A New Method to Determine Antigen-Specific CD8+ T Cell Activity in Vivo by Hydrodynamic Injection
Abstract
:1. Introduction
2. Experimental Section
2.1. Plasmid Vector

2.2. Recombinant Adenoviruses
2.3. Animals
2.4. Immunizations
2.5. In Vivo Depletion of CD8+ T Cells
2.6. Luciferase Expression by Noninvasive Bioluminescent Imaging
2.7. Statistical Analysis
3. Results
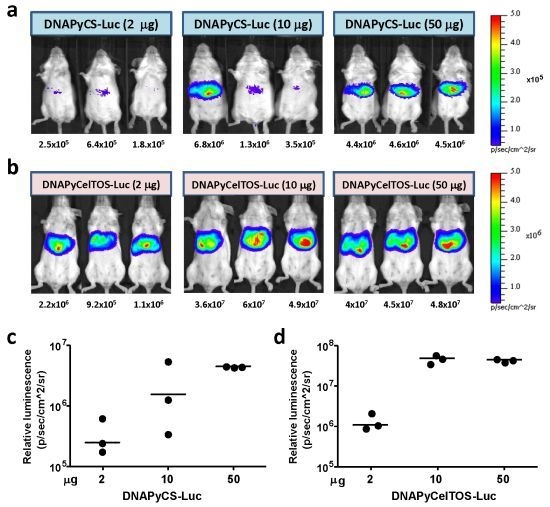
3.1. Level of Luciferase Expression in vivo after HTV Injection of Various Doses of Plasmid DNA Co-Encoding Malaria Antigen and Luciferase

3.2. Inhibition of DNAPyCS-Luc Induced Luciferase Expression by a Single Immunizing Dose of AdPyCS, but not AdPyCelTOS

3.3. Inhibition of DNAPyCelTOS-Luc Induced Luciferase Expression by a Single Immunizing Dose of AdPyCelTOS, but not AdPyCS

3.4. CD8+ T Cell-Mediated Inhibition of Luciferase Expression Induced by HTV Injection of DNA Co-Encoding Malaria Antigen and Luciferase in Mice Immunized with a rAd Expressing the Antigen

4. Discussion
5. Conclusions
Conflict of Interest
Acknowledgments
References
- Schofield, L.; Villaquiran, J.; Ferreira, A.; Schellekens, H.; Nussenzweig, R.; Nussenzweig, V. γ Interferon, CD8+ T cells and antibodies required for immunity to malaria sporozoites. Nature 1987, 330, 664–666. [Google Scholar]
- Weiss, W.R.; Sedegah, M.; Beaudoin, R.L.; Miller, L.H.; Good, M.F. CD8+ T cells (cytotoxic/suppressors) are required for protection in mice immunized with malaria sporozoites. Proc. Natl. Acad. Sci. USA 1988, 85, 573–576. [Google Scholar]
- Rodrigues, M.M.; Cordey, A.S.; Arreaza, G.; Corradin, G.; Romero, P.; Maryanski, J.L.; Nussenzweig, R.S.; Zavala, F. CD8+ cytolytic T cell clones derived against the Plasmodium yoelii circumsporozoite protein protect against malaria. Int. Immunol. 1991, 3, 579–585. [Google Scholar] [CrossRef]
- Romero, P.; Maryanski, J.L.; Corradin, G.; Nussenzweig, R.S.; Nussenzweig, V.; Zavala, F. Cloned cytotoxic T cells recognize an epitope in the circumsporozoite protein and protect against malaria. Nature 1989, 341, 323–326. [Google Scholar]
- Rodrigues, E.G.; Zavala, F.; Eichinger, D.; Wilson, J.M.; Tsuji, M. Single immunizing dose of recombinant adenovirus efficiently induces CD8+ T cell-mediated protective immunity against malaria. J. Immunol. 1997, 158, 1268–1274. [Google Scholar]
- Nardin, E.; Zavala, F.; Nussenzweig, V.; Nussenzweig, R.S. Pre-erythrocytic malaria vaccine: Mechanisms of protective immunity and human vaccine trials. Parassitologia 1999, 41, 397–402. [Google Scholar]
- Agnandji, S.T.; Lell, B.; Soulanoudjingar, S.S.; Fernandes, J.F.; Abossolo, B.P.; Conzelmann, C.; Methogo, B.G.; Doucka, Y.; Flamen, A.; Mordmüller, B.; et al. RTS,S Clinical Trials Partnership. First results of phase 3 trial of RTS,S/AS01 malaria vaccine in African children. N. Engl. J. Med. 2011, 365, 1863–1875. [Google Scholar]
- Doolan, D.L.; Southwood, S.; Freilich, D.A.; Sidney, J.; Graber, N.L.; Shatney, L.; Bebris, L.; Florens, L.; Dobano, C.; Witney, A.A.; et al. Identification of Plasmodium falciparum antigens by antigenic analysis of genomic and proteomic data. Proc. Natl. Acad. Sci. USA 2003, 100, 9952–9957. [Google Scholar]
- Bergmann-Leitner, E.S.; Legler, P.M.; Savranskaya, T.; Ockenhouse, C.F.; Angov, E. Cellular and humoral immune effector mechanisms required for sterile protection against sporozoite challenge induced with the novel malaria vaccine candidate CelTOS. Vaccine 2011, 29, 5940–5949. [Google Scholar]
- Wolff, J.A.; Malone, R.W.; Williams, P.; Chong, W.; Acsadi, G.; Jani, A.; Felgner, P.L. Direct gene transfer into mouse muscle in vivo. Science 1990, 247, 1465–1468. [Google Scholar]
- Budker, V.; Zhang, G.; Knechtle, S.; Wolff, J.A. Naked DNA delivered intraportally expresses efficiently in hepatocytes. Gene Ther. 1996, 3, 593–598. [Google Scholar]
- Zhang, G.; Vargo, D.; Budker, V.; Armstrong, N.; Knechtle, S.; Wolff, J.A. Expression of naked plasmid DNA injected into the afferent and efferent vessels of rodent and dog livers. Hum. Gene Ther. 1997, 8, 1763–1772. [Google Scholar] [CrossRef]
- Liu, F.; Song, Y.; Liu, D. Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Ther. 1999, 6, 1258–1266. [Google Scholar] [CrossRef]
- Zhang, G.; Budker, V.; Wolff, J.A. High levels of foreign gene expression in hepatocytes after tail vein injections of naked plasmid DNA. Hum. Gene Ther. 1999, 10, 1735–1737. [Google Scholar] [CrossRef]
- Mishra, S.; Rai, U.; Shiratsuchi, T.; Li, X.; Vanloubbeeck, Y.; Cohen, J.; Nussenzweig, R.S.; Winzeler, E.A.; Tsuji, M.; Nussenzweig, V. Identification of non-CSP antigens bearing CD8 epitopes in mice immunized with irradiated sporozoites. Vaccine 2011, 29, 7335–7342. [Google Scholar]
- Shiratsuchi, T.; Rai, U.; Krause, A.; Worgall, S.; Tsuji, M. Replacing adenoviral vector HVR1 with a malaria B cell epitope improves immunogenicity and circumvents preexisting immunity to adenovirus in mice. J. Clin. Invest. 2010, 120, 3688–3701. [Google Scholar] [CrossRef]
- Suda, T.; Liu, D. Hydrodynamic gene delivery: Its principles and applications. Mol. Ther. 2007, 15, 2063–2069. [Google Scholar] [CrossRef]
- Zhang, G.; Gao, X.; Song, Y.K.; Vollmer, R.; Stolz, D.B.; Gasiorowski, J.Z.; Dean, D.A.; Liu, D. Hydroporation as the mechanism of hydrodynamic delivery. Gene Ther. 2004, 11, 675–682. [Google Scholar] [CrossRef]
- Rehermann, B. Immunopathogenesis of Hepatitis C. In Hepatitis C (Biomedical Research Reports); Liang, T.J., Hofnagle, J.H., Gallin, J.I., Fauci, A.S., Eds.; Academic Press: San Diego, CA, USA, 2000; Volume 2, pp. 147–168. [Google Scholar]
- Gotch, F.; Rothbard, J.; Howland, K.; Townsend, A.; McMichael, A. Cytotoxic T lymphocytes recognize a fragment of influenza virus matrix protein in association with HLA-A2. Nature 1987, 326, 881–882. [Google Scholar]
- Cannon, M.J.; Openshaw, P.J.M.; Askonas, B.A. Cytotoxic T cells clear virus but augment lung pathology in mice infected with respiratory syncytial virus. J. Exp. Med. 1988, 168, 1163–1168. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Rai, U.; Huang, J.; Mishra, S.; Li, X.; Shiratsuchi, T.; Tsuji, M. A New Method to Determine Antigen-Specific CD8+ T Cell Activity in Vivo by Hydrodynamic Injection. Biomolecules 2012, 2, 23-33. https://doi.org/10.3390/biom2010023
Rai U, Huang J, Mishra S, Li X, Shiratsuchi T, Tsuji M. A New Method to Determine Antigen-Specific CD8+ T Cell Activity in Vivo by Hydrodynamic Injection. Biomolecules. 2012; 2(1):23-33. https://doi.org/10.3390/biom2010023
Chicago/Turabian StyleRai, Urvashi, Jing Huang, Satish Mishra, Xiangming Li, Takayuki Shiratsuchi, and Moriya Tsuji. 2012. "A New Method to Determine Antigen-Specific CD8+ T Cell Activity in Vivo by Hydrodynamic Injection" Biomolecules 2, no. 1: 23-33. https://doi.org/10.3390/biom2010023