The Cerebrospinal Fluid Free-Glycans Hex1 and HexNAc1Hex1Neu5Ac1 as Potential Biomarkers of Alzheimer’s Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Patient Population
2.3. Sample Collection and Characterization
2.4. Isolation and Purification of Free-Glycans
2.5. N-Glycan Release and Purification
2.6. O-Glycan Release and Purification
2.7. Permethylation
2.8. Mass Spectrometry
2.9. Statistical Analysis
3. Results
3.1. Human CSF Contains Free-, N- and O-Glycans
3.2. Inter-Day Reproducibility of Free-, N- and O-Glycans
3.3. The Glycan Profiles of Patients with AD Differ Significantly from Healthy Controls and Other Types of Dementia
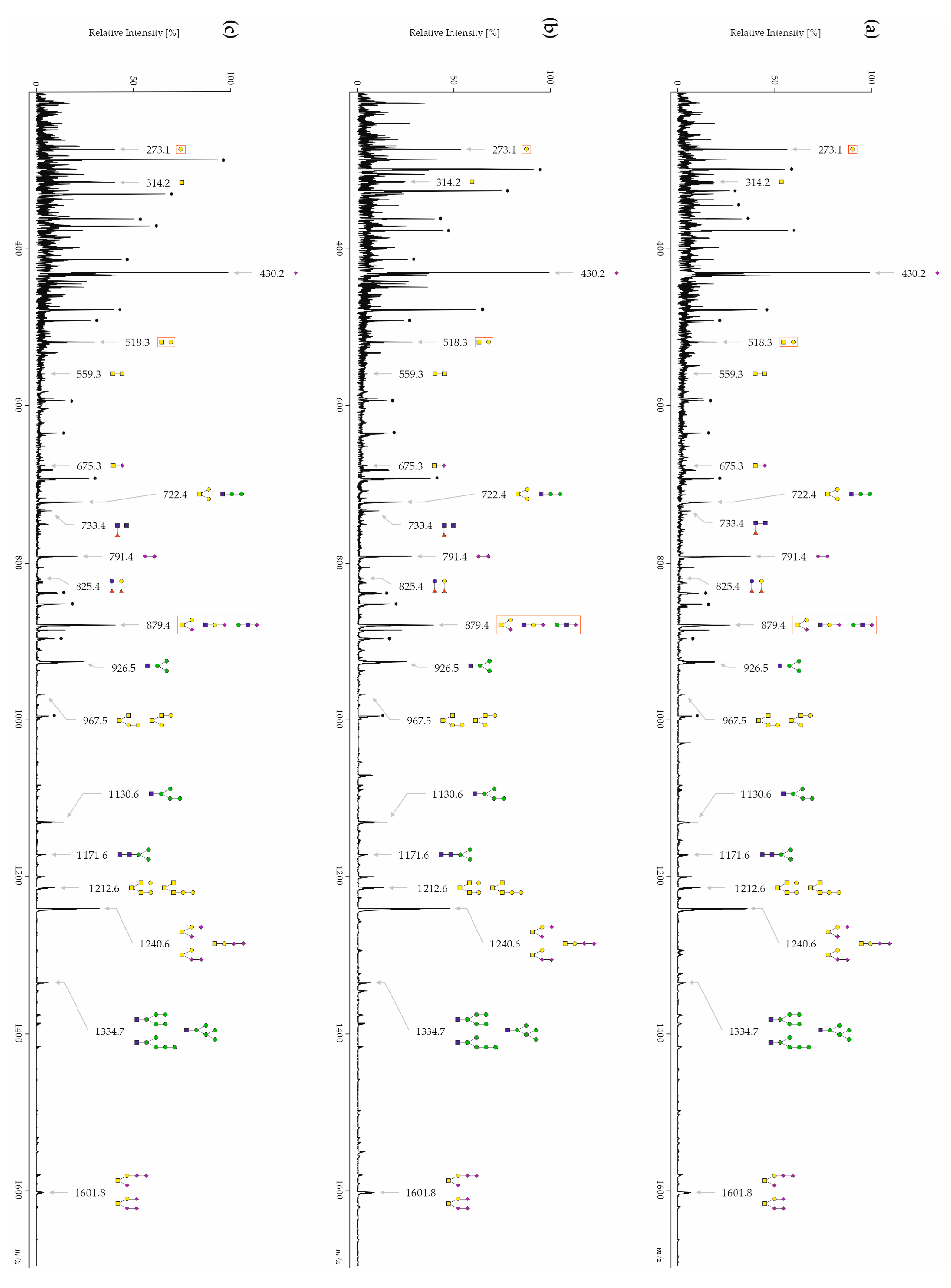
3.3.1. CSF Free-Glycans
3.3.2. CSF N-Glycans
3.3.3. CSF O-Glycans
3.4. The Sialic Acid Content Is Significantly Decreased in Free-Glycan Fragments and O-Glycans from Patients with AD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gilbert, B.J. The role of amyloid beta in the pathogenesis of Alzheimer’s disease. J. Clin. Pathol. 2013, 66, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.alzint.org/resource/world-alzheimer-report-2023/ (accessed on 10 May 2023).
- Winblad, B.; Amouyel, P.; Andrieu, S.; Ballard, C.; Brayne, C.; Brodaty, H.; Cedazo-Minguez, A.; Dubois, B.; Edvardsson, D.; Feldman, H.; et al. Defeating Alzheimer’s disease and other dementias: A priority for European science and society. Lancet Neurol. 2016, 15, 455–532. [Google Scholar] [CrossRef] [PubMed]
- Jeremic, D.; Jimenez-Diaz, L.; Navarro-Lopez, J.D. Past, present and future of therapeutic strategies against amyloid-beta peptides in Alzheimer’s disease: A systematic review. Ageing Res. Rev. 2021, 72, 101496. [Google Scholar] [CrossRef] [PubMed]
- McLaurin, J.; Yang, D.; Yip, C.M.; Fraser, P.E. Review: Modulating factors in amyloid-beta fibril formation. J. Struct. Biol. 2000, 130, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Yuzwa, S.A.; Vocadlo, D.J. O-GlcNAc modification and the tauopathies: Insights from chemical biology. Curr. Alzheimer Res. 2009, 6, 451–454. [Google Scholar] [CrossRef] [PubMed]
- Balana, A.T.; Pratt, M.R. Mechanistic roles for altered O-GlcNAcylation in neurodegenerative disorders. Biochem. J. 2021, 478, 2733–2758. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, T.; Ferreira, S.; Dupont-Wallois, L.; Bussiere, T.; Dupire, M.J.; Delacourte, A.; Michalski, J.C.; Caillet-Boudin, M.L. Evidence of a balance between phosphorylation and O-GlcNAc glycosylation of Tau proteins—A role in nuclear localization. Biochim. Et Biophys. Acta 2003, 1619, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Iqbal, K.; Grundke-Iqbal, I.; Hart, G.W.; Gong, C.X. O-GlcNAcylation regulates phosphorylation of tau: A mechanism involved in Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2004, 101, 10804–10809. [Google Scholar] [CrossRef]
- Alonso, A.D.; Grundke-Iqbal, I.; Barra, H.S.; Iqbal, K. Abnormal phosphorylation of tau and the mechanism of Alzheimer neurofibrillary degeneration: Sequestration of microtubule-associated proteins 1 and 2 and the disassembly of microtubules by the abnormal tau. Proc. Natl. Acad. Sci. USA 1997, 94, 298–303. [Google Scholar] [CrossRef]
- Iqbal, K.; Alonso Adel, C.; Chen, S.; Chohan, M.O.; El-Akkad, E.; Gong, C.X.; Khatoon, S.; Li, B.; Liu, F.; Rahman, A.; et al. Tau pathology in Alzheimer disease and other tauopathies. Biochim. Biophys. Acta 2005, 1739, 198–210. [Google Scholar] [CrossRef]
- Rogaev, E.I.; Sherrington, R.; Rogaeva, E.A.; Levesque, G.; Ikeda, M.; Liang, Y.; Chi, H.; Lin, C.; Holman, K.; Tsuda, T.; et al. Familial Alzheimer’s disease in kindreds with missense mutations in a gene on chromosome 1 related to the Alzheimer’s disease type 3 gene. Nature 1995, 376, 775–778. [Google Scholar] [CrossRef] [PubMed]
- Sherrington, R.; Rogaev, E.I.; Liang, Y.; Rogaeva, E.A.; Levesque, G.; Ikeda, M.; Chi, H.; Lin, C.; Li, G.; Holman, K.; et al. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer’s disease. Nature 1995, 375, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Guerreiro, R.; Brás, J.; Hardy, J. SnapShot: Genetics of Alzheimer’s Disease. Cell 2013, 155, 968–968.e961. [Google Scholar] [CrossRef]
- 2023 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2023, 17, 327–406. [CrossRef]
- Ryman, D.C.; Acosta-Baena, N.; Aisen, P.S.; Bird, T.; Danek, A.; Fox, N.C.; Goate, A.; Frommelt, P.; Ghetti, B.; Langbaum, J.B.S.; et al. Symptom onset in autosomal dominant Alzheimer disease: A systematic review and meta-analysis. Neurology 2014, 83, 253–260. [Google Scholar] [CrossRef]
- Corder, E.H.; Saunders, A.M.; Strittmatter, W.J.; Schmechel, D.E.; Gaskell, P.C.; Small, G.W.; Roses, A.D.; Haines, J.L.; Pericak-Vance, M.A. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 1993, 261, 921–923. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Knopman, D.S.; Jagust, W.J.; Petersen, R.C.; Weiner, M.W.; Aisen, P.S.; Shaw, L.M.; Vemuri, P.; Wiste, H.J.; Weigand, S.D.; et al. Tracking pathophysiological processes in Alzheimer’s disease: An updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013, 12, 207–216. [Google Scholar] [CrossRef]
- Hansson, O. Biomarkers for neurodegenerative diseases. Nat. Med. 2021, 27, 954–963. [Google Scholar] [CrossRef]
- Dunne, R.A.; Aarsland, D.; O’Brien, J.T.; Ballard, C.; Banerjee, S.; Fox, N.C.; Isaacs, J.D.; Underwood, B.R.; Perry, R.J.; Chan, D.; et al. Mild cognitive impairment: The Manchester consensus. Age Ageing 2021, 50, 72–80. [Google Scholar] [CrossRef]
- Caselli, R.J.; Beach, T.G.; Yaari, R.; Reiman, E.M. Alzheimer’s disease a century later. J. Clin. Psychiatry 2006, 67, 1784–1800. [Google Scholar] [CrossRef]
- Boeve, B.F.; Maraganore, D.M.; Parisi, J.E.; Ahlskog, J.E.; Graff-Radford, N.; Caselli, R.J.; Dickson, D.W.; Kokmen, E.; Petersen, R.C. Pathologic heterogeneity in clinically diagnosed corticobasal degeneration. Neurology 1999, 53, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Boeve, B.F.; Silber, M.H.; Ferman, T.J.; Lucas, J.A.; Parisi, J.E. Association of REM sleep behavior disorder and neurodegenerative disease may reflect an underlying synucleinopathy. Mov. Disord. 2001, 16, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Caselli, R.J.; Jack, C.R.; Petersen, R.C.; Wahner, H.W.; Yanagihara, T. Asymmetric cortical degenerative syndromes: Clinical and radiologic correlations. Neurology 1992, 42, 1462–1468. [Google Scholar] [CrossRef] [PubMed]
- Caselli, R.J.; Stelmach, G.E.; Caviness, J.N.; Timmann, D.; Royer, T.; Boeve, B.F.; Parisi, J.E. A kinematic study of progressive apraxia with and without dementia. Mov. Disord. 1999, 14, 276–287. [Google Scholar] [CrossRef] [PubMed]
- McKeith, I. Dementia with Lewy bodies. Dialogues Clin. Neurosci. 2004, 6, 333–341. [Google Scholar] [CrossRef] [PubMed]
- McKeith, I.G.; Dickson, D.W.; Lowe, J.; Emre, M.; O’Brien, J.T.; Feldman, H.; Cummings, J.; Duda, J.E.; Lippa, C.; Perry, E.K.; et al. Diagnosis and management of dementia with Lewy bodies: Third report of the DLB Consortium. Neurology 2005, 65, 1863–1872. [Google Scholar] [CrossRef]
- Tang-Wai, D.F.; Graff-Radford, N.R.; Boeve, B.F.; Dickson, D.W.; Parisi, J.E.; Crook, R.; Caselli, R.J.; Knopman, D.S.; Petersen, R.C. Clinical, genetic, and neuropathologic characteristics of posterior cortical atrophy. Neurology 2004, 63, 1168–1174. [Google Scholar] [CrossRef]
- Johnson, J.K.; Head, E.; Kim, R.; Starr, A.; Cotman, C.W. Clinical and pathological evidence for a frontal variant of Alzheimer disease. Arch. Neurol. 1999, 56, 1233–1239. [Google Scholar] [CrossRef]
- Heurling, K.; Leuzy, A.; Zimmer, E.R.; Lubberink, M.; Nordberg, A. Imaging beta-amyloid using [(18)F]flutemetamol positron emission tomography: From dosimetry to clinical diagnosis. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 362–373. [Google Scholar] [CrossRef]
- Leclerc, B.; Abulrob, A. Perspectives in molecular imaging using staging biomarkers and immunotherapies in Alzheimer’s disease. Sci. World J. 2013, 2013, 589308. [Google Scholar] [CrossRef]
- Sabri, O.; Seibyl, J.; Rowe, C.; Barthel, H. Beta-amyloid imaging with florbetaben. Clin. Transl. Imaging 2015, 3, 13–26. [Google Scholar] [CrossRef]
- Barthel, H.; Gertz, H.J.; Dresel, S.; Peters, O.; Bartenstein, P.; Buerger, K.; Hiemeyer, F.; Wittemer-Rump, S.M.; Seibyl, J.; Reininger, C.; et al. Cerebral amyloid-beta PET with florbetaben (18F) in patients with Alzheimer’s disease and healthy controls: A multicentre phase 2 diagnostic study. Lancet Neurol. 2011, 10, 424–435. [Google Scholar] [CrossRef]
- Herholz, K.; Ebmeier, K. Clinical amyloid imaging in Alzheimer’s disease. Lancet Neurol. 2011, 10, 667–670. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef]
- Blom, E.S.; Giedraitis, V.; Zetterberg, H.; Fukumoto, H.; Blennow, K.; Hyman, B.T.; Irizarry, M.C.; Wahlund, L.O.; Lannfelt, L.; Ingelsson, M. Rapid progression from mild cognitive impairment to Alzheimer’s disease in subjects with elevated levels of tau in cerebrospinal fluid and the APOE epsilon4/epsilon4 genotype. Dement. Geriatr. Cogn. Disord. 2009, 27, 458–464. [Google Scholar] [CrossRef]
- Samgard, K.; Zetterberg, H.; Blennow, K.; Hansson, O.; Minthon, L.; Londos, E. Cerebrospinal fluid total tau as a marker of Alzheimer’s disease intensity. Int. J. Geriatr. Psychiatry 2010, 25, 403–410. [Google Scholar] [CrossRef]
- Seubert, P.; Vigo-Pelfrey, C.; Esch, F.; Lee, M.; Dovey, H.; Davis, D.; Sinha, S.; Schlossmacher, M.; Whaley, J.; Swindlehurst, C.; et al. Isolation and quantification of soluble Alzheimer’s beta-peptide from biological fluids. Nature 1992, 359, 325–327. [Google Scholar] [CrossRef]
- Sadigh-Eteghad, S.; Sabermarouf, B.; Majdi, A.; Talebi, M.; Farhoudi, M.; Mahmoudi, J. Amyloid-beta: A crucial factor in Alzheimer’s disease. Med. Princ. Pract. 2015, 24, 1–10. [Google Scholar] [CrossRef]
- Jarrett, J.T.; Berger, E.P.; Lansbury, P.T., Jr. The carboxy terminus of the beta amyloid protein is critical for the seeding of amyloid formation: Implications for the pathogenesis of Alzheimer’s disease. Biochemistry 1993, 32, 4693–4697. [Google Scholar] [CrossRef] [PubMed]
- Shaw, L.M.; Vanderstichele, H.; Knapik-Czajka, M.; Clark, C.M.; Aisen, P.S.; Petersen, R.C.; Blennow, K.; Soares, H.; Simon, A.; Lewczuk, P.; et al. Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann. Neurol. 2009, 65, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Welge, V.; Fiege, O.; Lewczuk, P.; Mollenhauer, B.; Esselmann, H.; Klafki, H.W.; Wolf, S.; Trenkwalder, C.; Otto, M.; Kornhuber, J.; et al. Combined CSF tau, p-tau181 and amyloid-beta 38/40/42 for diagnosing Alzheimer’s disease. J. Neural Transm. 2009, 116, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.A.; Arvanitakis, Z.; Leurgans, S.E.; Bennett, D.A. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann. Neurol. 2009, 66, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Blennow, K.; Zetterberg, H.; Fagan, A.M. Fluid biomarkers in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2012, 2, a006221. [Google Scholar] [CrossRef] [PubMed]
- Peracaula, R.; Royle, L.; Tabarés, G.; Mallorquí-Fernández, G.; Barrabés, S.; Harvey, D.J.; Dwek, R.A.; Rudd, P.M.; de Llorens, R. Glycosylation of human pancreatic ribonuclease: Differences between normal and tumor states. Glycobiology 2003, 13, 227–244. [Google Scholar] [CrossRef] [PubMed]
- Peracaula, R.; Tabarés, G.; Royle, L.; Harvey, D.J.; Dwek, R.A.; Rudd, P.M.; de Llorens, R. Altered glycosylation pattern allows the distinction between prostate-specific antigen (PSA) from normal and tumor origins. Glycobiology 2003, 13, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Opdenakker, G.; Rudd, P.M.; Ponting, C.P.; Dwek, R.A. Concepts and principles of glycobiology. FASEB J. 1993, 7, 1330–1337. [Google Scholar] [CrossRef]
- Fukuda, M.N.; Sasaki, H.; Lopez, L.; Fukuda, M. Survival of recombinant erythropoietin in the circulation: The role of carbohydrates. Blood 1989, 73, 84–89. [Google Scholar] [CrossRef]
- Gu, J.; Isaji, T.; Xu, Q.; Kariya, Y.; Gu, W.; Fukuda, T.; Du, Y. Potential roles of N-glycosylation in cell adhesion. Glycoconj. J. 2012, 29, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Biskup, K.; Braicu, E.I.; Sehouli, J.; Fotopoulou, C.; Tauber, R.; Berger, M.; Blanchard, V. Serum glycome profiling: A biomarker for diagnosis of ovarian cancer. J. Proteome Res. 2013, 12, 4056–4063. [Google Scholar] [CrossRef]
- Gornik, O.; Royle, L.; Harvey, D.J.; Radcliffe, C.M.; Saldova, R.; Dwek, R.A.; Rudd, P.; Lauc, G. Changes of serum glycans during sepsis and acute pancreatitis. Glycobiology 2007, 17, 1321–1332. [Google Scholar] [CrossRef]
- Abd Hamid, U.M.; Royle, L.; Saldova, R.; Radcliffe, C.M.; Harvey, D.J.; Storr, S.J.; Pardo, M.; Antrobus, R.; Chapman, C.J.; Zitzmann, N.; et al. A strategy to reveal potential glycan markers from serum glycoproteins associated with breast cancer progression. Glycobiology 2008, 18, 1105–1118. [Google Scholar] [CrossRef]
- Akasaka-Manya, K.; Manya, H.; Sakurai, Y.; Wojczyk, B.S.; Kozutsumi, Y.; Saito, Y.; Taniguchi, N.; Murayama, S.; Spitalnik, S.L.; Endo, T. Protective effect of N -glycan bisecting GlcNAc residues on β-amyloid production in Alzheimer’s disease. Glycobiology 2010, 20, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Akasaka-Manya, K.; Manya, H.; Sakurai, Y.; Wojczyk, B.S.; Spitalnik, S.L.; Endo, T. Increased bisecting and core-fucosylated N-glycans on mutant human amyloid precursor proteins. Glycoconj. J. 2008, 25, 775–786. [Google Scholar] [CrossRef] [PubMed]
- Kitazume, S.; Nakagawa, K.; Oka, R.; Tachida, Y.; Ogawa, K.; Luo, Y.; Citron, M.; Shitara, H.; Taya, C.; Yonekawa, H.; et al. In vivo cleavage of alpha2,6-sialyltransferase by Alzheimer beta-secretase. J. Biol. Chem. 2005, 280, 8589–8595. [Google Scholar] [CrossRef] [PubMed]
- Maguire, T.M.; Breen, K.C. A decrease in neural sialyltransferase activity in Alzheimer’s disease. Dementia 1995, 6, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Maguire, T.M.; Gillian, A.M.; O’Mahony, D.; Coughlan, C.M.; Dennihan, A.; Breen, K.C. A decrease in serum sialyltransferase levels in Alzheimer’s disease. Neurobiol. Aging 1994, 15, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Kitazume, S.; Tachida, Y.; Oka, R.; Shirotani, K.; Saido, T.C.; Hashimoto, Y. Alzheimer’s β-secretase, β-site amyloid precursor protein-cleaving enzyme, is responsible for cleavage secretion of a Golgi-resident sialyltransferase. Proc. Natl. Acad. Sci. USA 2001, 98, 13554–13559. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.G.; Veillon, L.; Mechref, Y. N-Glycan Profile of Cerebrospinal Fluids from Alzheimer’s Disease Patients Using Liquid Chromatography with Mass Spectrometry. J. Proteome Res. 2019, 18, 3770–3779. [Google Scholar] [CrossRef] [PubMed]
- Goyallon, A.; Cholet, S.; Chapelle, M.; Junot, C.; Fenaille, F. Evaluation of a combined glycomics and glycoproteomics approach for studying the major glycoproteins present in biofluids: Application to cerebrospinal fluid. Rapid Commun. Mass. Spectrom. 2015, 29, 461–473. [Google Scholar] [CrossRef]
- Fogli, A.; Merle, C.; Roussel, V.; Schiffmann, R.; Ughetto, S.; Theisen, M.; Boespflug-Tanguy, O. CSF N-glycan profiles to investigate biomarkers in brain developmental disorders: Application to leukodystrophies related to eIF2B mutations. PLoS ONE 2012, 7, e42688. [Google Scholar] [CrossRef]
- Furukawa, J.-I.; Hanamatsu, H.; Yokota, I.; Hirayama, M.; Ando, T.; Kobayashi, H.; Ohnishi, S.; Miura, N.; Okada, K.; Sakai, S.; et al. Comprehensive Glycomic Approach Reveals Novel Low-Molecular-Weight Blood Group-Specific Glycans in Serum and Cerebrospinal Fluid. J. Proteome Res. 2021, 20, 2812–2822. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Seino, J.; Fujihira, H.; Sato, K.; Fujinawa, R.; Sumer-Bayraktar, Z.; Ishii, N.; Matsuo, I.; Nakaya, S.; Suzuki, T. Occurrence of free N-glycans with a single GlcNAc at the reducing termini in animal sera. Glycobiology 2022, 32, 314–332. [Google Scholar] [CrossRef] [PubMed]
- Iwatsuka, K.; Watanabe, S.; Kinoshita, M.; Kamisue, K.; Yamada, K.; Hayakawa, T.; Suzuki, T.; Kakehi, K. Free glycans derived from glycoproteins present in human sera. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2013, 928, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Hanzawa, K.; Tanaka-Okamoto, M.; Murakami, H.; Mukai, M.; Takahashi, H.; Omori, T.; Ikezawa, K.; Ohkawa, K.; Ohue, M.; Miyamoto, Y. Investigation of acidic free-glycans in urine and their alteration in cancer. Glycobiology 2021, 31, 391–409. [Google Scholar] [CrossRef] [PubMed]
- Hanzawa, K.; Tanaka-Okamoto, M.; Murakami, H.; Suzuki, N.; Mukai, M.; Takahashi, H.; Omori, T.; Ikezawa, K.; Ohkawa, K.; Ohue, M.; et al. Increased levels of acidic free-N-glycans, including multi-antennary and fucosylated structures, in the urine of cancer patients. PLoS ONE 2022, 17, e0266927. [Google Scholar] [CrossRef] [PubMed]
- Alonzi, D.S.; Su, Y.H.; Butters, T.D. Urinary glycan markers for disease. Biochem. Soc. Trans. 2011, 39, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Chalabi, S.; Easton, R.L.; Patankar, M.S.; Lattanzio, F.A.; Morrison, J.C.; Panico, M.; Morris, H.R.; Dell, A.; Clark, G.F. The expression of free oligosaccharides in human seminal plasma. J. Biol. Chem. 2002, 277, 32562–32570. [Google Scholar] [CrossRef] [PubMed]
- Boeddrich, A.; Haenig, C.; Neuendorf, N.; Blanc, E.; Ivanov, A.; Kirchner, M.; Schleumann, P.; Bayraktaroglu, I.; Richter, M.; Molenda, C.M.; et al. A proteomics analysis of 5xFAD mouse brain regions reveals the lysosome-associated protein Arl8b as a candidate biomarker for Alzheimer’s disease. Genome Med. 2023, 15, 50. [Google Scholar] [CrossRef] [PubMed]
- Reinke, S.O.; Bayer, M.; Berger, M.; Hinderlich, S.; Blanchard, V. The analysis of N-glycans of cell membrane proteins from human hematopoietic cell lines reveals distinctions in their pattern. Biol. Chem. 2012, 393, 731–747. [Google Scholar] [CrossRef]
- Ciucanu, I.; Kerek, F. Rapid and simultaneous methylation of fatty and hydroxy fatty acids for gas—Liquid chromatographic analysis. J. Chromatogr. A 1984, 284, 179–185. [Google Scholar] [CrossRef]
- Wedepohl, S.; Kaup, M.; Riese, S.B.; Berger, M.; Dernedde, J.; Tauber, R.; Blanchard, V. N-glycan analysis of recombinant L-Selectin reveals sulfated GalNAc and GalNAc-GalNAc motifs. J. Proteome Res. 2010, 9, 3403–3411. [Google Scholar] [CrossRef] [PubMed]
- Maass, K.; Ranzinger, R.; Geyer, H.; von der Lieth, C.W.; Geyer, R. “Glyco-peakfinder”—De novo composition analysis of glycoconjugates. Proteomics 2007, 7, 4435–4444. [Google Scholar] [CrossRef] [PubMed]
- Ceroni, A.; Maass, K.; Geyer, H.; Geyer, R.; Dell, A.; Haslam, S.M. GlycoWorkbench: A Tool for the Computer-Assisted Annotation of Mass Spectra of Glycans. J. Proteome Res. 2008, 7, 1650–1659. [Google Scholar] [CrossRef] [PubMed]
- Damerell, D.; Ceroni, A.; Maass, K.; Ranzinger, R.; Dell, A.; Haslam, S.M. The GlycanBuilder and GlycoWorkbench glycoinformatics tools: Updates and new developments. Biol. Chem. 2012, 393, 1357–1362. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.; Nimtz, M.; Getzlaff, R.; Conradt, H.S. ‘Brain-type’ N-glycosylation of asialo-transferrin from human cerebrospinal fluid. FEBS Lett. 1995, 359, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Palmigiano, A.; Barone, R.; Sturiale, L.; Sanfilippo, C.; Bua, R.O.; Romeo, D.A.; Messina, A.; Capuana, M.L.; Maci, T.; Le Pira, F.; et al. CSF N-glycoproteomics for early diagnosis in Alzheimer’s disease. J. Proteom. 2016, 131, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Stanta, J.L.; Saldova, R.; Struwe, W.B.; Byrne, J.C.; Leweke, F.M.; Rothermund, M.; Rahmoune, H.; Levin, Y.; Guest, P.C.; Bahn, S.; et al. Identification of N-glycosylation changes in the CSF and serum in patients with schizophrenia. J. Proteome Res. 2010, 9, 4476–4489. [Google Scholar] [CrossRef]
- Barone, R.; Sturiale, L.; Fiumara, A.; Palmigiano, A.; Bua, R.O.; Rizzo, R.; Zappia, M.; Garozzo, D. CSF N-glycan profile reveals sialylation deficiency in a patient with GM2 gangliosidosis presenting as childhood disintegrative disorder. Autism Res. 2016, 9, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, M.; Tillack, L.; de Carvalho, M.; Pinto, S.; Conradt, H.S.; Costa, J. Phosphoneurofilament heavy chain and N-glycomics from the cerebrospinal fluid in amyotrophic lateral sclerosis. Clin. Chim. Acta 2015, 438, 342–349. [Google Scholar] [CrossRef]
- Harada, Y.; Hirayama, H.; Suzuki, T. Generation and degradation of free asparagine-linked glycans. Cell Mol. Life Sci. 2015, 72, 2509–2533. [Google Scholar] [CrossRef]
- Moore, S.E. Transport of free polymannose-type oligosaccharides from the endoplasmic reticulum into the cytosol is inhibited by mannosides and requires a thapsigargin-sensitive calcium store. Glycobiology 1998, 8, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T. The cytoplasmic peptide:N-glycanase (Ngly1)-basic science encounters a human genetic disorder. J. Biochem. 2015, 157, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T. Catabolism of N-glycoproteins in mammalian cells: Molecular mechanisms and genetic disorders related to the processes. Mol. Asp. Med. 2016, 51, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Suttapitugsakul, S.; Stavenhagen, K.; Donskaya, S.; Bennett, D.A.; Mealer, R.G.; Seyfried, N.T.; Cummings, R.D. Glycoproteomics Landscape of Asymptomatic and Symptomatic Human Alzheimer’s Disease Brain. Mol. Cell Proteom. 2022, 21, 100433. [Google Scholar] [CrossRef] [PubMed]
- Murakami, Y.; Saito, K.; Ito, H.; Hashimoto, Y. Transferrin isoforms in cerebrospinal fluid and their relation to neurological diseases. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2019, 95, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Nakakita, S.; Natsuka, S.; Ikenaka, K.; Hase, S. Development-dependent expression of complex-type sugar chains specific to mouse brain. J. Biochem. 1998, 123, 1164–1168. [Google Scholar] [CrossRef]
- Kizuka, Y.; Kitazume, S.; Fujinawa, R.; Saito, T.; Iwata, N.; Saido, T.C.; Nakano, M.; Yamaguchi, Y.; Hashimoto, Y.; Staufenbiel, M.; et al. An aberrant sugar modification of BACE1 blocks its lysosomal targeting in Alzheimer’s disease. EMBO Mol. Med. 2015, 7, 175–189. [Google Scholar] [CrossRef] [PubMed]
- Kizuka, Y.; Taniguchi, N. Neural functions of bisecting GlcNAc. Glycoconj. J. 2018, 35, 345–351. [Google Scholar] [CrossRef]
- Wang, W.; Gopal, S.; Pocock, R.; Xiao, Z. Glycan Mimetics from Natural Products: New Therapeutic Opportunities for Neurodegenerative Disease. Molecules 2019, 24, 4604. [Google Scholar] [CrossRef]
- Schedin-Weiss, S.; Gaunitz, S.; Sui, P.; Chen, Q.; Haslam, S.M.; Blennow, K.; Winblad, B.; Dell, A.; Tjernberg, L.O. Glycan biomarkers for Alzheimer disease correlate with T-tau and P-tau in cerebrospinal fluid in subjective cognitive impairment. FEBS J. 2020, 287, 3221–3234. [Google Scholar] [CrossRef]
- Liu, F.; Zaidi, T.; Iqbal, K.; Grundke-Iqbal, I.; Gong, C.X. Aberrant glycosylation modulates phosphorylation of tau by protein kinase A and dephosphorylation of tau by protein phosphatase 2A and 5. Neuroscience 2002, 115, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Z.; Grundke-Iqbal, I.; Iqbal, K. Glycosylation of microtubule-associated protein tau: An abnormal posttranslational modification in Alzheimer’s disease. Nat. Med. 1996, 2, 871–875. [Google Scholar] [CrossRef] [PubMed]
- Kitazume, S.; Tachida, Y.; Oka, R.; Kotani, N.; Ogawa, K.; Suzuki, M.; Dohmae, N.; Takio, K.; Saido, T.C.; Hashimoto, Y. Characterization of α2,6-Sialyltransferase Cleavage by Alzheimer’s β-Secretase (BACE1)*. J. Biol. Chem. 2003, 278, 14865–14871. [Google Scholar] [CrossRef] [PubMed]
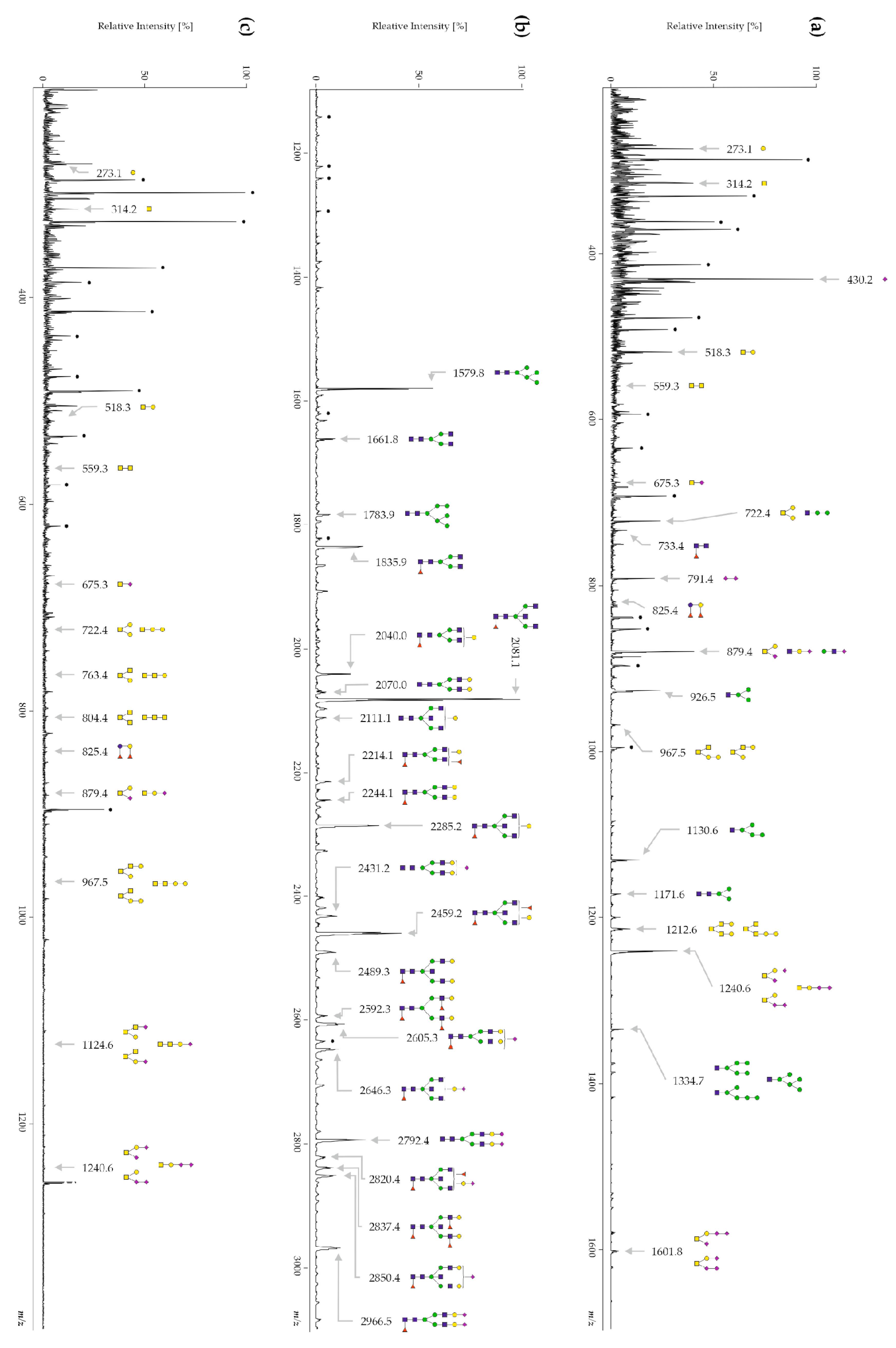

| Patients with AD | Disease Control Patients | Healthy Controls | |
|---|---|---|---|
| Number of patients (m/f) | 89 (43/46) | 86 (55/31) | 87 (48/39) |
| Age of the patient cohorts (years) | |||
| Mean (m/f) | 76 (75/76) | 73 (72/75) | 66 (67/65) |
| Median (m/f) | 77 (77/77) | 75 (72/77) | 66 (66/65) |
| SD | 6.7 | 8.3 | 8.5 |
| Range | 44–88 | 52–89 | 45–84 |
| MMSE | |||
| Mean (m/f) | 23.5 (23.6/23.5) | 23.6 (24.2/22.5) | 28.7 (28.8/28.7) |
| Median (m/f) | 24.0 (25.0/24.0) | 25.0 (25.0/23.5) | 29.0 (29.0/29.0) |
| SD | 3.8 | 4.6 | 1.1 |
| Range | 12.0–30.0 | 3.0–30.0 | 26.0–30.0 |
| Aβ ratio | |||
| Mean (m/f) | 0.051 (0.049/0.053) | 0.085 (0.085/0.085) | 0.088 (0.090/0.086) |
| Median (m/f) | 0.049 (0.048/0.051) | 0.095 (0.091/0.097) | 0.096 (0.097/0.094) |
| SD | 0.012 | 0.024 | 0.020 |
| Range | 0.025–0.103 | 0.027–0.120 | 0.034–0.115 |
| p-tau (pg/mL) | |||
| Mean (m/f) | 104.0 (112.0/96.4) | 53.1 (53.3/52.6) | 40.9 (41.2/40.6) |
| Median (m/f) | 98.1 (105.3/94.9) | 41.9 (42.0/40.6) | 37.2 (38.5/32.1) |
| SD | 42.9 | 37.4 | 14.4 |
| Range | 34.0–279.0 | 24.0–253.0 | 17.0–91.0 |
| t-tau (pg/mL) | |||
| Mean (m/f) | 674.6 (724.8/627.7) | 385.3 (381.5/391.9) | 282.8 (290.7/273.0) |
| Median (m/f) | 615.0 (625.0/612.5) | 308.0 (297.0/320.0) | 241.0 (267.5/237.0) |
| SD | 260.6 | 235.6 | 116.7 |
| Range | 231.0–1505.0 | 114.0–1349.0 | 96.0–583.0 |
| m/z | Composition | Patients with AD [%] | DC Patients [%] | HC Patients [%] | p Value AD/DC | p Value AD/HC | p Value DC/HC |
|---|---|---|---|---|---|---|---|
| 273.1 | Hex1 | 16.65 ± 5.27 | 11.46 ± 5.68 | 10.09 ± 3.95 | ≤0.01 | ≤0.01 | |
| 314.2 | HexNAc1 | 10.73 ± 2.40 | 11.61 ± 2.66 | 10.40 ± 2.68 | ≤0.05 | ≤0.01 | |
| 430.2 | Neu5Ac1 | 19.08 ± 3.32 | 18.60 ± 3.22 | 18.77 ± 4.24 | |||
| 518.3 | Hex1HexNAc1 | 5.62 ± 0.78 | 6.93 ± 1.04 | 6.68 ± 1.27 | ≤0.01 | ≤0.01 | |
| 559.3 | HexNAc2 | 1.19 ± 0.28 | 1.54 ± 0.49 | 1.31 ± 0.37 | ≤0.01 | ≤0.05 | ≤0.01 |
| 675.3 | Neu5Ac1HexNAc1 | 0.83 ± 0.14 | 1.12 ± 0.25 | 0.96 ± 0.18 | ≤0.01 | ≤0.01 | ≤0.01 |
| 722.4 | Hex2HexNAc1 | 3.98 ± 0.61 | 4.94 ± 0.83 | 4.39 ± 0.96 | ≤0.01 | ≤0.01 | ≤0.01 |
| 733.4 | Fuc1HexNAc2 | 1.73 ± 0.27 | 2.06 ± 0.49 | 2.07 ± 0.47 | ≤0.01 | ≤0.01 | |
| 791.4 | Neu5Ac2 | 3.71 ± 2.26 | 3.45 ± 2.07 | 4.46 ± 3.02 | |||
| 825.4 | Fuc2Hex2 | 0.95 ± 0.35 | 1.11 ± 0.49 | 1.05 ± 0.34 | ≤0.05 | ≤0.05 | |
| 879.4 | Neu5Ac1Hex1HexNAc1 | 6.49 ± 1.13 | 7.38 ± 1.28 | 7.69 ± 0.97 | ≤0.01 | ≤0.01 | ≤0.05 |
| 926.5 | Hex3HexNAc1 | 4.34 ± 0.76 | 5.17 ± 0.98 | 5.06 ± 1.15 | ≤0.01 | ≤0.01 | |
| 967.5 | Hex2HexNAc2 | 0.91 ± 0.15 | 1.04 ± 0.20 | 1.05 ± 0.26 | ≤0.01 | ≤0.01 | |
| 1130.6 | Hex4HexNAc1 | 2.46 ± 0.46 | 2.81 ± 0.60 | 2.89 ± 0.74 | ≤0.01 | ≤0.01 | |
| 1171.6 | Hex3HexNAc2 | 1.03 ± 0.20 | 1.08 ± 0.22 | 1.17 ± 0.31 | ≤0.01 | ||
| 1212.6 | Hex2HexNAc3 | 2.06 ± 0.53 | 2.24 ± 0.70 | 2.07 ± 0.52 | |||
| 1240.6 | Neu5Ac2Hex1HexNAc1 | 8.05 ± 2.16 | 8.17 ± 2.00 | 8.77 ± 1.87 | |||
| 1334.7 | Hex5HexNAc1 | 1.08 ± 0.26 | 1.17 ± 0.29 | 1.28 ± 0.39 | ≤0.01 | ||
| 1601.8 | Neu5Ac3Hex1HexNAc1 | 1.29 ± 0.52 | 0.95 ± 0.49 | 1.35 ± 0.55 | ≤0.01 | ≤0.01 | |
| Other | 7.81 | 7.18 | 8.51 |
| Male Patients | Female Patients | ||||||
|---|---|---|---|---|---|---|---|
| m/z | Composition | AD/DC | AD/HC | DC/HC | AD/DC | AD/HC | DC/HC |
| 1579.8 | Hex5HexNAc2 | ≤0.05 | ≤0.01 | ||||
| 1661.8 | Hex3HexNAc4 | ≤0.01 | |||||
| 1783.9 | Hex6HexNAc2 | ≤0.05 | |||||
| 1835.9 | Fuc1Hex3HexNAc4 | ≤0.01 | ≤0.05 | ||||
| 2040.0 | Fuc1Hex4HexNAc4 | ≤0.05 | |||||
| 2081.1 | Fuc1Hex3HexNAc5 | ≤0.01 | |||||
| 2111.1 | Hex4HexNAc5 | ≤0.01 | ≤0.05 | ||||
| 2214.1 | Fuc2Hex4HexNAc4 | ≤0.01 | ≤0.05 | ||||
| 2244.1 | Fuc1Hex5HexNAc4 | ≤0.05 | ≤0.01 | ||||
| 2431.2 | Neu5Ac1Hex5HexNAc4 | ≤0.05 | |||||
| 2459.2 | Fuc2Hex4HexNAc5 | ≤0.05 | ≤0.01 | ≤0.01 | ≤0.05 | ≤0.05 | ≤0.01 |
| 2489.3 | Fuc1Hex5HexNAc5 | ≤0.05 | |||||
| 2592.3 | Fuc3Hex5HexNAc4 | ≤0.05 | |||||
| 2605.3 | Neu5Ac1Fuc1Hex5HexNAc4 | ≤0.01 | ≤0.05 | ≤0.01 | ≤0.01 | ||
| 2646.3 | Neu5Ac1Fuc1Hex4HexNAc5 | ≤0.05 | ≤0.01 | ≤0.01 | ≤0.01 | ||
| 2837.4 | Fuc3Hex5HexNAc5 | ≤0.05 | ≤0.05 | ||||
| 2850.4 | Neu5Ac1Fuc1Hex5HexNAc5 | ≤0.05 | ≤0.05 | ≤0.01 | |||
| 2966.5 | Neu5Ac2Fuc1Hex5HexNAc4 | ≤0.01 | ≤0.01 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krüger, L.; Biskup, K.; Schipke, C.G.; Kochnowsky, B.; Schneider, L.-S.; Peters, O.; Blanchard, V. The Cerebrospinal Fluid Free-Glycans Hex1 and HexNAc1Hex1Neu5Ac1 as Potential Biomarkers of Alzheimer’s Disease. Biomolecules 2024, 14, 512. https://doi.org/10.3390/biom14050512
Krüger L, Biskup K, Schipke CG, Kochnowsky B, Schneider L-S, Peters O, Blanchard V. The Cerebrospinal Fluid Free-Glycans Hex1 and HexNAc1Hex1Neu5Ac1 as Potential Biomarkers of Alzheimer’s Disease. Biomolecules. 2024; 14(5):512. https://doi.org/10.3390/biom14050512
Chicago/Turabian StyleKrüger, Lynn, Karina Biskup, Carola G. Schipke, Bianca Kochnowsky, Luisa-Sophie Schneider, Oliver Peters, and Véronique Blanchard. 2024. "The Cerebrospinal Fluid Free-Glycans Hex1 and HexNAc1Hex1Neu5Ac1 as Potential Biomarkers of Alzheimer’s Disease" Biomolecules 14, no. 5: 512. https://doi.org/10.3390/biom14050512





