Decorin Content and Near Infrared Spectroscopy Analysis of Dried Collagenous Biomaterial Samples
Abstract
:1. Introduction
2. Results and Discussion
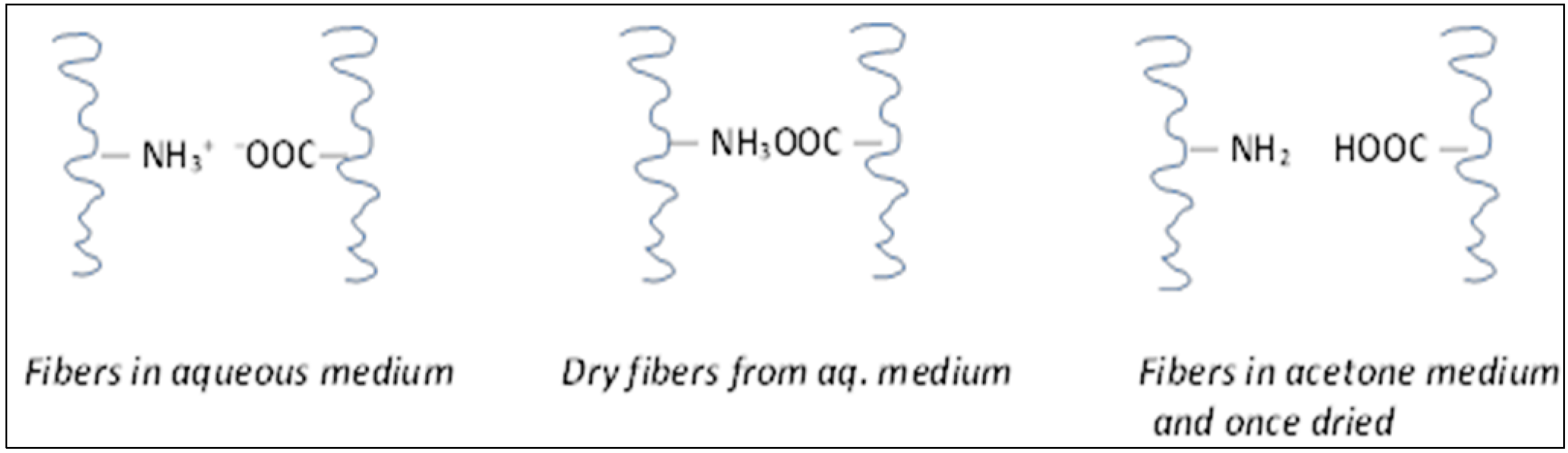
2.1. Acetone Drying Efficiency
| Sample code | Sample washing / drying tretament | Water content in acetone washing float (%) |
|---|---|---|
| 0S | Acetone dehydrated / 1 wash within 60 min | 24.5 ± 0.10 |
| 1S | Acetone dehydrated / 2 wash within 60 min | 6.8 ± 0.15 |
| 2S | Acetone dehydrated / 3 wash within 60 min | 3.0 ± 0.00 |
| 3S | Acetone dehydrated / 4 wash within 60 min | 1.8 ± 0.05 |
| 4S | Acetone dehydrated / 5 wash within 60 min | 1.3 ± 0.00 |
| 5S | No Acetone dehydrated / Air dried | N/A |
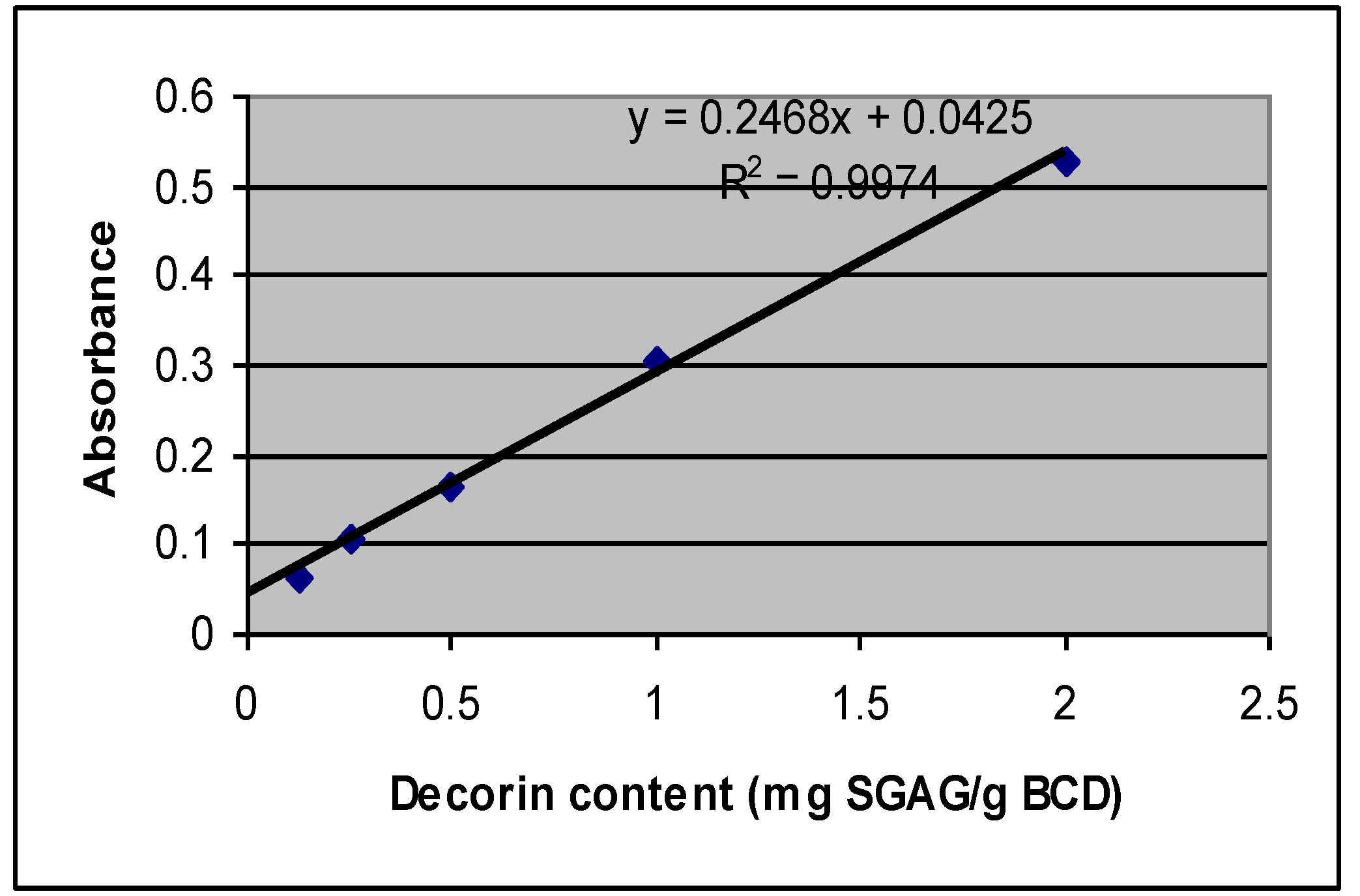
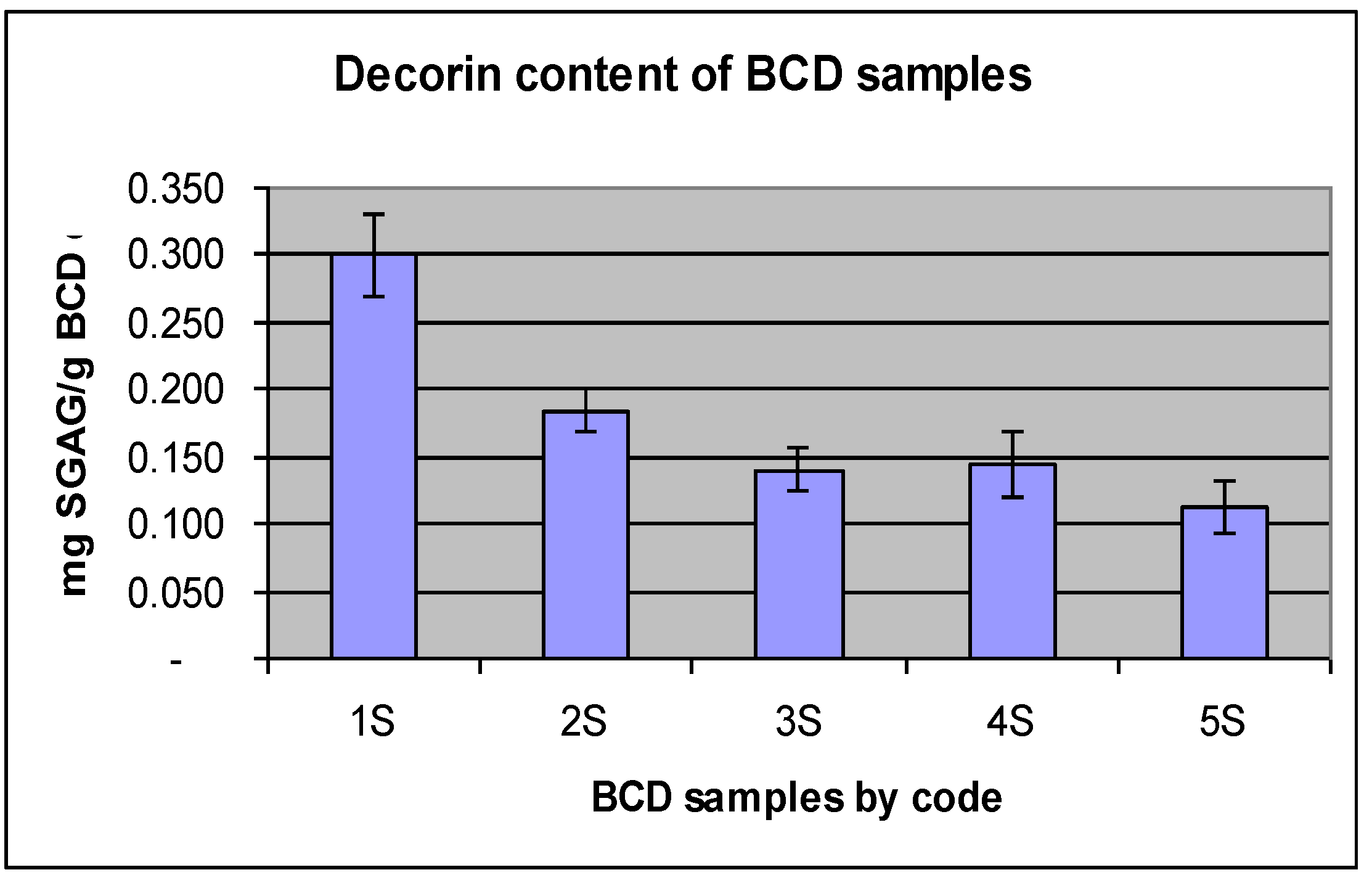
2.2. Decorin Analysis Based on SGAG Portion of the Molecule
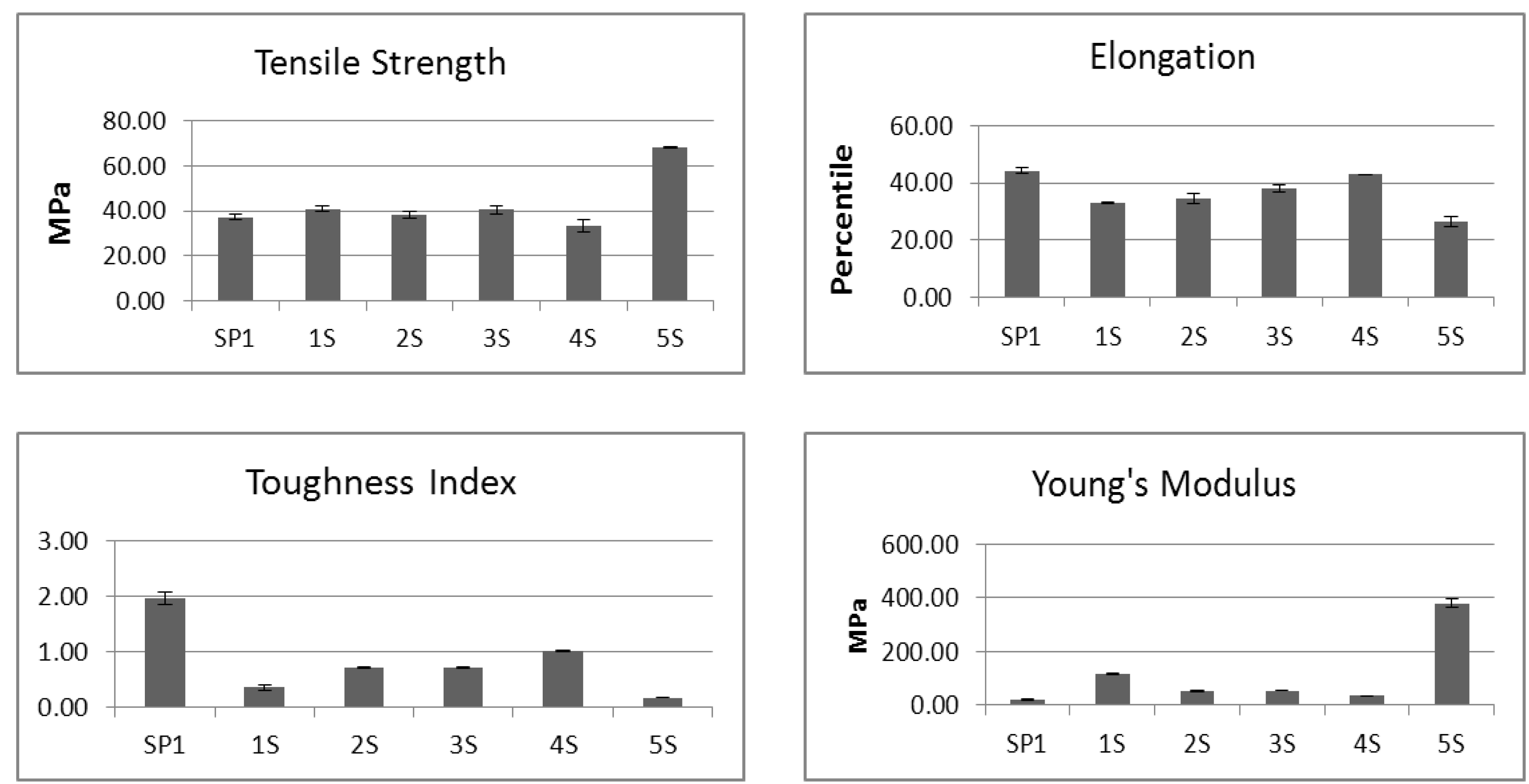
2.3. Mechanical Properties of BCD Samples
| Sample Code | Sample Acetonedrying Treatment | Elongation-to-break, % | Tensile Strength, N/m² | mgSGAG/g BCD |
|---|---|---|---|---|
| 1S | Acetone dehydrated /2 washes | 67 | 386 | 0.30 ± 0.07 |
| 2S | Acetone dehydrated /3 washes | 68 | 369 | 0.18 ± 0.04 |
| 3S | Acetone dehydrated /4 washes | 86 | 405 | 0.14 ± 0.05 |
| 4S | Acetone dehydrated /5 washes | 76 | 361 | 0.14 ± 0.06 |
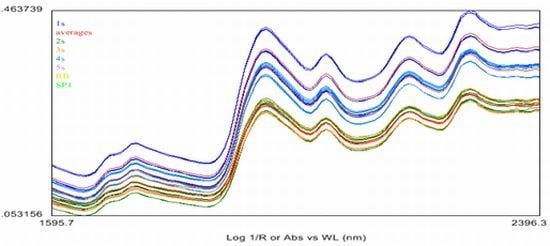
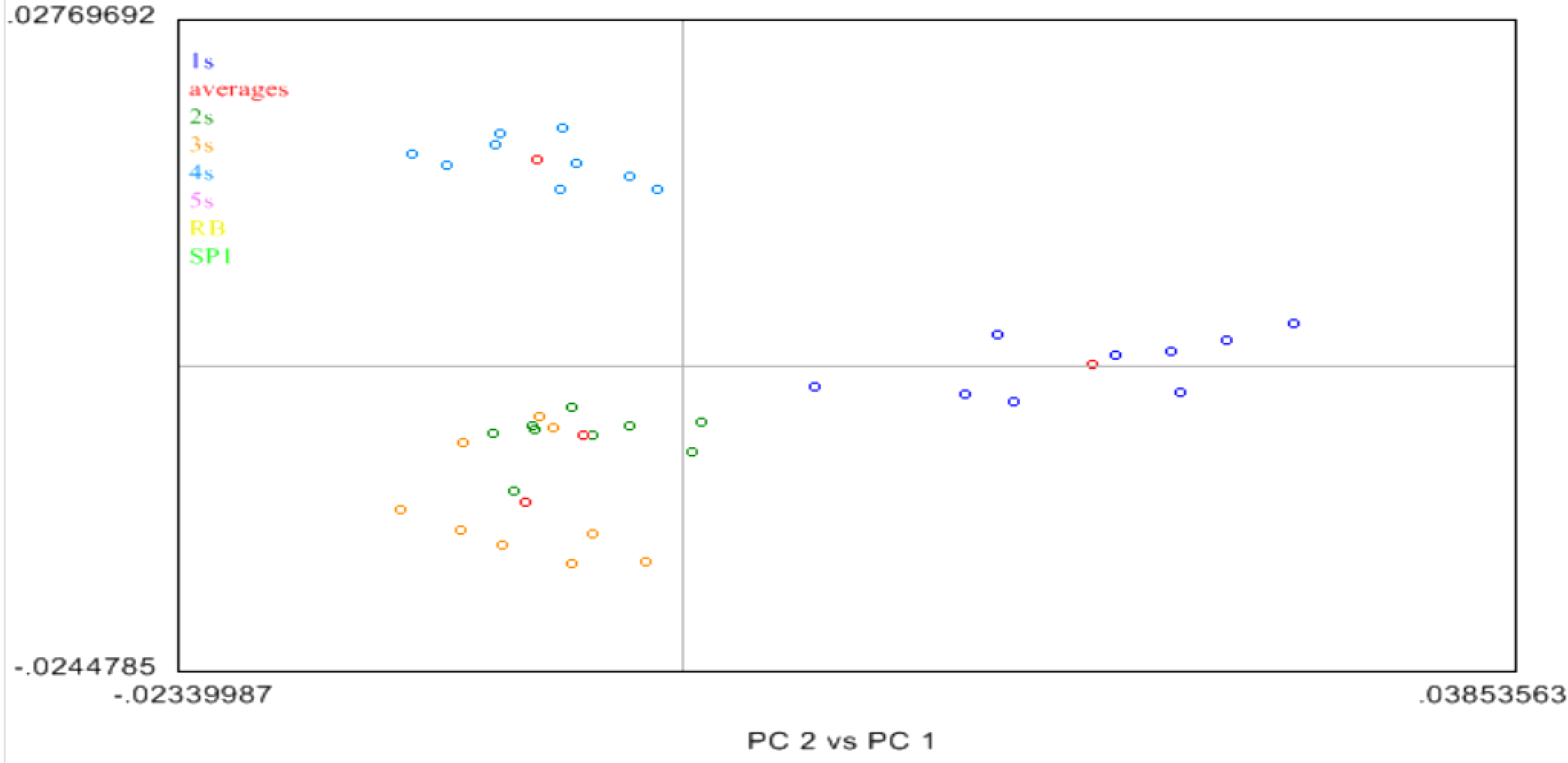
2.4. Near Infrared (NIR) Spectroscopy
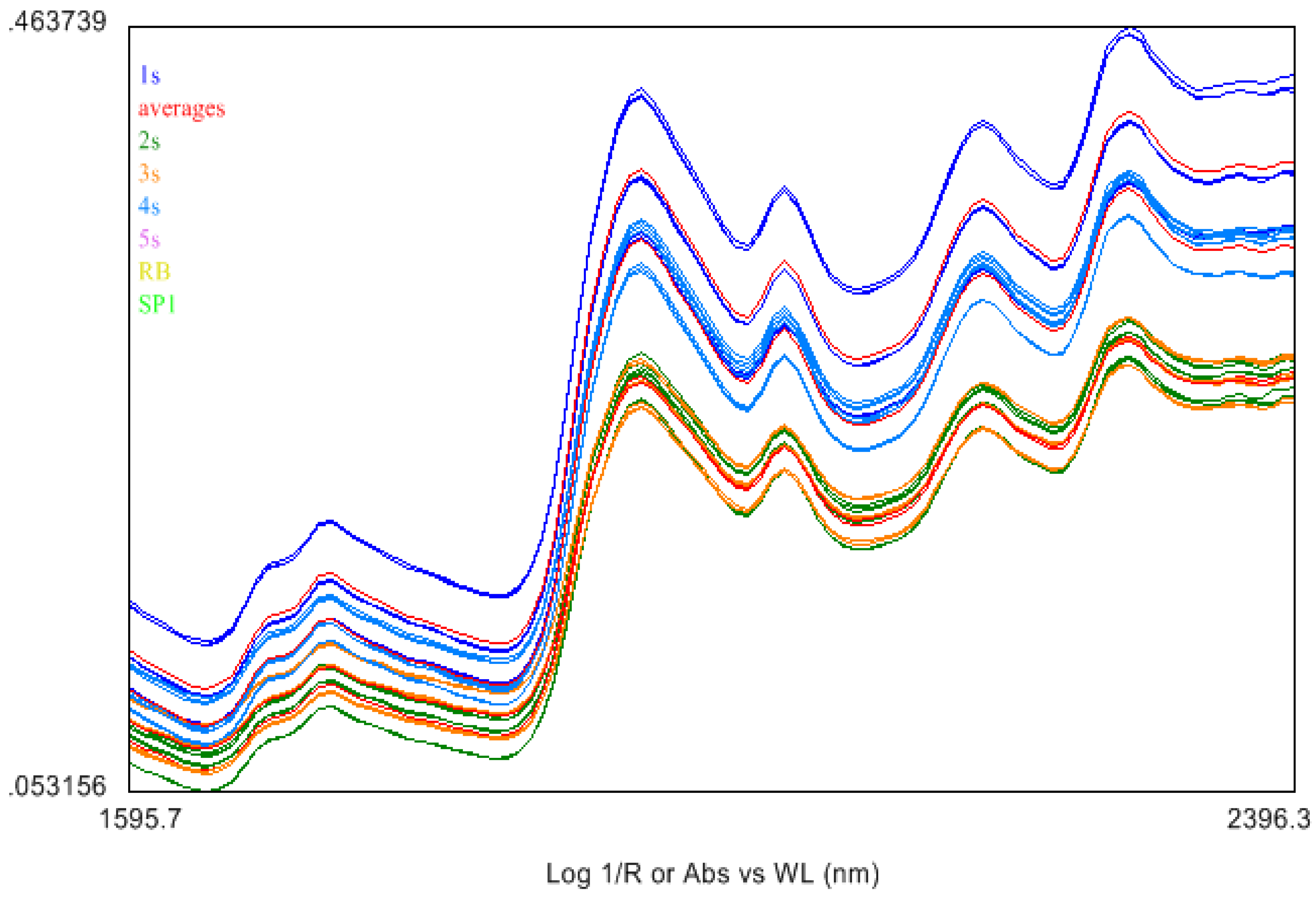
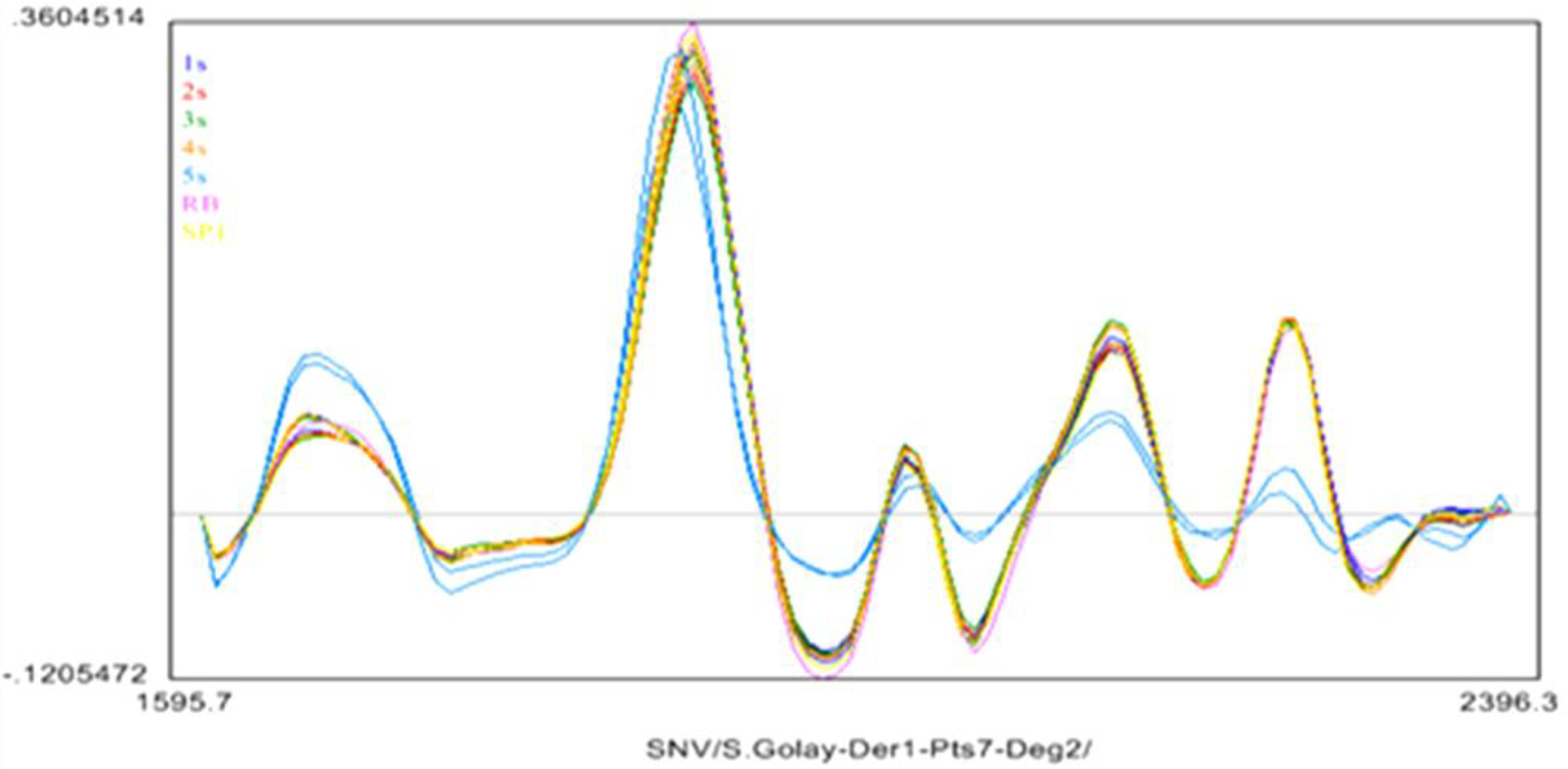
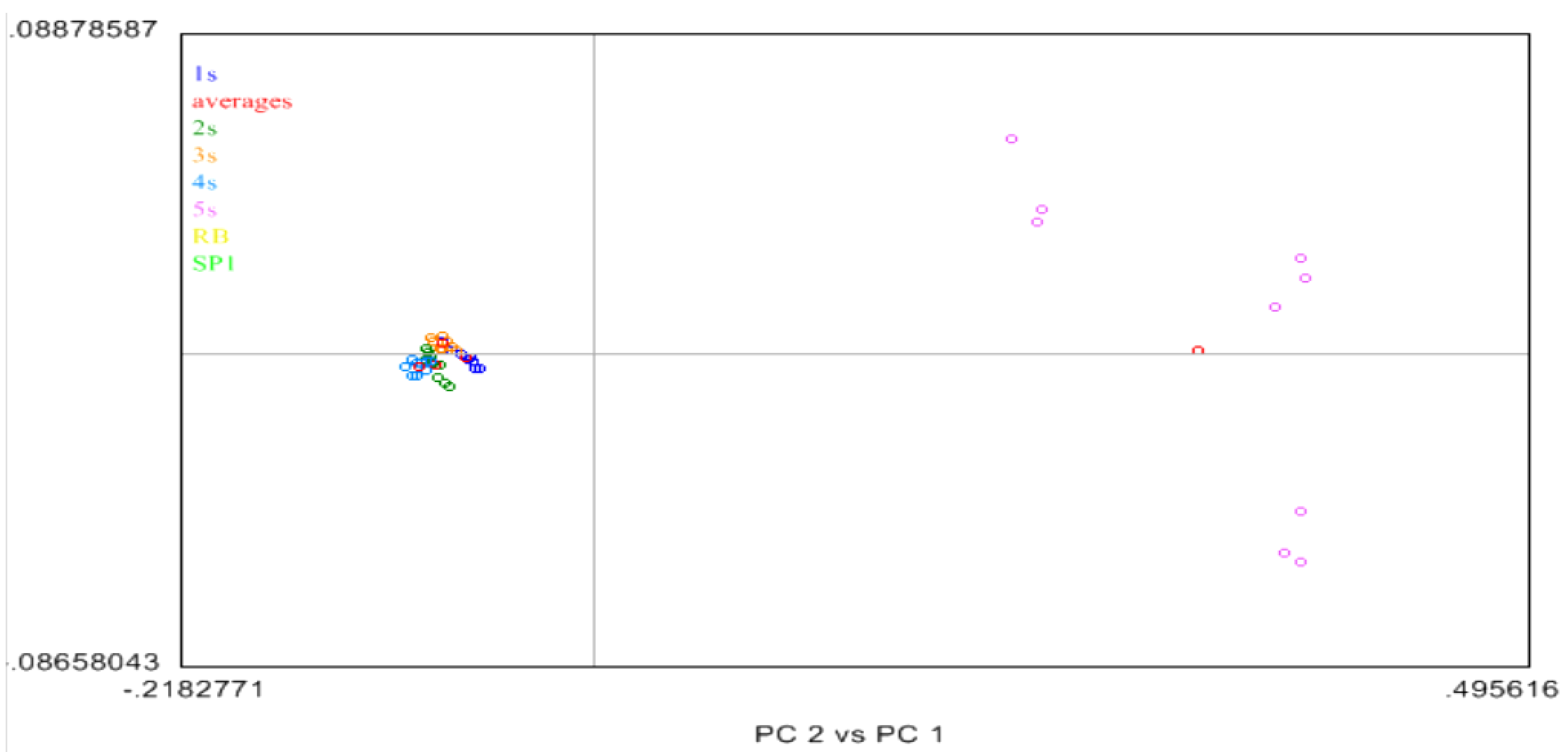
3. Experimental Section
3.1. Materials and Method
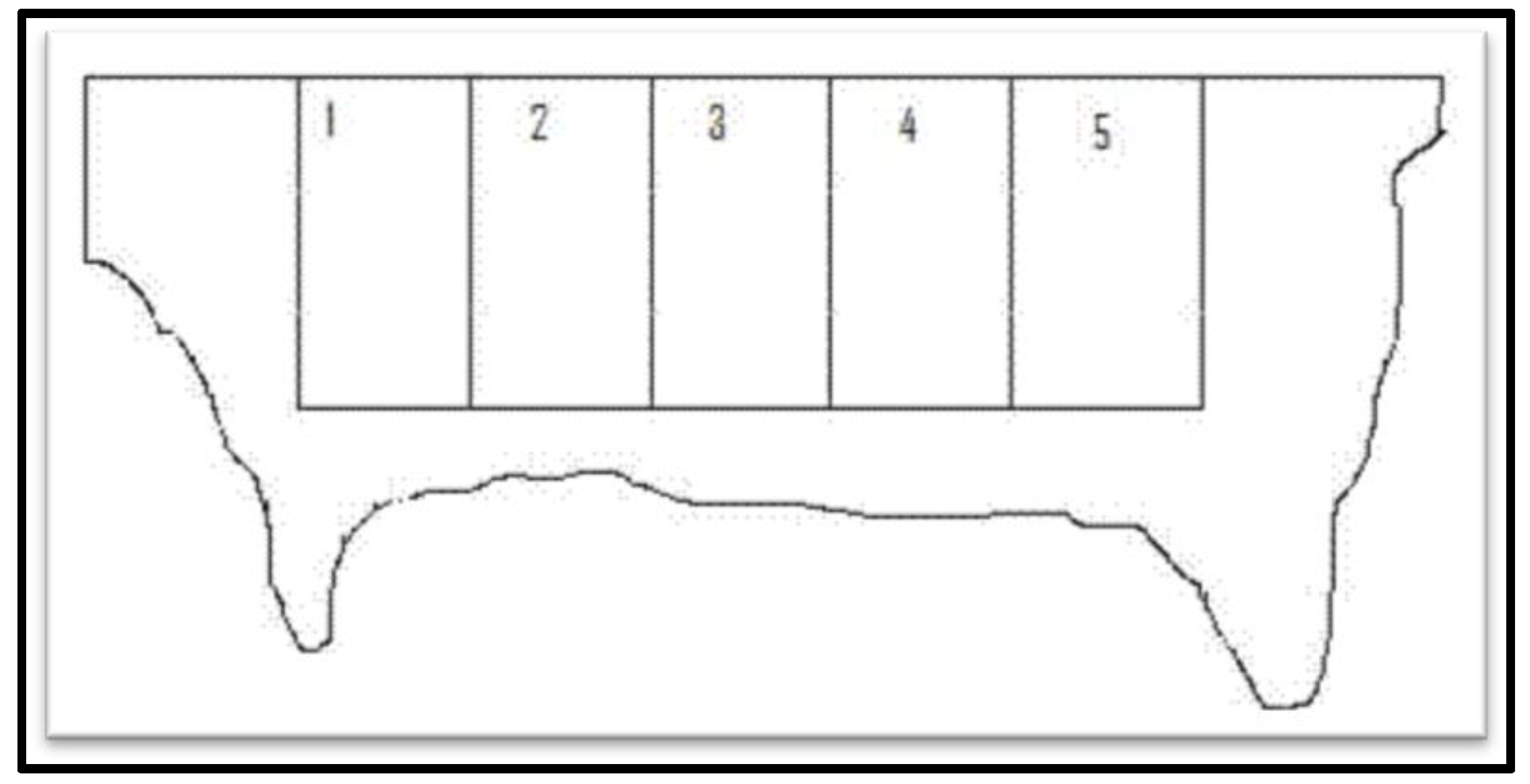
3.2. Preparation of Dried Collagenous Biomaterial (BCD)
3.3. Sample Preparation
3.3.1. Pulverization of BCD Samples
3.4. Extraction of the Proteoglycan, Decorin, from Powdered BCD Samples
3.5. Decorin Analysis Based on sGAG Portion of the Molecule
3.6. Determination of Mechanical Properties
3.7. The Near Infrared Spectroscopy
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Castell, J.C.; Adzet, J.M.; Sabe, R. International patent number PCT/IB2009/055733.
- Castell, J.C.; Adzet, J.M.; Sabe, R. Dry biomaterial production from fresh hides and skins. In 105th ALCA Annual Meeting, Wheeling, West Virginia, USA, 18-21 June 2009.
- Castell, J.C.; Adzet, J.M.; Sabe, R. Fast chrome tanning by immersion of BCD. In Proceedings of the XXX-International Union Leather Technician and Chemists Society (IULTCS) Congress, Beijing, China, 11-14 October 2009.
- Scott, P.G.; McEwan, P.A.; Dodd, C.M.; Bergmann, E.M.; Bishop, P.N.; Bella, J. Crystal structure of the dimeric protein core of decorin, the archetypal small leucine-rich repeat proteoglycan. Proc. Natl. Acad. Sci. USA 2004, 101, 15633–15638. [Google Scholar]
- Ramamurthy, P.; Hocking, A.M.; McQuillan, D.J. Recombinant decorin glycoforms-Purification and structure. ASBMB 1996, 271, 19578–19584. [Google Scholar]
- Aldema-Ramos, M.L.; Latona, R.J.; Fortis, R.L; Marmer, W.N. Identification of decorin and other proteins in bovine hides as they are processed to leather. JALCA 2008, 103, 324–329. [Google Scholar]
- Aldema-Ramos, M.L.; Liu, C. A novel system of removing decorin, a minor proteoglycan of bovine hides, to improve the quality of leather. JALCA 2010, 105, 222–228. [Google Scholar]
- Castell, J.C.; Aldema-Ramos, M.L.; Adzet, J.M.; Sabe, R. Decorin content in various BCD (Dried Collagenous Biomaterial) samples. In Proc. XXXI-IULTCS Congress, Valencia, Spain, 27-30 September 2011.
- Mozersky, S.M.; Wildermuth, R.J.; Marmer, W.N. Estimation of the sulfated glycosaminoglycan content of bovine skin with Alcian blue. JALCA 2003, 98, 337–343. [Google Scholar]
- Karlsson, M.; Bjornsson, S. Quantitation of Proteoglycans in Biological Fluids Using Alcian Blue. Proteoglycan Protocol. Meth. Mol. Biol. 2001, 171, 159–172. [Google Scholar] [CrossRef]
- ASTM D2813-03(2008). Standard Practice for Sampling Leather for Physical and Chemical Tests. ASTM Int. 2007, 15, 266–267. [CrossRef]
- SGAG Assay Kit manual. Kamiya Biochemical Company: Seattle, Washington, USA, 2006.
- Workman, J.; Weyer, L. Practical Guide to Interpretive Near-Infrared Spectroscopy. CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Barton, F. Theory and principles of near infrared spectroscopy, Spectroscopy Europe. Wiley: Chicester, UK, 2002; pp. 12–18. [Google Scholar]
- MicroPhazir version 3.101. Portable Analytical Instruments. Thermo Fisher Scientific, Tewksbury, MA, USA. Available online: http://www.thermofisher.com/ (accessed on July-December 2011).
- Esbensen, K.H.; Geladi, P. Principal Component Analysis: Concept, Geometrical Interpretation, Mathematical Background, Algorithms, History, Practice. In Comprehensive Chemometrics; Brown, S.D., Tauler, R., Walczak, B., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2009; pp. 211–226. [Google Scholar]
- Aldema-Ramos, M.L.; Muir, Z.E.; Solaiman, D.K.Y.; Schreyer, S. Microbiological and near IR studies of the leather from hides presoaked in formulations that can remove hardened bovine manure. JALCA 2012, 107, 302–310. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Aldema-Ramos, M.L.; Castell, J.C.; Muir, Z.E.; Adzet, J.M.; Sabe, R.; Schreyer, S. Decorin Content and Near Infrared Spectroscopy Analysis of Dried Collagenous Biomaterial Samples. Biomolecules 2012, 2, 622-634. https://doi.org/10.3390/biom2040622
Aldema-Ramos ML, Castell JC, Muir ZE, Adzet JM, Sabe R, Schreyer S. Decorin Content and Near Infrared Spectroscopy Analysis of Dried Collagenous Biomaterial Samples. Biomolecules. 2012; 2(4):622-634. https://doi.org/10.3390/biom2040622
Chicago/Turabian StyleAldema-Ramos, Mila L., Joan Carles Castell, Zerlina E. Muir, Jose Maria Adzet, Rosa Sabe, and Suzanne Schreyer. 2012. "Decorin Content and Near Infrared Spectroscopy Analysis of Dried Collagenous Biomaterial Samples" Biomolecules 2, no. 4: 622-634. https://doi.org/10.3390/biom2040622
APA StyleAldema-Ramos, M. L., Castell, J. C., Muir, Z. E., Adzet, J. M., Sabe, R., & Schreyer, S. (2012). Decorin Content and Near Infrared Spectroscopy Analysis of Dried Collagenous Biomaterial Samples. Biomolecules, 2(4), 622-634. https://doi.org/10.3390/biom2040622