G.; Villa, D.; et al. Fresh Lemon Juice Supplementation for the Prevention of Recurrent Stones in Calcium Oxalate Nephrolithiasis: A Pragmatic, Prospective, Randomised, Open, Blinded Endpoint (PROBE) Trial. EClinicalMedicine 2022, 43, 101227. [Google Scholar] [CrossRef]
- Asplin, J.R. The Management of Patients with Enteric Hyperoxaluria. Urolithiasis 2016, 44, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Glew, R.H.; Sun, Y.; Horowitz, B.L.; Konstantinov, K.N.; Barry, M.; Fair, J.R.; Massie, L.; Tzamaloukas, A.H. Nephropathy in Dietary Hyperoxaluria: A Potentially Preventable Acute or Chronic Kidney Disease. World J. Nephrol. 2014, 3, 122–142. [Google Scholar] [CrossRef]
- Arvans, D.; Jung, Y.-C.; Antonopoulos, D.; Koval, J.; Granja, I.; Bashir, M.; Karrar, E.; Roy-Chowdhury, J.; Musch, M.; Asplin, J.; et al. Oxalobacter Formigenes-Derived Bioactive Factors Stimulate Oxalate Transport by Intestinal Epithelial Cells. J. Am. Soc. Nephrol. 2017, 28, 876–887. [Google Scholar] [CrossRef]
- Verhulst, A.; Dehmel, B.; Lindner, E.; Akerman, M.E.; D’Haese, P.C. Oxalobacter Formigenes Treatment Confers Protective Effects in a Rat Model of Primary Hyperoxaluria by Preventing Renal Calcium Oxalate Deposition. Urolithiasis 2022, 50, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Milliner, D.; Hoppe, B.; Groothoff, J. A Randomised Phase II/III Study to Evaluate the Efficacy and Safety of Orally Administered Oxalobacter Formigenes to Treat Primary Hyperoxaluria. Urolithiasis 2018, 46, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, B.; Pellikka, P.A.; Dehmel, B.; Banos, A.; Lindner, E.; Herberg, U. Effects of Oxalobacter Formigenes in Subjects with Primary Hyperoxaluria Type 1 and End-Stage Renal Disease: A Phase II Study. Nephrol. Dial. Transplant. 2021, 36, 1464–1473. [Google Scholar] [CrossRef]
- Goldfarb, D.S.; Modersitzki, F.; Asplin, J.R. A Randomized, Controlled Trial of Lactic Acid Bacteria for Idiopathic Hyperoxaluria. Clin. J. Am. Soc. Nephrol. 2007, 2, 745–749. [Google Scholar] [CrossRef]
- Mehra, Y.; Viswanathan, P. High-Quality Whole-Genome Sequence Analysis of Lactobacillus Paragasseri UBLG-36 Reveals Oxalate-Degrading Potential of the Strain. PLoS ONE 2021, 16, e0260116. [Google Scholar] [CrossRef]
- Mehra, Y.; Rajesh, N.G.; Viswanathan, P. Analysis and Characterization of Lactobacillus Paragasseri and Lacticaseibacillus Paracasei: Two Probiotic Bacteria That Can Degrade Intestinal Oxalate in Hyperoxaluric Rats. Probiotics Antimicrob. Proteins 2022, 14, 854–872. [Google Scholar] [CrossRef] [PubMed]
- Ariceta, G.; Collard, L.; Abroug, S.; Moochhala, S.H.; Gould, E.; Boussetta, A.; Ben Hmida, M.; De, S.; Hunley, T.E.; Jarraya, F.; et al. EPHex: A Phase 3, Double-Blind, Placebo-Controlled, Randomized Study to Evaluate Long-Term Efficacy and Safety of Oxalobacter Formigenes in Patients with Primary Hyperoxaluria. Pediatr. Nephrol. 2023, 38, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Peck, A.B.; Canales, B.K.; Nguyen, C.Q. Oxalate-Degrading Microorganisms or Oxalate-Degrading Enzymes: Which Is the Future Therapy for Enzymatic Dissolution of Calcium-Oxalate Uroliths in Recurrent Stone Disease? Urolithiasis 2016, 44, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Grujic, D.; Salido, E.C.; Shenoy, B.C.; Langman, C.B.; McGrath, M.E.; Patel, R.J.; Rashid, A.; Mandapati, S.; Jung, C.W.; Margolin, A.L. Hyperoxaluria Is Reduced and Nephrocalcinosis Prevented with an Oxalate-Degrading Enzyme in Mice with Hyperoxaluria. Am. J. Nephrol. 2009, 29, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Lingeman, J.E.; Pareek, G.; Easter, L.; Pease, R.; Grujic, D.; Brettman, L.; Langman, C.B. ALLN-177, Oral Enzyme Therapy for Hyperoxaluria. Int. Urol. Nephrol. 2019, 51, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Massey, L. Magnesium Therapy for Nephrolithiasis. Magnes. Res. 2005, 18, 123–126. [Google Scholar] [PubMed]
- Gheissari, A.; Ziaee, A.; Farhang, F.; Farhang, F.; Talaei, Z.; Merrikhi, A.; Ghafghazi, T.; Moslehi, M. Evaluating the Effectiveness of Adding Magnesium Chloride to Conventional Protocol of Citrate Alkali Therapy in Children with Urolithiasis. Int. J. Prev. Med. 2012, 3, 791–797. [Google Scholar] [PubMed]
- Spasov, A.A.; Iezhitsa, I.N.; Kharitonova, M.V.; Kravchenko, M.S.; Snigur, G.L.; Pisarev, V.B. Experimental evidence of magnesium salts for treatment of calcium oxalate nephrolithiasis in an animal model based on sodium oxalate and a cyclooxygenase 2 selective inhibitor. Patol. Fiziol. Eksp. Ter. 2013, 50–55. [Google Scholar]
- Robijn, S.; Vervaet, B.A.; Hoppe, B.; D’Haese, P.C.; Verhulst, A. Lanthanum Carbonate Inhibits Intestinal Oxalate Absorption and Prevents Nephrocalcinosis after Oxalate Loading in Rats. J. Urol. 2013, 189, 1960–1966. [Google Scholar] [CrossRef]
- Hutchison, A.J.; Wilson, R.J.; Garafola, S.; Copley, J.B. Lanthanum Carbonate: Safety Data after 10 Years. Nephrology 2016, 21, 987–994. [Google Scholar] [CrossRef]
- Pozdzik, A.; David, C.; Vekeman, J.; Tielens, F.; Daudon, M. Lanthanum Carbonate to Control Plasma and Urinary Oxalate Level in Type 1 Primary Hyperoxaluria? IJU Case Rep. 2021, 4, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Cheng, Y.; Zhou, J.; Liu, X.; Lin, R.; Xiang, S.; Liu, Z.; Wang, C. Zn2+ Regulates Human Oxalate Metabolism by Manipulating Oxalate Decarboxylase to Treat Calcium Oxalate Stones. Int. J. Biol. Macromol. 2023, 234, 123320. [Google Scholar] [CrossRef]
- Fargue, S.; Rumsby, G.; Danpure, C.J. Multiple Mechanisms of Action of Pyridoxine in Primary Hyperoxaluria Type 1. Biochim. Biophys. Acta 2013, 1832, 1776–1783. [Google Scholar] [CrossRef]
- Cellini, B.; Montioli, R.; Oppici, E.; Astegno, A.; Voltattorni, C.B. The Chaperone Role of the Pyridoxal 5′-Phosphate and Its Implications for Rare Diseases Involving B6-Dependent Enzymes. Clin. Biochem. 2014, 47, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, B.; Latta, K.; von Schnakenburg, C.; Kemper, M.J. Primary Hyperoxaluria—The German Experience. Am. J. Nephrol. 2005, 25, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Toussaint, C. Pyridoxine-Responsive PH1: Treatment. J. Nephrol. 1998, 11 (Suppl. 1), 49–50. [Google Scholar] [PubMed]
- Mandrile, G.; Beck, B.; Acquaviva, C.; Rumsby, G.; Deesker, L.; Garrelfs, S.; Gupta, A.; Bacchetta, J.; Groothoff, J. OxalEurope Consortium/Erknet Guideline Workgroup On Hyperoxaluria Genetic Assessment in Primary Hyperoxaluria: Why It Matters. Pediatr. Nephrol. 2023, 38, 625–634. [Google Scholar] [CrossRef]
- Hoyer-Kuhn, H.; Kohbrok, S.; Volland, R.; Franklin, J.; Hero, B.; Beck, B.B.; Hoppe, B. Vitamin B6 in Primary Hyperoxaluria I: First Prospective Trial after 40 Years of Practice. Clin. J. Am. Soc. Nephrol. CJASN 2014, 9, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Garrelfs, S.F.; van Harskamp, D.; Peters-Sengers, H.; van den Akker, C.H.P.; Wanders, R.J.A.; Wijburg, F.A.; van Goudoever, J.B.; Groothoff, J.W.; Schierbeek, H.; Oosterveld, M.J.S. Endogenous Oxalate Production in Primary Hyperoxaluria Type 1 Patients. J. Am. Soc. Nephrol. 2021, 32, 3175–3186. [Google Scholar] [CrossRef]
- Montioli, R.; Oppici, E.; Dindo, M.; Roncador, A.; Gotte, G.; Cellini, B.; Voltattorni, C.B. Misfolding Caused by the Pathogenic Mutation G47R on the Minor Allele of Alanine: Glyoxylate Aminotransferase and Chaperoning Activity of Pyridoxine. Biochim. Biophys. Acta 2015, 1854, 1280–1289. [Google Scholar] [CrossRef]
- Oppici, E.; Roncador, A.; Montioli, R.; Bianconi, S.; Cellini, B. Gly161 Mutations Associated with Primary Hyperoxaluria Type I Induce the Cytosolic Aggregation and the Intracellular Degradation of the Apo-Form of Alanine: Glyoxylate Aminotransferase. Biochim. Biophys. Acta 2013, 1832, 2277–2288. [Google Scholar] [CrossRef] [PubMed]
- Oppici, E.; Fargue, S.; Reid, E.S.; Mills, P.B.; Clayton, P.T.; Danpure, C.J.; Cellini, B. Pyridoxamine and Pyridoxal Are More Effective than Pyridoxine in Rescuing Folding-Defective Variants of Human Alanine: Glyoxylate Aminotransferase Causing Primary Hyperoxaluria Type I. Hum. Mol. Genet. 2015, 24, 5500–5511. [Google Scholar] [CrossRef] [PubMed]
- Dobrijević, D.; Pastor, K.; Nastić, N.; Özogul, F.; Krulj, J.; Kokić, B.; Bartkiene, E.; Rocha, J.M.; Kojić, J. Betaine as a Functional Ingredient: Metabolism, Health-Promoting Attributes, Food Sources, Applications and Analysis Methods. Molecules 2023, 28, 4824. [Google Scholar] [CrossRef] [PubMed]
- Lumb, M.J.; Birdsey, G.M.; Danpure, C.J. Correction of an Enzyme Trafficking Defect in Hereditary Kidney Stone Disease in Vitro. Biochem. J. 2003, 374, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Santana, A.; Salido, E.; Torres, A.; Shapiro, L.J. Primary Hyperoxaluria Type 1 in the Canary Islands: A Conformational Disease Due to I244T Mutation in the P11L-Containing Alanine:Glyoxylate Aminotransferase. Proc. Natl. Acad. Sci. USA 2003, 100, 7277–7282. [Google Scholar] [CrossRef]
- Coulter-Mackie, M.B.; Lian, Q. Partial Trypsin Digestion as an Indicator of Mis-Folding of Mutant Alanine: Glyoxylate Aminotransferase and Chaperone Effects of Specific Ligands. Study of a Spectrum of Missense Mutants. Mol. Genet. Metab. 2008, 94, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Miyata, N.; Steffen, J.; Johnson, M.E.; Fargue, S.; Danpure, C.J.; Koehler, C.M. Pharmacologic Rescue of an Enzyme-Trafficking Defect in Primary Hyperoxaluria 1. Proc. Natl. Acad. Sci. USA 2014, 111, 14406–14411. [Google Scholar] [CrossRef] [PubMed]
- Belostotsky, R.; Lyakhovetsky, R.; Sherman, M.Y.; Shkedy, F.; Tzvi-Behr, S.; Bar, R.; Hoppe, B.; Reusch, B.; Beck, B.B.; Frishberg, Y. Translation Inhibition Corrects Aberrant Localization of Mutant Alanine-Glyoxylate Aminotransferase: Possible Therapeutic Approach for Hyperoxaluria. J. Mol. Med. 2018, 96, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Illies, F.; Bonzel, K.-E.; Wingen, A.-M.; Latta, K.; Hoyer, P.F. Clearance and Removal of Oxalate in Children on Intensified Dialysis for Primary Hyperoxaluria Type 1. Kidney Int. 2006, 70, 1642–1648. [Google Scholar] [CrossRef]
- Kohli, H.; Kurtz, M.P. Primary Hyperoxaluria Type 1: Urologic and Therapeutic Management. Clin. Kidney J. 2022, 15, i14–i16. [Google Scholar] [CrossRef]
- Al-Abadi, E.; Hulton, S.-A. Extracorporal Shock Wave Lithotripsy in the Management of Stones in Children with Oxalosis—Still the First Choice? Pediatr. Nephrol. 2013, 28, 1085–1089. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.C.; Saw, K.C.; Paterson, R.F.; Hatt, E.K.; McAteer, J.A.; Lingeman, J.E. Variability of Renal Stone Fragility in Shock Wave Lithotripsy. Urology 2003, 61, 1092–1096. [Google Scholar] [CrossRef] [PubMed]
- Esposito, C.; Masieri, L.; Blanc, T.; Lendvay, T.; Escolino, M. Robot-Assisted Laparoscopic Surgery for Treatment of Urinary Tract Stones in Children: Report of a Multicenter International Experience. Urolithiasis 2021, 49, 575–583. [Google Scholar] [CrossRef]
- Carrasco, A.; Granberg, C.F.; Gettman, M.T.; Milliner, D.S.; Krambeck, A.E. Surgical Management of Stone Disease in Patients with Primary Hyperoxaluria. Urology 2015, 85, 522–526. [Google Scholar] [CrossRef]
- Rule, A.D.; Krambeck, A.E.; Lieske, J.C. Chronic Kidney Disease in Kidney Stone Formers. Clin. J. Am. Soc. Nephrol. 2011, 6, 2069–2075. [Google Scholar] [CrossRef] [PubMed]
- Bergstralh, E.J.; Monico, C.G.; Lieske, J.C.; Herges, R.M.; Langman, C.B.; Hoppe, B.; Milliner, D.S. Transplantation Outcomes in Primary Hyperoxaluria. Am. J. Transplant. 2010, 10, 2493–2501. [Google Scholar] [CrossRef] [PubMed]
- Metry, E.L.; van Dijk, L.M.M.; Peters-Sengers, H.; Oosterveld, M.J.S.; Groothoff, J.W.; Ploeg, R.J.; Stel, V.S.; Garrelfs, S.F. Transplantation Outcomes in Patients with Primary Hyperoxaluria: A Systematic Review. Pediatr. Nephrol. 2021, 36, 2217–2226. [Google Scholar] [CrossRef]
- Büscher, R.; Büscher, A.K.; Cetiner, M.; Treckmann, J.W.; Paul, A.; Vester, U.; Hoyer, P.F. Combined Liver and Kidney Transplantation and Kidney after Liver Transplantation in Children: Indication, Postoperative Outcome, and Long-Term Results. Pediatr. Transplant. 2015, 19, 858–865. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Chen, Z.; Xu, F.; Mei, S.; Li, Z.; Zhou, J.; Dong, Y.; Gu, Y.; Huang, Z.; Hu, Z. Outcomes of Liver–Kidney Transplantation in Patients with Primary Hyperoxaluria: An Analysis of the Scientific Registry of Transplant Recipients Database. BMC Gastroenterol. 2020, 20, 208. [Google Scholar] [CrossRef]
- Horoub, R.; Shamsaeefar, A.; Dehghani, M.; Nikoopour, H.; Entezari, M.; Moradi, A.; Kazemi, K.; Eshraghian, A.; Nikeghbalian, S.; Malek-Hosseini, S.A. Liver Transplant for Primary Hyperoxaluria Type 1: Results of Sequential, Combined Liver and Kidney, and Preemptive Liver Transplant. Exp. Clin. Transplant. Off. J. Middle East Soc. Organ Transplant. 2021, 19, 445–449. [Google Scholar] [CrossRef]
- Loos, S.; Kemper, M.J.; Schmaeschke, K.; Herden, U.; Fischer, L.; Hoppe, B.; Levart, T.K.; Grabhorn, E.; Schild, R.; Oh, J.; et al. Long-Term Outcome after Combined or Sequential Liver and Kidney Transplantation in Children with Infantile and Juvenile Primary Hyperoxaluria Type 1. Front. Pediatr. 2023, 11, 1157215. [Google Scholar] [CrossRef] [PubMed]
- Metry, E.L.; Garrelfs, S.F.; Peters-Sengers, H.; Hulton, S.-A.; Acquaviva, C.; Bacchetta, J.; Beck, B.B.; Collard, L.; Deschênes, G.; Franssen, C.; et al. Long-Term Transplantation Outcomes in Patients with Primary Hyperoxaluria Type 1 Included in the European Hyperoxaluria Consortium (OxalEurope) Registry. Kidney Int. Rep. 2022, 7, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Shasha-Lavsky, H.; Avni, A.; Paz, Z.; Kalfon, L.; Dror, A.A.; Yakir, O.; Zaccai, T.F.; Weissman, I. Long-Term Outcomes after Pre-Emptive Liver Transplantation in Primary Hyperoxaluria Type 1. Pediatr. Nephrol. 2023, 38, 1811–1820. [Google Scholar] [CrossRef] [PubMed]
- Joher, N.; Moktefi, A.; Grimbert, P.; Pagot, E.; Jouan, N.; El Karoui, K.; Champy, C.M.; Matignon, M.; Stehlé, T. Early Post-Transplant Recurrence of Oxalate Nephropathy in a Patient with Primary Hyperoxaluria Type 1, despite Pretransplant Lumasiran Therapy. Kidney Int. 2022, 101, 185–186. [Google Scholar] [CrossRef] [PubMed]
- Sellier-Leclerc, A.-L.; Metry, E.; Clave, S.; Perrin, P.; Acquaviva-Bourdain, C.; Levi, C.; Crop, M.; Caillard, S.; Moulin, B.; Groothoff, J.; et al. Isolated Kidney Transplantation under Lumasiran Therapy in Primary Hyperoxaluria Type 1: A Report of Five Cases. Nephrol. Dial. Transplant. 2023, 38, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Metry, E.L.; Deesker, L.J.; Garrelfs, S.F.; Oosterveld, M.J.S.; Beekman, K.M.; Cornelissen, M.E.A.; Koster-Kamphuis, L.; Groothoff, J.W. Successful Kidney-Alone Transplantation in a Patient with PH1 on Combination RNA-Interference Therapy. Kidney Int. 2023, 104, 203–204. [Google Scholar] [CrossRef] [PubMed]
- Cornell, L.D.; Amer, H.; Viehman, J.K.; Mehta, R.A.; Lieske, J.C.; Lorenz, E.C.; Heimbach, J.K.; Stegall, M.D.; Milliner, D.S. Posttransplant Recurrence of Calcium Oxalate Crystals in Patients with Primary Hyperoxaluria: Incidence, Risk Factors, and Effect on Renal Allograft Function. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2022, 22, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Naderi, G.; Latif, A.; Tabassomi, F.; Esfahani, S.T. Failure of Isolated Kidney Transplantation in a Pediatric Patient with Primary Hyperoxaluria Type 2. Pediatr. Transplant. 2014, 18, E69–E73. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Gao, B.; Wang, G.; Wang, W.; Lian, X.; Wu, S.; Yu, J.; Fu, Y.; Zhou, H. Recurrent Primary Hyperoxaluria Type 2 Leads to Early Post-Transplant Renal Function Loss: A Case Report. Exp. Ther. Med. 2018, 15, 3169–3172. [Google Scholar] [CrossRef]
- Jia, Z.; Zhong, Q.; Lin, T.; Song, T. Subsequent Liver Transplantation Did Not Reverse Recurrence of Oxalate Nephropathy after Isolated Kidney Transplantation for Primary Type 2 Hyperoxaluria. Asian J. Surg. 2022, 45, 483–485. [Google Scholar] [CrossRef]
- Bello, A.D.; Cointault, O.; Delas, A.; Kamar, N. Primary Hyperoxaluria Type 2 Successfully Treated with Combined Liver-Kidney Transplantation after Failure of Isolated Kidney Transplantation. Am. J. Transplant. 2020, 20, 1752–1753. [Google Scholar] [CrossRef] [PubMed]
- Dhondup, T.; Lorenz, E.C.; Milliner, D.S.; Lieske, J.C. Combined Liver-Kidney Transplantation for Primary Hyperoxaluria Type 2: A Case Report. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2018, 18, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Hosaagrahara Ramakrishna, S.; Hassan, A.; Kasala, M.B.; Perumal, K.; Venkategowda, C.; Malleeswaran, S.; Periasamy, M.; Kaliyaperumal, M.; Patcha, R.V.; Varghese, J.; et al. Pediatric Combined Living Donor Liver and Kidney Transplantation for Primary Hyperoxaluria Type 2. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2023, 23, 1622–1625. [Google Scholar] [CrossRef] [PubMed]
- Traber, G.M.; Yu, A.-M. RNAi-Based Therapeutics and Novel RNA Bioengineering Technologies. J. Pharmacol. Exp. Ther. 2023, 384, 133–154. [Google Scholar] [CrossRef] [PubMed]
- Dejban, P.; Lieske, J.C. New Therapeutics for Primary Hyperoxaluria Type 1. Curr. Opin. Nephrol. Hypertens. 2022, 31, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Liebow, A.; Li, X.; Racie, T.; Hettinger, J.; Bettencourt, B.R.; Najafian, N.; Haslett, P.; Fitzgerald, K.; Holmes, R.P.; Erbe, D.; et al. An Investigational RNAi Therapeutic Targeting Glycolate Oxidase Reduces Oxalate Production in Models of Primary Hyperoxaluria. J. Am. Soc. Nephrol. 2017, 28, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Roberts, T.C.; Langer, R.; Wood, M.J.A. Advances in Oligonucleotide Drug Delivery. Nat. Rev. Drug Discov. 2020, 19, 673–694. [Google Scholar] [CrossRef] [PubMed]
- Martin-Higueras, C.; Luis-Lima, S.; Salido, E. Glycolate Oxidase Is a Safe and Efficient Target for Substrate Reduction Therapy in a Mouse Model of Primary Hyperoxaluria Type I. Mol. Ther. 2016, 24, 719–725. [Google Scholar] [CrossRef]
- Frishberg, Y.; Deschênes, G.; Groothoff, J.W.; Hulton, S.-A.; Magen, D.; Harambat, J.; Van’T Hoff, W.G.; Lorch, U.; Milliner, D.S.; Lieske, J.C.; et al. Phase 1/2 Study of Lumasiran for Treatment of Primary Hyperoxaluria Type 1: A Placebo-Controlled Randomized Clinical Trial. Clin. J. Am. Soc. Nephrol. 2021, 16, 1025–1036. [Google Scholar] [CrossRef]
- Garrelfs, S.F.; Frishberg, Y.; Hulton, S.A.; Koren, M.J.; O’Riordan, W.D.; Cochat, P.; Deschênes, G.; Shasha-Lavsky, H.; Saland, J.M.; Van’t Hoff, W.G.; et al. Lumasiran, an RNAi Therapeutic for Primary Hyperoxaluria Type 1. N. Engl. J. Med. 2021, 384, 1216–1226. [Google Scholar] [CrossRef]
- Sas, D.J.; Magen, D.; Hayes, W.; Shasha-Lavsky, H.; Michael, M.; Schulte, I.; Sellier-Leclerc, A.-L.; Lu, J.; Seddighzadeh, A.; Habtemariam, B.; et al. Phase 3 Trial of Lumasiran for Primary Hyperoxaluria Type 1: A New RNAi Therapeutic in Infants and Young Children. Genet. Med. 2022, 24, 654–662. [Google Scholar] [CrossRef]
- Hayes, W.; Sas, D.J.; Magen, D.; Shasha-Lavsky, H.; Michael, M.; Sellier-Leclerc, A.-L.; Hogan, J.; Ngo, T.; Sweetser, M.T.; Gansner, J.M.; et al. Efficacy and Safety of Lumasiran for Infants and Young Children with Primary Hyperoxaluria Type 1: 12-Month Analysis of the Phase 3 ILLUMINATE-B Trial. Pediatr. Nephrol. 2023, 38, 1075–1086. [Google Scholar] [CrossRef]
- Biebuyck, N.; Destombes, C.; Prakash, R.; Boyer, O. Is Withdrawal of Nocturnal Hyperhydration Possible in Children with Primary Hyperoxaluria Treated with RNAi? J. Nephrol. 2023, 36, 1473–1476. [Google Scholar] [CrossRef]
- Michael, M.; Groothoff, J.W.; Shasha-Lavsky, H.; Lieske, J.C.; Frishberg, Y.; Simkova, E.; Sellier-Leclerc, A.-L.; Devresse, A.; Guebre-Egziabher, F.; Bakkaloglu, S.A.; et al. Lumasiran for Advanced Primary Hyperoxaluria Type 1: Phase 3 ILLUMINATE-C Trial. Am. J. Kidney Dis. 2023, 81, 145–155.e1. [Google Scholar] [CrossRef]
- Lai, C.; Pursell, N.; Gierut, J.; Saxena, U.; Zhou, W.; Dills, M.; Diwanji, R.; Dutta, C.; Koser, M.; Nazef, N.; et al. Specific Inhibition of Hepatic Lactate Dehydrogenase Reduces Oxalate Production in Mouse Models of Primary Hyperoxaluria. Mol. Ther. 2018, 26, 1983–1995. [Google Scholar] [CrossRef]
- Hoppe, B.; Koch, A.; Cochat, P.; Garrelfs, S.F.; Baum, M.A.; Groothoff, J.W.; Lipkin, G.; Coenen, M.; Schalk, G.; Amrite, A.; et al. Safety, Pharmacodynamics, and Exposure-Response Modeling Results from a First-in-Human Phase 1 Study of Nedosiran (PHYOX1) in Primary Hyperoxaluria. Kidney Int. 2022, 101, 626–634. [Google Scholar] [CrossRef]
- Baum, M.A.; Langman, C.; Cochat, P.; Lieske, J.C.; Moochhala, S.H.; Hamamoto, S.; Satoh, H.; Mourani, C.; Ariceta, G.; Torres, A.; et al. PHYOX2: A Pivotal Randomized Study of Nedosiran in Primary Hyperoxaluria Type 1 or 2. Kidney Int. 2023, 103, 207–217. [Google Scholar] [CrossRef]
- Goldfarb, D.S.; Lieske, J.C.; Groothoff, J.; Schalk, G.; Russell, K.; Yu, S.; Vrhnjak, B. Nedosiran in Primary Hyperoxaluria Subtype 3: Results from a Phase I, Single-Dose Study (PHYOX4). Urolithiasis 2023, 51, 80. [Google Scholar] [CrossRef]
- Verrotti, A.; Prezioso, G.; Stagi, S.; Paolino, M.C.; Parisi, P. Pharmacological Considerations in the Use of Stiripentol for the Treatment of Epilepsy. Expert Opin. Drug Metab. Toxicol. 2016, 12, 345–352. [Google Scholar] [CrossRef]
- Sada, N.; Lee, S.; Katsu, T.; Otsuki, T.; Inoue, T. Epilepsy Treatment. Targeting LDH Enzymes with a Stiripentol Analog to Treat Epilepsy. Science 2015, 347, 1362–1367. [Google Scholar] [CrossRef]
- Le Dudal, M.; Huguet, L.; Perez, J.; Vandermeersch, S.; Bouderlique, E.; Tang, E.; Martori, C.; Chemaly, N.; Nabbout, R.; Haymann, J.-P.; et al. Stiripentol Protects against Calcium Oxalate Nephrolithiasis and Ethylene Glycol Poisoning. J. Clin. Investig. 2019, 129, 2571–2577. [Google Scholar] [CrossRef]
- Violier, P.; Boyer, O.; Berthaud, R.; Dorval, G. Treatment with Stiripentol in a Patient with Primary Hyperoxaluria Type 1: Lesson for the Clinical Nephrologist. J. Nephrol. 2022, 35, 1049–1051. [Google Scholar] [CrossRef]
- Martin-Higueras, C.; Feldkötter, M.; Hoppe, B. Is Stiripentol Truly Effective for Treating Primary Hyperoxaluria? Clin. Kidney J. 2021, 14, 442–444. [Google Scholar] [CrossRef]
- Kempf, C.; Pfau, A.; Holle, J.; Müller-Schlüter, K.; Bufler, P.; Knauf, F.; Müller, D. Stiripentol Fails to Lower Plasma Oxalate in a Dialysis-Dependent PH1 Patient. Pediatr. Nephrol. 2020, 35, 1787–1789. [Google Scholar] [CrossRef]
- Lombardi, Y.; Isnard, P.; Chavarot, N.; Chauvet, S.; Martinez, F.; Thervet, É.; Anglicheau, D.; Karras, A. Stiripentol and Lumasiran as a Rescue Therapy for Oxalate Nephropathy Recurrence After Kidney Transplantation in an Adult Patient with Primary Hyperoxaluria Type 1. Am. J. Kidney Dis. 2023, 82, 113–116. [Google Scholar] [CrossRef]
- Alejo-Armijo, A.; Cuadrado, C.; Altarejos, J.; Fernandes, M.X.; Salido, E.; Diaz-Gavilan, M.; Salido, S. Lactate Dehydrogenase A Inhibitors with a 2,8-Dioxabicyclo[3.3.1]Nonane Scaffold: A Contribution to Molecular Therapies for Primary Hyperoxalurias. Bioorg. Chem. 2022, 129, 106127. [Google Scholar] [CrossRef]
- Cabrera, N.; Cuesta, S.A.; Mora, J.R.; Paz, J.L.; Márquez, E.A.; Espinoza-Montero, P.J.; Marrero-Ponce, Y.; Pérez, N.; Contreras-Torres, E. Searching Glycolate Oxidase Inhibitors Based on QSAR, Molecular Docking, and Molecular Dynamic Simulation Approaches. Sci. Rep. 2022, 12, 19969. [Google Scholar] [CrossRef]
- Ding, J.; Gumpena, R.; Boily, M.-O.; Caron, A.; Chong, O.; Cox, J.H.; Dumais, V.; Gaudreault, S.; Graff, A.H.; King, A.; et al. Dual Glycolate Oxidase/Lactate Dehydrogenase a Inhibitors for Primary Hyperoxaluria. ACS Med. Chem. Lett. 2021, 12, 1116–1123. [Google Scholar] [CrossRef]
- Moya-Garzon, M.D.; Rodriguez-Rodriguez, B.; Martin-Higueras, C.; Franco-Montalban, F.; Fernandes, M.X.; Gomez-Vidal, J.A.; Pey, A.L.; Salido, E.; Diaz-Gavilan, M. New Salicylic Acid Derivatives, Double Inhibitors of Glycolate Oxidase and Lactate Dehydrogenase, as Effective Agents Decreasing Oxalate Production. Eur. J. Med. Chem. 2022, 237, 114396. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, S.; Kwaku Dad, A.-B.; Beloor, J.; Gopalappa, R.; Lee, S.-K.; Kim, H. Gene Disruption by Cell-Penetrating Peptide-Mediated Delivery of Cas9 Protein and Guide RNA. Genome Res. 2014, 24, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
- Gaj, T.; Epstein, B.E.; Schaffer, D.V. Genome Engineering Using Adeno-Associated Virus: Basic and Clinical Research Applications. Mol. Ther. J. Am. Soc. Gene Ther. 2016, 24, 458–464. [Google Scholar] [CrossRef]
- Berardo, C.; Siciliano, V.; Di Pasqua, L.G.; Richelmi, P.; Vairetti, M.; Ferrigno, A. Comparison between Lipofectamine RNAiMAX and GenMute Transfection Agents in Two Cellular Models of Human Hepatoma. Eur. J. Histochem. EJH 2019, 63, 3048. [Google Scholar] [CrossRef]
- Zheng, R.; Li, Y.; Wang, L.; Fang, X.; Zhang, J.; He, L.; Yang, L.; Li, D.; Geng, H. CRISPR/Cas9-Mediated Metabolic Pathway Reprogramming in a Novel Humanized Rat Model Ameliorates Primary Hyperoxaluria Type 1. Kidney Int. 2020, 98, 947–957. [Google Scholar] [CrossRef]
- Zheng, R.; Fang, X.; Chen, X.; Huang, Y.; Xu, G.; He, L.; Li, Y.; Niu, X.; Yang, L.; Wang, L.; et al. Knockdown of Lactate Dehydrogenase by Adeno-associated Virus-delivered CRISPR/Cas9 System Alleviates Primary Hyperoxaluria Type 1. Clin. Transl. Med. 2020, 10, e261. [Google Scholar] [CrossRef]
- Zheng, R.; Zhang, D.-X.; Shao, Y.-J.; Fang, X.-L.; Yang, L.; Huo, Y.-N.; Li, D.-L.; Geng, H.-Q. Multiplex Gene Editing Reduces Oxalate Production in Primary Hyperoxaluria Type 1. Zool. Res. 2023, 44, 993–1002. [Google Scholar] [CrossRef]
- Rabinowitz, J.; Chan, Y.K.; Samulski, R.J. Adeno-Associated Virus (AAV) versus Immune Response. Viruses 2019, 11, 102. [Google Scholar] [CrossRef]
- Uddin, F.; Rudin, C.M.; Sen, T. CRISPR Gene Therapy: Applications, Limitations, and Implications for the Future. Front. Oncol. 2020, 10, 1387. [Google Scholar] [CrossRef]
- Hu, H.; Mosca, R.; Gomero, E.; van de Vlekkert, D.; Campos, Y.; Fremuth, L.E.; Brown, S.A.; Weesner, J.A.; Annunziata, I.; d’Azzo, A. AAV-Mediated Gene Therapy for Galactosialidosis: A Long-Term Safety and Efficacy Study. Mol. Ther. Methods Clin. Dev. 2021, 23, 644–658. [Google Scholar] [CrossRef]
- Roncador, A.; Oppici, E.; Talelli, M.; Pariente, A.N.; Donini, M.; Dusi, S.; Voltattorni, C.B.; Vicent, M.J.; Cellini, B. Use of Polymer Conjugates for the Intraperoxisomal Delivery of Engineered Human Alanine:Glyoxylate Aminotransferase as a Protein Therapy for Primary Hyperoxaluria Type I. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Salido, E.; Rodriguez-Pena, M.; Santana, A.; Beattie, S.G.; Petry, H.; Torres, A. Phenotypic Correction of a Mouse Model for Primary Hyperoxaluria with Adeno-Associated Virus Gene Transfer. Mol. Ther. 2011, 19, 870–875. [Google Scholar] [CrossRef] [PubMed]
- Castello, R.; Borzone, R.; D’Aria, S.; Annunziata, P.; Piccolo, P.; Brunetti-Pierri, N. Helper-Dependent Adenoviral Vectors for Liver-Directed Gene Therapy of Primary Hyperoxaluria Type 1. Gene Ther. 2016, 23, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Kukreja, A.; Lasaro, M.; Cobaugh, C.; Forbes, C.; Tang, J.-P.; Gao, X.; Martin-Higueras, C.; Pey, A.L.; Salido, E.; Sobolov, S.; et al. Systemic Alanine Glyoxylate Aminotransferase mRNA Improves Glyoxylate Metabolism in a Mouse Model of Primary Hyperoxaluria Type 1. Nucleic Acid Ther. 2019, 29, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Estève, J.; Blouin, J.-M.; Lalanne, M.; Azzi-Martin, L.; Dubus, P.; Bidet, A.; Harambat, J.; Llanas, B.; Moranvillier, I.; Bedel, A.; et al. Targeted Gene Therapy in Human-Induced Pluripotent Stem Cells from a Patient with Primary Hyperoxaluria Type 1 Using CRISPR/Cas9 Technology. Biochem. Biophys. Res. Commun. 2019, 517, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Estève, J.; Blouin, J.-M.; Lalanne, M.; Azzi-Martin, L.; Dubus, P.; Bidet, A.; Harambat, J.; Llanas, B.; Moranvillier, I.; Bedel, A.; et al. Generation of Induced Pluripotent Stem Cells-Derived Hepatocyte-like Cells for Ex Vivo Gene Therapy of Primary Hyperoxaluria Type 1. Stem Cell Res. 2019, 38, 101467. [Google Scholar] [CrossRef] [PubMed]
- Beck, B.B.; Habbig, S.; Dittrich, K.; Stippel, D.; Kaul, I.; Koerber, F.; Goebel, H.; Salido, E.C.; Kemper, M.; Meyburg, J.; et al. Liver Cell Transplantation in Severe Infantile Oxalosis--a Potential Bridging Procedure to Orthotopic Liver Transplantation? Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2012, 27, 2984–2989. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Salido, E.C.; Guha, C.; Wang, X.; Moitra, R.; Liu, L.; Roy-Chowdhury, J.; Roy-Chowdhury, N. Correction of Hyperoxaluria by Liver Repopulation with Hepatocytes in a Mouse Model of Primary Hyperoxaluria Type-1. Transplantation 2008, 85, 1253–1260. [Google Scholar] [CrossRef]
- Swanson, K.V.; Deng, M.; Ting, J.P.Y. The NLRP3 Inflammasome: Molecular Activation and Regulation to Therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef]
- Mulay, S.R. Multifactorial Functions of the Inflammasome Component NLRP3 in Pathogenesis of Chronic Kidney Diseases. Kidney Int. 2019, 96, 58–66. [Google Scholar] [CrossRef]
- Mulay, S.R.; Kulkarni, O.P.; Rupanagudi, K.V.; Migliorini, A.; Darisipudi, M.N.; Vilaysane, A.; Muruve, D.; Shi, Y.; Munro, F.; Liapis, H.; et al. Calcium Oxalate Crystals Induce Renal Inflammation by NLRP3-Mediated IL-1β Secretion. J. Clin. Investig. 2013, 123, 236–246. [Google Scholar] [CrossRef]
- Song, Z.; Zhang, Y.; Gong, B.; Xu, H.; Hao, Z.; Liang, C. Long Noncoding RNA LINC00339 Promotes Renal Tubular Epithelial Pyroptosis by Regulating the miR-22-3p/NLRP3 Axis in Calcium Oxalate–Induced Kidney Stone. J. Cell. Biochem. 2019, 120, 10452–10462. [Google Scholar] [CrossRef]
- Ludwig-Portugall, I.; Bartok, E.; Dhana, E.; Evers, B.D.G.; Primiano, M.J.; Hall, J.P.; Franklin, B.S.; Knolle, P.A.; Hornung, V.; Hartmann, G.; et al. An NLRP3-Specific Inflammasome Inhibitor Attenuates Crystal-Induced Kidney Fibrosis in Mice. Kidney Int. 2016, 90, 525–539. [Google Scholar] [CrossRef]
- Mulay, S.R.; Desai, J.; Kumar, S.V.; Eberhard, J.N.; Thomasova, D.; Romoli, S.; Grigorescu, M.; Kulkarni, O.P.; Popper, B.; Vielhauer, V.; et al. Cytotoxicity of Crystals Involves RIPK3-MLKL-Mediated Necroptosis. Nat. Commun. 2016, 7, 10274. [Google Scholar] [CrossRef]
- Mulay, S.R.; Eberhard, J.N.; Desai, J.; Marschner, J.A.; Kumar, S.V.R.; Weidenbusch, M.; Grigorescu, M.; Lech, M.; Eltrich, N.; Müller, L.; et al. Hyperoxaluria Requires TNF Receptors to Initiate Crystal Adhesion and Kidney Stone Disease. J. Am. Soc. Nephrol. 2017, 28, 761. [Google Scholar] [CrossRef]
| Clinical Features | PH1 (Approximately 80%) | PH2 (Approximately 7.9–10%) | PH3 (Approximately 8.4–17%) |
|---|---|---|---|
| Estimated incidence rate | 1:121,499 | 1:196,952 | 1:79,499 |
| Affected enzyme | AGT | GRHPR | HOGA1 |
| Enzyme distribution | Hepatic-specific distribution (peroxisome) | Predominantly distributed in liver (predominantly in the cytoplasm and a smaller portion in mitochondria) | Predominantly distributed in liver and kidneys (mitochondria) |
| Coding gene (chromosomal position) | AGXT (2q37.3) | GRHPR (9p13.2) | HOGA1 (10q24.2) |
| Biochemical markers | Glyoxylate | L-glycerate | 4OHGlu, DHG, HOG |
| Age of initial symptom onset | 4.9 years | 5.7 years | 2.7 years |
| Clinical performance | Infantile oxalosis, nephrocalcinosis, systemic oxalosis and ESRD | Nephrocalcinosis, systemic oxalosis and ESRD (approximately 25%) | Recurrent calcium oxalate renal stones and ESRD (case reports) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Zhu, W.; Zhou, J.; Huang, Q.; Zeng, G. Navigating the Evolving Landscape of Primary Hyperoxaluria: Traditional Management Defied by the Rise of Novel Molecular Drugs. Biomolecules 2024, 14, 511. https://doi.org/10.3390/biom14050511
Huang Y, Zhu W, Zhou J, Huang Q, Zeng G. Navigating the Evolving Landscape of Primary Hyperoxaluria: Traditional Management Defied by the Rise of Novel Molecular Drugs. Biomolecules. 2024; 14(5):511. https://doi.org/10.3390/biom14050511
Chicago/Turabian StyleHuang, Yueqi, Wei Zhu, Jia Zhou, Qiulin Huang, and Guohua Zeng. 2024. "Navigating the Evolving Landscape of Primary Hyperoxaluria: Traditional Management Defied by the Rise of Novel Molecular Drugs" Biomolecules 14, no. 5: 511. https://doi.org/10.3390/biom14050511




